Abstract
Intractable bleeding from gastric and duodenal ulcers is associated with significant morbidity and mortality. Aggressive treatment with early endoscopic hemostasis is essential for a favourable outcome. In as many as 12%-17% of patients, endoscopy is either not available or unsuccessful. Endovascular therapy with selective catheterization of the culprit vessel and injection of embolic material has emerged as an alternative to emergent operative intervention in high-risk patients. There has not been a systematic literature review to assess the role for embolotherapy in the treatment of acute upper gastrointestinal bleeding from gastroduodenal ulcers after failed endoscopic hemostasis. Here, we present an overview of indications, techniques, and clinical outcomes after endovascular embolization of acute peptic-ulcer bleeding. Topics of particular relevance to technical and clinical success are also discussed. Our review shows that transcatheter arterial embolization is a safe alternative to surgery for massive gastroduodenal bleeding that is refractory to endoscopic treatment, can be performed with high technical and clinical success rates, and should be considered the salvage treatment of choice in patients at high surgical risk.
Keywords: Peptic ulcer, Massive bleeding, Endoscopy, Angiography, Embolization
INTRODUCTION
Acute bleeding is the most common complication of peptic ulcer disease and about half the cases of upper gastrointestinal bleeding (UGI) are caused by gastric and duodenal ulcers[1,2]. First-line endoscopy achieves bleeding control in as many as 98% of patients[3,4]. Despite these measures, the mortality rate in patients with bleeding peptic ulcers remains as high as 5% to 10%[5,6] due to a combination of advanced age, multiple co-morbidities, and high transfusion requirements[7]. Current treatment algorithms for massive UGI bleeding recommend aggressive correction of coagulation disorders followed by endoscopy[8,9]. Endoscopic therapy with epinephrine injection and heat probe coagulation is the most reliable method. Re-bleeding is usually managed with a second endoscopic attempt. Severe bleeding despite conservative medical treatment or endoscopic intervention occurs in 5% of patients[10] and requires surgery or transcatheter arterial embolization. Surgery is associated with mortality rates as high as 20% to 40%[11]. Although endovascular management is not included in the treatment algorithms for UGI bleeding described in surgical textbooks, selective catheter-directed embolization has been proposed as a less hazardous alternative to surgery, especially for high-risk patients[12,13], and is now considered in many institutions as the first-line intervention for massive gastroduodenal bleeding after failed endoscopic treatment[12-15]. The obvious advantage of transcatheter embolization is avoidance of a laparotomy in a critically ill patient. With the advent of metallic coils, gelfoam, and surgical glue, outcomes after embolization have compared favourably with those of surgery. The purpose of this review is to review the data on the indications, safety, effectiveness, and outcomes of embolotherapy in the treatment of acute UGI from gastroduodenal ulcers.
KEYWORD SEARCH
We searched PubMed for studies of embolization for peptic ulcer bleeding published in English from 1992 to 2009. Further studies were sought by manually searching the reference lists of articles retrieved via PubMed. We then selected the articles that had well-defined indications for the intervention and offered a detailed description of the outcomes, including technical and clinical success rates, re-bleeding, re-intervention, need for surgery for bleeding control, and morbidity and mortality rates. To avoid selection bias associated with a small series, we excluded studies with fewer than 10 patients and anecdotal case-reports. Studies of patients with bleeding from causes other than peptic ulcers were also excluded. The results were tabulated as absolute numbers and percentages. Mean values of the outcome variables of interest were computed. Information on indications, technique, complications, and a variety of other topics of interest is presented as a narrative, in order to provide a better understanding of the current status and controversial aspects of the endovascular treatment of UGI bleeding from peptic ulcers.
INDICATIONS
Transcatheter arterial embolization as an alternative to surgery for the control of UGI bleeding was introduced by Rösch et al[16] in 1972. Since then, arterial catheterization has become a useful diagnostic and therapeutic tool in selected populations[17]. The typical candidate presents with massive bleeding (transfusion requirement of at least four units of blood per 24 h) or hemodynamic instability (hypotension with systolic pressure less than 100 mmHg and heart rate of 100/min or clinical shock secondary to blood loss) that has not responded to conservative medical treatment combining volume replacement, proton pump inhibitors, and at least one endoscopic procedure aimed at controlling the bleeding[18]. At this point, surgery is offered to low-risk patients and percutaneous embolotherapy to high-risk patients. Finally, endovascular treatment can be used if the bleeding recurs after surgery[19].
TECHNIQUE
A transfemoral approach was used in most of the case-series retrieved by our literature search. A 5-French sheath is placed in the common femoral artery. Brachial access may be necessary when there is an acute angulation at the origin of the celiac axis. A variety of selective catheters can be used to cannulate the celiac artery and to access the common hepatic artery. Once access is obtained, arteriography is performed to delineate the arterial anatomy and to identify contrast extravasation. If no extravasation is seen, then superselective catheterization of the gastroduodenal artery (GDA) (Figure 1), left gastric artery (Figure 2), or splenic artery (Figure 3) is performed, depending on the endoscopic evidence concerning the probable bleeding site. During this step, a microcatheter is useful but not indispensable. Arteriography after superselective cannulation might reveal extravasation that was missed during contrast injection into the main hepatic artery. When a dual supply to the bleeding area is suspected, both arterial sources must be embolized. This typically occurs with ulcers that erode the GDA: embolization in this case needs to start distally to prevent persistent “backdoor” bleeding from the right gastroepiploic and superior pancreaticoduodenal arteries, and should then move to the proximal side of the erosion. If no evidence of bleeding is found on the pre-embolization arteriogram, then blind embolization is performed, typically guided by the endoscopic findings regarding the bleeding site (Figure 4). Another useful manoeuvre in this scenario is the placement, during the pre-embolization endoscopy, of clips around the bleeding site. The clips remain in position for several hours and allow for an educated guess about the location of the bleeding arterial branch[20]. If, despite the injection of a contrast agent, no extravasation is seen, then the branches terminating at each clip are selectively catheterized using microcatheter techniques and embolized. Arteriography with multiple projections is necessary at this step to assess the relationship between each clip and the adjacent branches. Infusion of a fibrinolytic agent such as t-PA, intra-arterial anticoagulants, or vasodilators to temporarily increase the bleeding rate during angiography has been reported to facilitate the angiographic identification and localisation of the bleeding vessel[21].
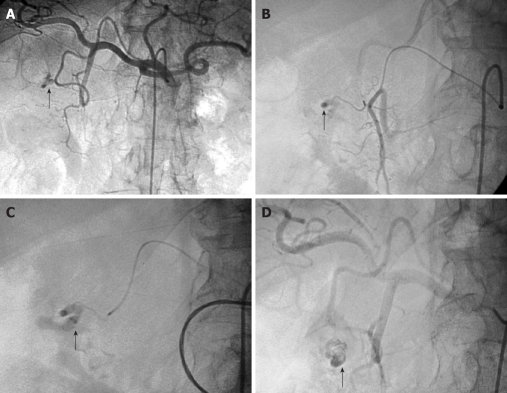
Figure 1.
Arteriogram images of bleeding from a bulbar duodenal ulcer in a 76-year-old man. A, B: Arteriogram showing contrast medium extravasated from a slender branch of the gastroduodenal artery (GDA) into the duodenum (arrows); C, D: After microcatheterization, selective glue embolization (radiopaque because of associated lipiodol (arrows) preserving the GDA ensured control of the bleeding, with no early or late recurrences.
Figure 2.
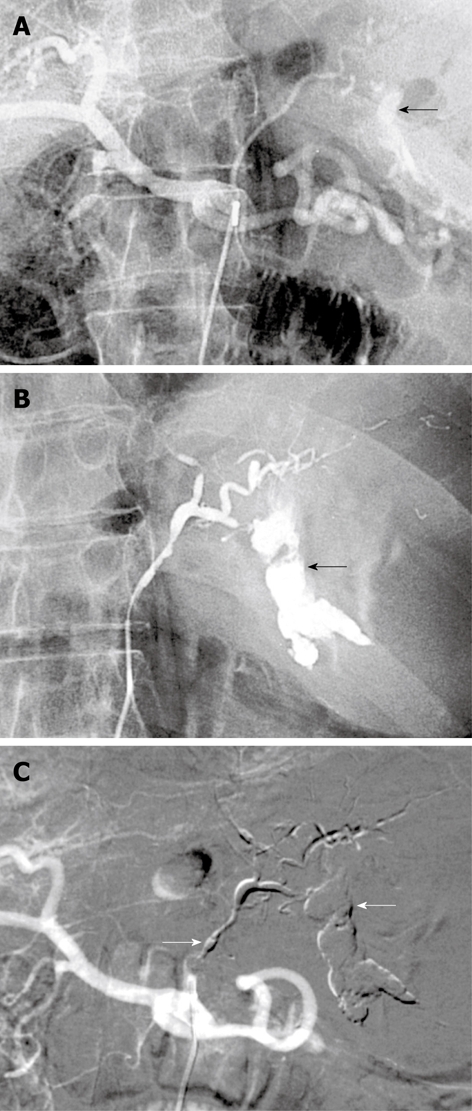
Bleeding Dieulafoy lesion in an 87-year-old man. A, B: Selective angiography shows contrast medium extravasation from the left gastric artery at the celiac trunk, indicating active bleeding (arrows); C: After arterial microcatheterization, bleeding was controlled after embolization of the left gastric artery using a Glubran/Lipidol mixture (1:3) (arrows).
Figure 3.
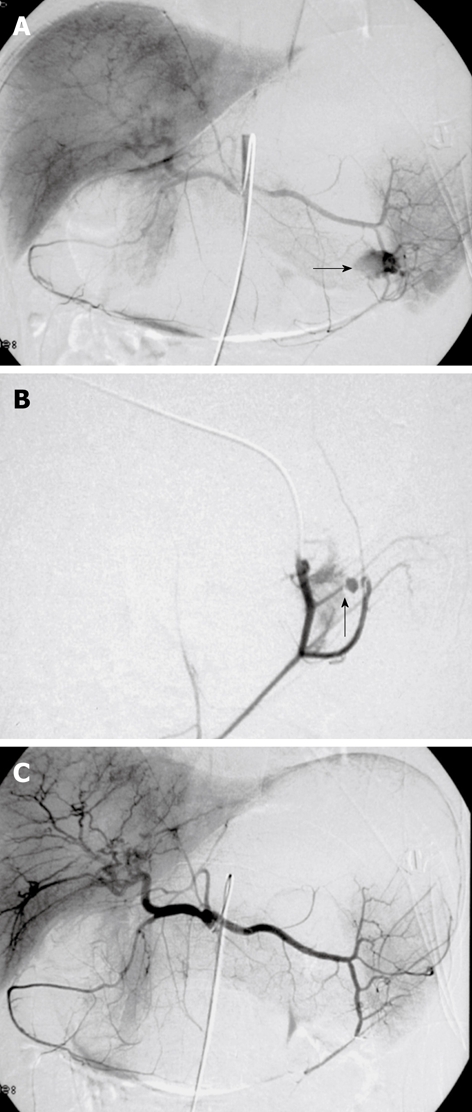
Digital subtraction images from a 37-year-old man with massive hematemesis. A, B: Selective angiography shows a bleeding ulcer in the fundus of the stomach. Extravasation of contrast medium from a branch of the left gastroepiploic artery is seen (arrows); C: The control angiogram after glue embolization throughout the splenic artery shows complete and selective occlusion of the bleeding branch, with no active bleeding. The patient was discharged from the hospital 4 d later.
Figure 4.
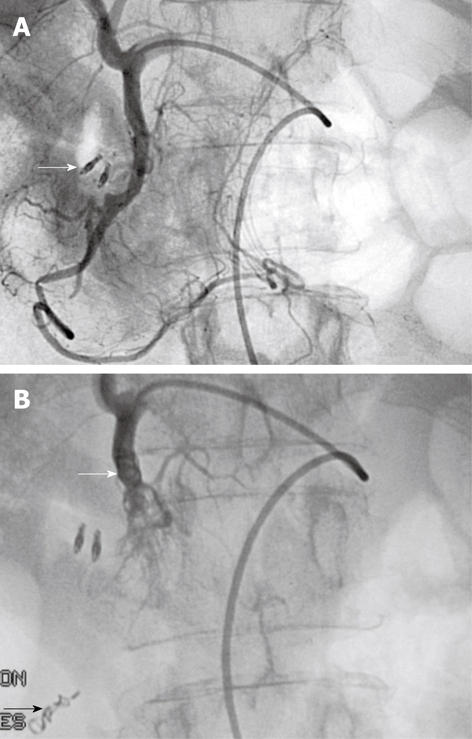
Typical sandwich embolization in a 75-year-old woman with bleeding from a postbulbar duodenal ulcer at endoscopy. A: Angiography before embolization, guided by clip position (arrow): no evidence of active bleeding; B: Result after coil embolization of the distal and proximal GDA (with gelatine sponge in the arterial trunk), including the anterior and posterior superior pancreaticoduodenal arteries and the right gastroepiploic artery, to prevent retrograde flow (arrows). No ischemic complications were reported.
COMPLICATIONS
Groin hematomas and contrast-related complications occur with the same frequency as during other endovascular procedures. Acute renal failure may develop as a result of multiple factors including contrast injection and intravascular volume depletion. Duodenal ischemia can result from embolization of terminal muscular branches or from embolization of the main GDA with polyvinyl alcohol (PVA) particles. Typical symptoms include persistent epigastric pain, nausea and, occasionally, vomiting. Endoscopy shows small multiple duodenal erosions consistent with healing ischemic lesions. Predisposing factors include previous abdominal surgery and/or radiation therapy. Conservative treatment with proton pump inhibitors and maintenance of NPO status is usually sufficient. Inadvertent embolization of the main hepatic artery can result in a broad spectrum of manifestations, ranging from temporary liver enzyme elevation to life-threatening hepatic failure, for which risk factors include cirrhosis and associated portal vein compromise[12]. Inadvertent placement of coils in the main branches of the celiac axis has been reported. Given the rich collateral circulation, however, coils in the left gastric or splenic artery rarely produce organ-threatening ischemia[22]. Duodenal stenosis can become a serious problem after GDA embolization. It is more common after superselective embolization of terminal muscular branches with surgical glue and can occur several years after successful embolization. Balloon dilatation can be attempted initially, although surgery is required in patients with persistent symptoms of duodenal obstruction[23].
OUTCOMES IN CASE-SERIES
We identified 13 studies (422 patients; mean age, 69 years) on the endovascular management of intractable gastrointestinal bleeding from gastroduodenal ulcers. Endoscopy was performed unsuccessfully in 98% of these patients (Table 1). The vast majority of the patients with major co-morbidities and were considered at high surgical risk. Endovascular embolization was technically successful in 392 (93%) patients. A variety of embolic materials including coils, PVA particles, blood clot, gelfoam, and cyanoacrylate glue were used. The “sandwich” technique with placement of embolic material on either side of the bleeding vessel was used in most case-series to minimize the risk of recurrent bleeding due to collaterals. Active extravasation was present at the time of embolization in 53% of patients. The other patients underwent blind embolization guided by the endoscopy findings or by clips placed around the bleeding site. In the subgroup with technically successful embolization, the rate of bleeding cessation (clinical success rate) was 81% (Table 2).
Table 1.
Synopsis of the studies under review
| Ref., yr | Patients (n) | Mean age (yr) | Previous endoscopy (%) | Active extravasation (%) | Technical success (%) |
| Lang et al[23], 1992 | 57 | 52 | NA | 100 | 91 |
| Toyoda et al[33], 1995 | 11 | 65 | 100 | 54 | 100 |
| Toyoda et al[37], 1996 | 30 | 62 | 100 | NA | 100 |
| Walsh et al[43], 1999 | 50 | 64 | 100 | 50 | 92 |
| De Wispelaere et al[40], 2002 | 28 | 69 | 100 | 39 | 89 |
| Ljungdahl et al[19], 2002 | 18 | 78 | 72 | 50 | 72 |
| Ripoll et al[24], 2004 | 31 | 75 | 100 | NA | 100 |
| Holme et al[25], 2006 | 40 | 70 | 100 | 30 | 100 |
| Eriksson et al[20], 2006 | 10 | 75 | 100 | 10 | 100 |
| Loffroy et al[13], 2008 | 35 | 71 | 100 | 66 | 94 |
| Larssen et al[15], 2008 | 36 | 80 | 100 | 42 | 92 |
| van Vugt et al[35], 2009 | 16 | 71 | 100 | 75 | 88 |
| Loffroy et al[26], 2009 | 60 | 69 | 100 | 63 | 95 |
| All studies | 422 | 69 | 98 | 53 | 93 |
NA: Not available.
Table 2.
Outcomes in case-series that included more than 10 patients treated with endovascular embolization for peptic ulcer bleeding over a 17-year period
| Ref., yr | Clinical success (%) | Re-bleeding rate (%) | Need for surgery (%) | Complication rate (%) | 30-d mortality (%) |
| Lang et al[23], 1992 | 86 | 56 | 2 | 16 | 4 |
| Toyoda et al[33], 1995 | 91 | 18 | 18 | 0 | 27 |
| Toyoda et al[37], 1996 | 80 | 23 | 13 | NA | 23 |
| Walsh et al[43], 1999 | 52 | 52 | 37 | 4 | 40 |
| De Wispelaere et al[40], 2002 | 64 | 36 | 21 | 0 | 46 |
| Ljungdahl et al[19], 2002 | 67 | 8 | 8 | 0 | 6 |
| Ripoll et al[24], 2004 | 71 | 29 | 16 | 0 | 26 |
| Holme et al[25], 2006 | 65 | 28 | 35 | 0 | 25 |
| Eriksson et al[20], 2006 | 80 | 0 | 20 | NA | NA |
| Loffroy et al[13], 2008 | 94 | 17 | 14 | 6 | 21 |
| Larssen et al[15], 2008 | 72 | 9 | 30 | 8 | 17 |
| van Vugt et al[35], 2009 | 81 | 19 | 12 | NA | 38 |
| Loffroy et al[26], 2009 | 72 | 28 | 12 | 10 | 27 |
| All studies | 75 | 25 | 18 | 4 | 25 |
The table shows the rates of clinical success, recurrent bleeding after technically successful embolization, need for surgery to control the bleeding, complications, and peri-procedural mortality.
Overall, 25% (106/422) of patients had persistent bleeding. However, almost half of them responded to repeat embolization. Finally, 18% of patients overall underwent surgery for bleeding control (Table 2). Major and minor embolization-related complications developed in 4% of patients and included access-site complications, dissection of the target vessel, and hepatic or splenic infarction. The most significant long-term complication was duodenal stenosis, particularly after glue embolization of terminal muscular branches of the GDA. Overall 30-d mortality was 25% (Table 2). The data available in the study reports did not allow us to assess the causes of death or their relationship with the result of the embolization or need for further intervention. Although the mortality rates seem as high as those in several case-series of emergent surgery for UGI bleeding, they should be interpreted with the knowledge that most of the patients treated with embolotherapy had been turned down for surgery due to major co-morbidities and advanced age.
Given the variability in the way the results are reported and incomplete data on risk factors in the patient populations, we cannot draw conclusions regarding the impact of embolotherapy on mortality. Nevertheless, several important points can be made. First, mortality and complication rates varied widely across the series, highlighting the influence of individual expertise and center volume on the outcomes. Second, the visualization of active extravasation followed by selective embolization was not consistently associated with a higher short-term clinical success rate. Possible explanations are the intermittent nature of gastrointestinal bleeding and the presence of bleeders missed by highly selective embolotherapy. Lastly, only 18% of the patients who initially underwent embolization finally needed surgery to control recurrent bleeding. Thus, embolotherapy considerably diminishes the need for laparotomy in patients with acute UGI bleeding from peptic ulcers.
A few of the most noteworthy case-series that raised interesting points are briefly summarized below. Ripoll et al[24] compared outcomes after embolization (31 patients) or surgery (39 patients) for bleeding from UGI peptic ulcers. Patients treated with embolotherapy were older (mean age, about 10 years older) and had higher rates of cardiovascular disease and anticoagulation treatment. Otherwise, co-morbidities were not different between the two groups. In the embolization group, the technical failure rate was 6.5% (n = 2) and the re-bleeding rate was 29% (n = 9). Four of the patients with persistent bleeding underwent surgical exploration. In the surgery group, the re-bleeding rate was 23.1% (n = 9); five of these patients underwent a repeat surgical procedure for bleeding control. In addition, seven other patients required repeat surgery for complications from the initial operation. Overall, the embolization and surgery groups were not significantly different regarding the need for additional surgery (16.1% vs 30.8%, respectively) or survival (25.8% vs 20.5%, respectively). However, the re-intervention rate was considerably higher in the surgery group and the difference would perhaps have been statistically significant had the sample sizes been larger. Holme et al[25] reported on 40 consecutive patients who were referred for embolotherapy after unsuccessful endoscopic or surgical treatment. Long-term bleeding control was achieved in 26 (65%) patients. Of the 12 patients with active bleeding from a duodenal ulcer at the time of embolization, 10 (83%) had the bleeding controlled; one of these patients experienced re-bleeding, which was managed by surgery. In this subgroup, lasting hemostasis was achieved in eight (75%) patients. The 28 other patients had no signs of active bleeding on the diagnostic angiogram and underwent blind GDA coil embolization. Among them, 11 (39%) experienced re-bleeding. The inability to accurately identify and selectively embolize the culprit vessel in this subgroup is the most likely explanation for the high re-bleeding rate. The group with active bleeding on the angiogram had a higher likelihood of lasting hemostasis (75% vs 66%), underlining the limited effectiveness of blind coil embolization of the GDA. In this series, 10 patients died, including five as a result of continuous bleeding. Of note, this series included 13 patients who underwent duodenotomy for surgical bleeding control then experienced re-bleeding that was managed endovascularly. Eriksson et al[20] reported a series of 10 patients who were referred for embolotherapy after endoscopy failed to control bleeding from acute duodenal ulcers. To guide the endovascular treatment, a metallic clip was placed to mark the edge of the ulcer next to the bleeding site. Embolization ensured bleeding control in eight patients; the two remaining patients required surgery. In six patients, the clip played a crucial role in identifying the bleeding vessel, as there was no evidence of contrast extravasation on the angiogram. The clip was particularly useful in three patients: two patients had bleeding from a supraduodenal artery without connection to the GDA, and the remaining patient had bleeding from an erosion in the inferior pancreaticoduodenal artery that arose from the superior mesenteric artery. One of the series with the best follow-up data was described by Lang et al[23], who reported immediate and long-term results in 57 patients with bleeding duodenal ulcers. Control of bleeding was achieved in 52 of 57 patients. Superselective terminal muscular branch embolization was as effective as embolization of the main GDA. In eight of these patients, a second catheter-based intervention was needed to achieve complete bleeding cessation. Importantly, 29 of the 52 patients whose embolization procedure was successful experienced re-bleeding during follow-up (up to 7 years), underlining the need for aggressive long-term risk factor modification and treatment of the underlying peptic ulcer disease. Long-term bleeding control was more common in the subgroup of patients who underwent selective terminal muscular branch embolization, compared to the individuals treated with embolization of the main GDA trunk (53% vs 27%, P = 0.084). Long-term success in this series was related to the embolic material used. For occlusion of the muscular branch arteries, 6-cyanoacrylate had the highest success rate, whereas for occlusion of the main GDA, an epsilon-aminocaproic acid-induced blood clot was superior over the other modalities (coils, gelatine sponge particles, or PVA particles). Together with re-bleeding, duodenal stenosis was the most troublesome complication and developed in nine patients between 8 mo and 7 years after the embolization procedure. This complication was more common after superselective embolization of terminal muscular branches. Surgical correction of the stenosis was necessary in eight patients to address persistent symptoms. Another patient required multiple balloon dilatations for duodenal stenosis. Balloon dilatations were performed in one additional patient for recurrent symptoms of duodenal obstruction after surgical resection. We reported our experience managing 60 patients with peptic ulcer bleeding[26]. The technical success rate was 95%. In 37% (n = 22) of patients, the angiography showed no contrast extravasation and, therefore, empiric embolization was performed based on the endoscopic findings prior to the procedure. Approximately 28% (n = 16) of these patients experienced re-bleeding, and only three underwent repeat embolization. Interestingly, the univariate analysis showed that early re-bleeding was associated with several of the study variables including coagulation disorders, a longer time from shock onset to angiography, a larger number of red-blood-cell units transfused before angiography, having two or more co-morbid conditions, and being treated with coils as the only embolic agent. The multivariate analysis identified two factors that significantly predicted failure of embolization, namely, the presence of coagulation disorders (P = 0.027) and the use of coils as the only embolic agent (P = 0.022). The mortality rate was not different in the patient group with clinically successful embolization and in the group with failed embolization (22% vs 37%)[26].
TOPICS OF INTEREST
Predictors of favourable outcome
In surgical case-series, mortality rates in patients who have UGI bleeding from gastroduodenal ulcers and who do not respond to conservative therapy have ranged from 17% to 43%[27,28]. Factors influencing mortality include advanced age, trauma or sepsis, recent major operation, lung or liver disease, and massive blood transfusions[28,29]. After embolization in patients who are too sick to undergo surgery, mortality rates were similar, with a range of 10% to 45%. A number of factors have been identified as influencing post-embolization mortality. One of the most important and common factors is the absence of early re-bleeding, especially after selective embolization of a vessel with contrast extravasation on the initial angiography. Patients with angiographic extravasation and successful embolization have considerably lower mortality rates compared to patients who require surgery after failed embolization (38% vs 83%, respectively)[30]. Coagulopathy correlates closely with clinical failure and death after embolization. Thus, patients with impaired coagulation are three times more likely to re-bleed after initially successful embolization and 10 times more likely to die as a result of bleeding, compared to those with normal coagulation[2,12]. Rescue surgery after a failed embolization attempt has a very high mortality rate that exceeds even the 50% rate associated with emergent surgery[31,32]. In other series, underlying medical problems such as cirrhosis and malignancy, had major impacts on the mortality rate. Finally, in patients with multiorgan failure, clinically successful embolization appears to offer the only chance for survival. In the case-series reported by Schenker et al[22], the mortality rate was 96% in patients with multiorgan failure who did not respond to embolization vs 31% in those who did.
Choice of embolic agent
A focus of greater controversy is the influence of the type of embolic agent on the clinical outcome. There is general agreement that embolic therapy is superior over vasopressin infusion for the treatment of UGI bleeding from gastroduodenal ulcers[17]. The choice of the best embolic agent remains a matter of debate. Coils alone inserted into the GDA or super selectively in the pancreaticoduodenal arteries have been used successfully by several authors[33-35]. Lang et al[23] compared several embolic agents in a case-series of 57 patients. Safety and efficacy were best with autologous blood clot for proximal GDA embolization and with tissue adhesive for occlusion of the distal vessels from the GDA. These authors reported a 40% rate of duodenal stricture with tissue adhesives, a finding that may be related to the use of tissue adhesives to embolize the terminal muscular branches, and not to the nature of the embolization agent. The same group reported a high rate of re-bleeding when PVA particles or gelfoam were used alone. Similarly, Encarnacion et al[2] obtained a low success rate (62%) in their case-series, which chiefly included patients embolized with gelatine sponge alone. Good results have also been reported with cyanoacrylate[36,37] and with the combination of gelatine sponge and coils[38]. Most of these series included small study populations; therefore, no statistical conclusions can be drawn. Finally, Aina et al[12] compared embolization with coils alone vs coils combined with PVA particles or gelfoam. By multivariate regression analysis, the use of coils alone was associated with re-bleeding in patients with coagulopathy, a finding that supports the use of PVA or gelfoam in combination with coils in this patient subgroup. We also found that using coils alone was significantly associated with early re-bleeding[26]. Otherwise, the nature of the embolic agent does not seem to affect the clinical response or re-bleeding rate.
Blind or empirical embolization
Blind embolization, defined as embolization without angiographic proof of extravasation, is also controversial. In a study comparing several groups of patients, Dempsey et al[30] found that blind embolization was not helpful in achieving bleeding control. The proportion of patients who required surgery for bleeding control was similar in the patients without angiographic evidence of contrast extravasation who did not undergo embolization and in the patients who underwent blind embolization. However, endoscopy - a crucial procedure for selecting the target vessel for blind embolization - was non-diagnostic in 39% of patients in this case-series[30]. Massive bleeding is often intermittent[39]; therefore, most groups perform embolization based on the endoscopic findings, even when no extravasation is visible on the angiogram. In the case-series by Aina et al[12] and us[26], outcomes were not different between patients who underwent blind embolization and those who underwent embolization after angiographic identification of a bleeding site. Other researchers also advocate endoscopy-directed blind embolization[2,33,40]. Based on the data in the literature and our own experience, we believe that blind embolization is appropriate. The GDA should be embolized using the “sandwich technique”, in which both ends of the artery are filled with coils to avoid retrograde bleeding from the superior mesenteric circulation. If smaller muscular branches terminating at a clip are suspected culprits, then they should be embolized with any of the available materials.
Marking with a metallic clip
Clip placement during endoscopy can help to localize the vessel feeding the bleeding ulcer, even when there is no contrast medium extravasation after injection with the catheter into the common hepatic artery or the main trunk of the GDA. Clip placement is also helpful when the bleeding artery arises separately from the proper hepatic artery or the GDA. Superselective angiography guided by clip position is more likely to visualize the extravasation, thus making blind coil placement unnecessary, increasing the efficacy of the procedure, and decreasing the risk of coil misplacement and inadvertent hepatic embolization[20,26]. The only limitation of this technique is the need for around-the-clock availability of an experienced interventionalist and gastroenterologist, which is easy to achieve only at high-volume centres. This approach increases the likelihood of successful embolization and is now routinely used at our centre. Even when extravasation is not visualized, the clips can guide the blind embolization procedure, as pointed out previously.
Risk for gastrointestinal tract necrosis
Arterial embolization in the UGI tract above the ligament of Treitz is generally considered very safe because of the rich collateral supply to the stomach and duodenum. However, the risk of significant ischemia after embolization is increased in patients with a history of surgery in the same area[41] or with embolic agents that can advance far into the vascular bed such as liquid agents (e.g. tissue adhesives such as cyanoacrylate) or very small particles (e.g. gelatine sponge powder)[41-44]. Although cases have been reported at the acute phase, post-embolization ischemia usually presents as duodenal stenosis at the chronic phase. Lang et al[23] reported duodenal stenosis in seven of 28 patients after embolization of terminal vessels, mostly when tissue adhesive was used. In this series, duodenal stenosis after GDA embolization was far less common and occurred in only two of 29 patients who underwent more proximal GDA occlusion. No major gastric or duodenal ischemic events occurred in our case-series; coils were the most often used single embolic agents, and gelatine sponge plugs were used instead of powder. A tissue adhesive was used only when angiographic extravasation was considered massive, and one part of cyanoacrylate was then diluted in two parts of lipiodol to ensure rapid polymerization. In addition, the mixture was injected selectively into the bleeding vessel while taking care not to fill the normal branches[12].
Angiographic embolization vs surgery
To date, there has been no controlled trial comparing angiographic embolization to surgery as a salvage procedure for failed endoscopic therapy. Two retrospective comparisons showed at least similar efficacy in terms of rates of re-bleeding, morbidity, and mortality. Ripoll et al[24] retrospectively assessed the outcomes of 70 patients with refractory peptic ulcer bleeding: 31 patients underwent angiographic embolization, and 39 patients were managed with surgery. Although the patients treated with angiographic embolization were 10 years older on average and more often had heart disease, there were no major differences in the rates of re-bleeding (29% vs 23%) or mortality (26% vs 21%). Another retrospective comparison, by Eriksson et al[45], included 40 patients who underwent angiographic embolization and 51 patients who underwent surgery after failed endoscopic therapy. The angiographic embolization group was older and had a higher co-morbidity rate. Nevertheless, 30-d mortality was lower in the angiographic embolization group (3% vs 14%). These results are promising, and we are eagerly awaiting the results of randomized, controlled trials.
CONCLUSION
Massive bleeding from a peptic ulcer remains a challenge. Optimal management required a multidisciplinary team of skilled endoscopists, intensivists, experienced UGI surgeons, and interventional radiologists. Endoscopy is the first-line treatment. The role for early elective surgery or angiographic embolization in selected high-risk patients to prevent re-bleeding remains controversial. However, technological advances including lower-profile catheter systems will probably broaden the indications for endovascular treatment of UGI bleeding from gastroduodenal ulcers after failed endoscopy. Although prospective studies are needed to compare these management strategies, the available data suggest that transcatheter arterial embolization is not only a good alternative to surgery, but should now be considered the salvage treatment of choice after failed endoscopic treatment. However, only high volume centers, with experienced and skillful interventional radiologists, have the opportunity to use this technique as an alternative treatment.
Footnotes
Peer reviewers: Tamara Alempijevic, MD, PhD, Assistant Professor, Clinic for Gastroenterology and Hepatology, Clinical Centre of Serbia, 2 Dr Koste Todorovica St., 11000 Belgrade, Serbia; Dr. Herwig H Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29 A-8036 Graz, Austria
S- Editor Tian L L- Editor Stewart GJ E- Editor Zheng XM
References
- 1.Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717–727. doi: 10.1056/NEJM199409153311107. [DOI] [PubMed] [Google Scholar]
- 2.Encarnacion CE, Kadir S, Beam CA, Payne CS. Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology. 1992;183:505–508. doi: 10.1148/radiology.183.2.1561358. [DOI] [PubMed] [Google Scholar]
- 3.Consensus conference: Therapeutic endoscopy and bleeding ulcers. JAMA. 1989;262:1369–1372. [PubMed] [Google Scholar]
- 4.Liou TC, Lin SC, Wang HY, Chang WH. Optimal injection volume of epinephrine for endoscopic treatment of peptic ulcer bleeding. World J Gastroenterol. 2006;12:3108–3113. doi: 10.3748/wjg.v12.i19.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman ML, LAND WC, Bradley EL, Anderson RT. Transcatheter therapeutic embolization in the management of massive upper gastrointestinal bleeding. Radiology. 1976;120:513–521. doi: 10.1148/120.3.513. [DOI] [PubMed] [Google Scholar]
- 6.Rollhauser C, Fleischer DE. Nonvariceal upper gastrointestinal bleeding: an update. Endoscopy. 1997;29:91–105. doi: 10.1055/s-2007-1004082. [DOI] [PubMed] [Google Scholar]
- 7.Branicki FJ, Coleman SY, Pritchett CJ, Cheung WL, Tuen H, Fok PJ, Fan ST, Lai EC, Lau PW, Mok FP. Emergency surgical treatment for nonvariceal bleeding of the upper part of the gastrointestinal tract. Surg Gynecol Obstet. 1991;172:113–120. [PubMed] [Google Scholar]
- 8.Barkun A, Fallone CA, Chiba N, Fishman M, Flook N, Martin J, Rostom A, Taylor A. A Canadian clinical practice algorithm for the management of patients with nonvariceal upper gastrointestinal bleeding. Can J Gastroenterol. 2004;18:605–609. doi: 10.1155/2004/595470. [DOI] [PubMed] [Google Scholar]
- 9.Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, Troisi R, Kunnen M. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218:739–748. doi: 10.1148/radiology.218.3.r01mr05739. [DOI] [PubMed] [Google Scholar]
- 10.Qvist P, Arnesen KE, Jacobsen CD, Rosseland AR. Endoscopic treatment and restrictive surgical policy in the management of peptic ulcer bleeding. Five years' experience in a central hospital. Scand J Gastroenterol. 1994;29:569–576. doi: 10.3109/00365529409092474. [DOI] [PubMed] [Google Scholar]
- 11.Cheynel N, Peschaud F, Hagry O, Rat P, Ognois-Ausset P, Favre JP. [Bleeding gastroduodenal ulcer: results of surgical management] Ann Chir. 2001;126:232–235. doi: 10.1016/s0003-3944(01)00505-3. [DOI] [PubMed] [Google Scholar]
- 12.Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, Soulez G. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195–200. doi: 10.1016/s1051-0443(07)61825-9. [DOI] [PubMed] [Google Scholar]
- 13.Loffroy R, Guiu B, Cercueil JP, Lepage C, Latournerie M, Hillon P, Rat P, Ricolfi F, Krausé D. Refractory bleeding from gastroduodenal ulcers: arterial embolization in high-operative-risk patients. J Clin Gastroenterol. 2008;42:361–367. doi: 10.1097/MCG.0b013e3180319177. [DOI] [PubMed] [Google Scholar]
- 14.Poultsides GA, Kim CJ, Orlando R 3rd, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008;143:457–461. doi: 10.1001/archsurg.143.5.457. [DOI] [PubMed] [Google Scholar]
- 15.Larssen L, Moger T, Bjørnbeth BA, Lygren I, Kløw NE. Transcatheter arterial embolization in the management of bleeding duodenal ulcers: a 5.5-year retrospective study of treatment and outcome. Scand J Gastroenterol. 2008;43:217–222. doi: 10.1080/00365520701676443. [DOI] [PubMed] [Google Scholar]
- 16.Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- 17.Gomes AS, Lois JF, McCoy RD. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol. 1986;146:1031–1037. doi: 10.2214/ajr.146.5.1031. [DOI] [PubMed] [Google Scholar]
- 18.Parente F, Anderloni A, Bargiggia S, Imbesi V, Trabucchi E, Baratti C, Gallus S, Bianchi Porro G. Outcome of non-variceal acute upper gastrointestinal bleeding in relation to the time of endoscopy and the experience of the endoscopist: a two-year survey. World J Gastroenterol. 2005;11:7122–7130. doi: 10.3748/wjg.v11.i45.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljungdahl M, Eriksson LG, Nyman R, Gustavsson S. Arterial embolization in management of massive bleeding from gastric and duodenal ulcers. Eur J Surg. 2002;168:384–390. doi: 10.1080/110241502320789050. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson LG, Sundbom M, Gustavsson S, Nyman R. Endoscopic marking with a metallic clip facilitates transcatheter arterial embolization in upper peptic ulcer bleeding. J Vasc Interv Radiol. 2006;17:959–964. doi: 10.1097/01.RVI.0000223719.79371.46. [DOI] [PubMed] [Google Scholar]
- 21.Sandstede J, Wittenberg G, Schmitt S, Scheppach W, Hahn D. [The diagnostic localization of acute gastrointestinal bleeding in a primarily negative angiographic finding] Rofo. 1997;166:258–259. doi: 10.1055/s-2007-1015420. [DOI] [PubMed] [Google Scholar]
- 22.Schenker MP, Duszak R Jr, Soulen MC, Smith KP, Baum RA, Cope C, Freiman DB, Roberts DA, Shlansky-Goldberg RD. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263–1271. doi: 10.1016/s1051-0443(07)61549-8. [DOI] [PubMed] [Google Scholar]
- 23.Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992;182:703–707. doi: 10.1148/radiology.182.3.1535883. [DOI] [PubMed] [Google Scholar]
- 24.Ripoll C, Bañares R, Beceiro I, Menchén P, Catalina MV, Echenagusia A, Turegano F. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004;15:447–450. doi: 10.1097/01.rvi.0000126813.89981.b6. [DOI] [PubMed] [Google Scholar]
- 25.Holme JB, Nielsen DT, Funch-Jensen P, Mortensen FV. Transcatheter arterial embolization in patients with bleeding duodenal ulcer: an alternative to surgery. Acta Radiol. 2006;47:244–247. doi: 10.1080/02841850600550690. [DOI] [PubMed] [Google Scholar]
- 26.Loffroy R, Guiu B, D'Athis P, Mezzetta L, Gagnaire A, Jouve JL, Ortega-Deballon P, Cheynel N, Cercueil JP, Krausé D. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7:515–523. doi: 10.1016/j.cgh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim B, Wright HK, Bordan D, Fielding LP, Swaney R. Risks of surgery for upper gastrointestinal hemorrhage: 1972 versus 1982. Am J Surg. 1985;149:474–476. doi: 10.1016/s0002-9610(85)80042-8. [DOI] [PubMed] [Google Scholar]
- 28.Larson G, Schmidt T, Gott J, Bond S, O'Connor CA, Richardson JD. Upper gastrointestinal bleeding: predictors of outcome. Surgery. 1986;100:765–773. [PubMed] [Google Scholar]
- 29.Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding. II. Clinical prognostic factors. Gastrointest Endosc. 1981;27:80–93. doi: 10.1016/s0016-5107(81)73156-0. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey DT, Burke DR, Reilly RS, McLean GK, Rosato EF. Angiography in poor-risk patients with massive nonvariceal upper gastrointestinal bleeding. Am J Surg. 1990;159:282–286. doi: 10.1016/s0002-9610(05)81218-8. [DOI] [PubMed] [Google Scholar]
- 31.Welch CE, Rodkey GV, von Ryll Gryska P. A thousand operations for ulcer disease. Ann Surg. 1986;204:454–467. doi: 10.1097/00000658-198610000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheatley KE, Dykes PW. Upper gastrointestinal bleeding--when to operate. Postgrad Med J. 1990;66:926–931. doi: 10.1136/pgmj.66.781.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toyoda H, Nakano S, Takeda I, Kumada T, Sugiyama K, Osada T, Kiriyama S, Suga T. Transcatheter arterial embolization for massive bleeding from duodenal ulcers not controlled by endoscopic hemostasis. Endoscopy. 1995;27:304–307. doi: 10.1055/s-2007-1005697. [DOI] [PubMed] [Google Scholar]
- 34.Ledermann HP, Schoch E, Jost R, Decurtins M, Zollikofer CL. Superselective coil embolization in acute gastrointestinal hemorrhage: personal experience in 10 patients and review of the literature. J Vasc Interv Radiol. 1998;9:753–760. doi: 10.1016/s1051-0443(98)70387-2. [DOI] [PubMed] [Google Scholar]
- 35.van Vugt R, Bosscha K, van Munster IP, de Jager CP, Rutten MJ. Embolization as treatment of choice for bleeding peptic ulcers in high-risk patients. Dig Surg. 2009;26:37–42. doi: 10.1159/000193476. [DOI] [PubMed] [Google Scholar]
- 36.Goldman ML, Freeny PC, Tallman JM, Galambos JT, Bradley EL 3rd, Salam A, Oen KT, Gordon IJ, Mennemeyer R. Transcatheter vascular occlusion therapy with isobutyl 2-cyanoacrylate (bucrylate) for control of massive upper-gastrointestinal bleeding. Radiology. 1978;129:41–49. doi: 10.1148/129.1.41. [DOI] [PubMed] [Google Scholar]
- 37.Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, Kiriyama S. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J Gastroenterol Hepatol. 1996;11:252–258. doi: 10.1111/j.1440-1746.1996.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 38.Loffroy R, Guiu B, Mezzetta L, Minello A, Michiels C, Jouve JL, Cheynel N, Rat P, Cercueil JP, Krausé D. Short- and long-term results of transcatheter embolization for massive arterial hemorrhage from gastroduodenal ulcers not controlled by endoscopic hemostasis. Can J Gastroenterol. 2009;23:115–120. doi: 10.1155/2009/795460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sos TA, Lee JG, Wixson D, Sniderman KW. Intermittent bleeding from minute to minute in acute massive gastrointestinal hemorrhage: arteriographic demonstration. AJR Am J Roentgenol. 1978;131:1015–1017. doi: 10.2214/ajr.131.6.1015. [DOI] [PubMed] [Google Scholar]
- 40.De Wispelaere JF, De Ronde T, Trigaux JP, de Cannière L, De Geeter T. Duodenal ulcer hemorrhage treated by embolization: results in 28 patients. Acta Gastroenterol Belg. 2002;65:6–11. [PubMed] [Google Scholar]
- 41.Shapiro N, Brandt L, Sprayregan S, Mitsudo S, Glotzer P. Duodenal infarction after therapeutic Gelfoam embolization of a bleeding duodenal ulcer. Gastroenterology. 1981;80:176–180. [PubMed] [Google Scholar]
- 42.Rösch J, Keller FS, Kozak B, Niles N, Dotter CT. Gelfoam powder embolization of the left gastric artery in treatment of massive small-vessel gastric bleeding. Radiology. 1984;151:365–370. doi: 10.1148/radiology.151.2.6608749. [DOI] [PubMed] [Google Scholar]
- 43.Walsh RM, Anain P, Geisinger M, Vogt D, Mayes J, Grundfest-Broniatowski S, Henderson JM. Role of angiography and embolization for massive gastroduodenal hemorrhage. J Gastrointest Surg. 1999;3:61–65; discussion 66. doi: 10.1016/s1091-255x(99)80010-9. [DOI] [PubMed] [Google Scholar]
- 44.Loffroy R, Guiu B, Cercueil JP, Krausé D. Endovascular therapeutic embolization: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009;7:250–263. doi: 10.2174/157016109787455617. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008;19:1413–1418. doi: 10.1016/j.jvir.2008.06.019. [DOI] [PubMed] [Google Scholar]