Abstract
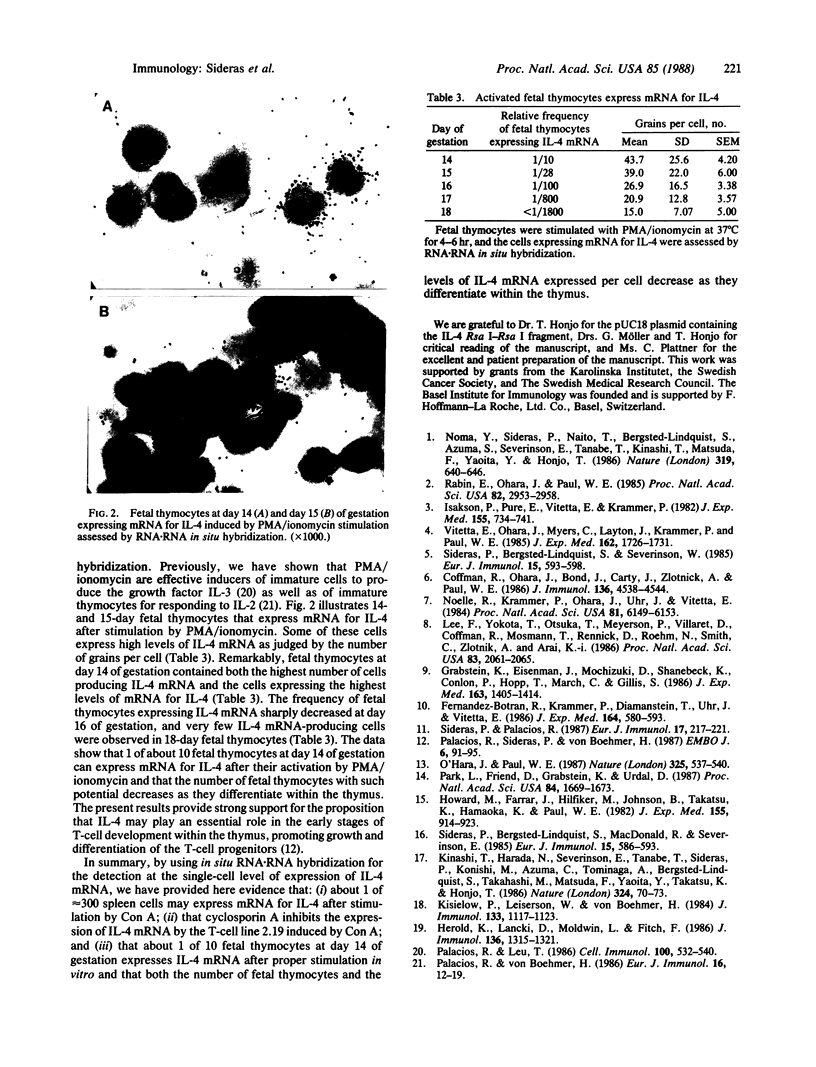
We have made use of RNA.RNA in situ hybridization to study the presence of cells producing mRNA for interleukin 4 (IL-4) in the developing thymus, spleen, and T-cell line 2.19. Approximately 1 of 300-400 spleen cells expressed detectable IL-4 mRNA 24 hr after their stimulation by the lectin concanavalin A. Spleen cells were also induced to express mRNA for IL-4 by stimulation with alloantigens. Splenocytes producing mRNA for IL-4 were detected 4 hr after stimulation by concanavalin A; the response peaked at approximately equal to 24 hr and was undetectable by 72 hr. Cyclosporin A inhibited the synthesis of IL-4 mRNA in the T-cell line 2.19, which had been induced by concanavalin A. Approximately 1 of 10 fetal thymocytes at day 14 of gestation expressed mRNA for IL-4 after their stimulation by phorbol 12-myristate 13-acetate and ionomycin. Both the frequency of fetal thymocytes expressing IL-4 mRNA and the amount of mRNA for IL-4 synthesized per cell sharply decreased at day 16 of gestation, and less than 1 of 1800 fetal thymocytes at day 18 of gestation expressed detectable IL-4 mRNA. Our results define the relative frequency of cells capable of expressing IL-4 mRNA after stimulation in vitro in the spleen and in the developing thymus. The data strongly argue for an important role of IL-4 in growth and differentiation of lymphoid cells, notably during T-cell development within the thymus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K., Eisenman J., Mochizuki D., Shanebeck K., Conlon P., Hopp T., March C., Gillis S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J Exp Med. 1986 Jun 1;163(6):1405–1414. doi: 10.1084/jem.163.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold K. C., Lancki D. W., Moldwin R. L., Fitch F. W. Immunosuppressive effects of cyclosporin A on cloned T cells. J Immunol. 1986 Feb 15;136(4):1315–1321. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Leiserson W., Von Boehmer H. Differentiation of thymocytes in fetal organ culture: analysis of phenotypic changes accompanying the appearance of cytolytic and interleukin 2-producing cells. J Immunol. 1984 Sep;133(3):1117–1123. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987 Feb 5;325(6104):537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Leu T. Splenocytes and bone marrow cells from T-cell deficient Nu/Nu mice secrete interleukin 3 activity after stimulation in vitro. Cell Immunol. 1986 Jul;100(2):532–540. doi: 10.1016/0008-8749(86)90051-1. [DOI] [PubMed] [Google Scholar]
- Palacios R., Sideras P., von Boehmer H. Recombinant interleukin 4/BSF-1 promotes growth and differentiation of intrathymic T cell precursors from fetal mice in vitro. EMBO J. 1987 Jan;6(1):91–95. doi: 10.1002/j.1460-2075.1987.tb04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Von Boehmer H. Requirements for growth of immature thymocytes from fetal and adult mice in vitro. Eur J Immunol. 1986 Jan;16(1):12–19. doi: 10.1002/eji.1830160104. [DOI] [PubMed] [Google Scholar]
- Park L. S., Friend D., Grabstein K., Urdal D. L. Characterization of the high-affinity cell-surface receptor for murine B-cell-stimulating factor 1. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1669–1673. doi: 10.1073/pnas.84.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Bergstedt-Lindqvist S., MacDonald H. R., Severinson E. Secretion of IgG1 induction factor by T cell clones and hybridomas. Eur J Immunol. 1985 Jun;15(6):586–593. doi: 10.1002/eji.1830150611. [DOI] [PubMed] [Google Scholar]
- Sideras P., Bergstedt-Lindqvist S., Severinson E. Partial biochemical characterization of IgG1-inducing factor. Eur J Immunol. 1985 Jun;15(6):593–598. doi: 10.1002/eji.1830150612. [DOI] [PubMed] [Google Scholar]
- Sideras P., Palacios R. Bone marrow pro-T and pro-B lymphocyte clones express functional receptors for interleukin (IL) 3 and IL 4/BSF-1 and nonfunctional receptors for IL 2. Eur J Immunol. 1987 Feb;17(2):217–221. doi: 10.1002/eji.1830170211. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]