Abstract
AIM: To analyze the effect of chemotherapeutic drugs and specific kinase inhibitors, in combination with the death receptor ligand tumor necrosis factor-related apoptosis inducing ligand (TRAIL), on overcoming TRAIL resistance in hepatocellular carcinoma (HCC) and to study the efficacy of agonistic TRAIL antibodies, as well as the commitment of antiapoptotic BCL-2 proteins, in TRAIL-induced apoptosis.
METHODS: Surface expression of TRAIL receptors (TRAIL-R1-4) and expression levels of the antiapoptotic BCL-2 proteins MCL-1 and BCL-xL were analyzed by flow cytometry and Western blotting, respectively. Knock-down of MCL-1 and BCL-xL was performed by transfecting specific small interfering RNAs. HCC cells were treated with kinase inhibitors and chemotherapeutic drugs. Apoptosis induction and cell viability were analyzed via flow cytometry and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
RESULTS: TRAIL-R1 and -R2 were profoundly expressed on the HCC cell lines Huh7 and Hep-G2. However, treatment of Huh7 and Hep-G2 with TRAIL and agonistic antibodies only induced minor apoptosis rates. Apoptosis resistance towards TRAIL could be considerably reduced by adding the chemotherapeutic drugs 5-fluorouracil and doxorubicin as well as the kinase inhibitors LY294002 [inhibition of phosphoinositol-3-kinase (PI3K)], AG1478 (epidermal growth factor receptor kinase), PD98059 (MEK1), rapamycin (mammalian target of rapamycin) and the multi-kinase inhibitor Sorafenib. Furthermore, the antiapoptotic BCL-2 proteins MCL-1 and BCL-xL play a major role in TRAIL resistance: knock-down by RNA interference increased TRAIL-induced apoptosis of HCC cells. Additionally, knock-down of MCL-1 and BCL-xL led to a significant sensitization of HCC cells towards inhibition of both c-Jun N-terminal kinase and PI3K.
CONCLUSION: Our data identify the blockage of survival kinases, combination with chemotherapeutic drugs and targeting of antiapoptotic BCL-2 proteins as promising ways to overcome TRAIL resistance in HCC.
Keywords: Hepatocellular carcinoma, Apoptosis, Tumor necrosis factor-related apoptosis inducing ligand, BCL-xL, MCL-1, 5-fluorouracil, Doxorubicin, Sorafenib, Phosphoinositol-3-kinase, (Mitogen-activated protein kinase)/(extracellular signal regulated kinase) kinase, c-Jun N-terminal kinase
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide. It ranks at third place in the list of malignancies leading to death. Over the past decades the incidence of HCC has increased worldwide, especially in eastern Asia and sub-Saharan Africa[1,2]. HCC is clinically characterized by its invasiveness, poor prognosis and limited therapeutic opportunities, mostly due to the high resistance of HCC cells towards chemotherapeutic agents. Today, surgery is considered to be the only curative treatment procedure for most HCC patients[3]. However, in many patients, HCC is diagnosed at an advanced or metastasized stage. For the treatment of these patients, the Food and Drug Administration approved the multi-kinase inhibitor Sorafenib in 2007[4,5], which highlights the fact that specific inhibition of survival pathways is an effective treatment option in HCC[6].
Apoptosis is a genetically determined process of controlled cellular suicide[7]. Dysregulation of apoptosis is involved in the pathophysiology of liver diseases including hepatocarcinogenesis[8]. Resistance of HCC cells to apoptosis is a crucial aspect in cancer treatment because it impairs the efficacy of different therapy regimens[9].
Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) is a promising anti-tumor agent since it is capable of killing tumor cells via receptor-mediated apoptosis[10,11]. TRAIL ligates two different types of receptors: (1) death receptors triggering TRAIL-induced apoptosis, and (2) decoy receptors possibly inhibiting the TRAIL death-signaling pathway. Receptors TRAIL-R1 and -R2 contain an intracellular death domain (DD) motif essential for signal transduction. In contrast, TRAIL-R3 (DcR1) and -R4 (DcR2) appear to act as “decoys”, lacking a DD. Due to this fact they are capable of binding the ligand without effecting a death signal. Under certain conditions, a relative TRAIL resistance occurs in cells expressing high levels of DcR1 or DcR2.
Binding of an agonistic ligand or mAb to TRAIL-R1 or -R2 leads to the intracellular formation of a protein complex termed death inducing signaling complex (DISC). DISC formation includes the activation of the apical activator caspase 8, representing the initial point of receptor-related apoptosis signaling.
In addition to this receptor-related extrinsic pathway, there is an intrinsic pathway of apoptosis, which is crucial as a cellular response to DNA damage and oxidative stress. Central organelles for the intrinsic pathway are mitochondria, where a delicate balance between pro- and antiapoptotic BCL-2 proteins decides cell destiny. If DNA damage or other intrinsic triggers occur, proapoptotic BCL-2 proteins and mitochondria are activated. Subsequently, a multimeric protein complex, designated as an apoptosome, is formed. The apoptosome cleaves caspase 9, which in turn activates the downstream effector caspase 3, where intrinsic and extrinsic pathways of apoptosis converge.
Notably, receptor-mediated caspase 8 activation can promote an activation of mitochondria by cleavage and subsequent activation of the proapoptotic BCL-2 protein, BID[12]. The crosstalk between extrinsic and intrinsic apoptosis pathways amplifies a death signal mediated by TRAIL, leading to a more effective execution of apoptosis.
MCL-1 and BCL-xL are antiapoptotic members of the BCL-2 family serving as protective factors against several death stimuli. Both proteins were found to be expressed at a high level in different solid tumor entities, including HCC[13-15]. Antiapoptotic BCL-2 proteins interact with proapoptotic BCL-2 proteins BAX and BAK, thereby inhibiting the activation of mitochondria. It appears that high expression levels of MCL-1 and BCL-xL provide resistance of tumor cells to chemotherapeutic drugs and TRAIL[16,17].
Resistance towards TRAIL can be due to failure at any step in the death signaling cascade. For example, TRAIL resistance can be located at receptor level due to an inappropriate expression or at DISC level mediated by proteins counteracting DISC formation[18-20]. Furthermore, an inability to activate mitochondria during apoptosis, due to high expression levels of antiapoptotic proteins (e.g. MCL-1), can cause resistance towards TRAIL[16,21]. Finally, antiapoptotic pathways, such as phosphoinositol-3-kinase (PI3K)/Akt signaling, are aberrantly activated in various tumor cells, thus contributing to TRAIL resistance[22,23].
In our study, we investigated whether TRAIL resistance in HCC cells can be overcome by combining TRAIL with chemotherapeutic drugs, inhibitors of survival signaling or targeted therapies against antiapoptotic BCL-2 proteins.
MATERIALS AND METHODS
Reagents and cell lines
HCC cell lines, Hep-G2 and Huh7, were purchased from ECACC. Cells were cultured in DMEM (Invitrogen, Karlsruhe, Germany), supplemented with 10% fetal calf serum (FCS, Biochrom, Berlin, Germany), 1% Pen/Strep (PAA laboratories, Pasching, Austria), 1% HEPES and 1% L-Glutamine (Cambrex, Verviers, Belgium). Cells were cultivated at 37°C with a concentration of 5% CO2. Transfection experiments were performed in OPTIMEM (Invitrogen).
Reagents were purchased from the following suppliers: recombinant TRAIL (with Enhancer applied in a concentration of 1 μg/mL) and SuperKillerTRAIL (SkTRAIL) from Alexis Biochemicals (SanDiego, CA, USA), goat anti-human IgG F(ab)’2 from Meridian Life Science (Cincinnati, USA), 5-fluorouracil (5-FU), doxorubicin (Doxo) from Sigma (Deisenhofen, Germany), SP600125, AG1478, PD98059, LY294002 and rapamycin (RAPA) from Calbiochem (Schwalbach, Germany). LBY135 was supplied from Novartis (Basel, Switzerland), Sorafenib (BAY 43-9006) from Bayer (Leverkusen, Germany).
Viability test
Cell viability was determined by a colorimetric 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HCC cell lines were seeded onto 96-well plates. On day one after seeding, cells were treated as indicated. We added 10 μL MTT (5 mg/mL) 48 h after treatment and incubated cells for a further 3 h at 37°C. Next, supernatant was discarded and cells were lysed by adding 100 μL 1-propanol to each well followed by shaking plates till complete lysis. Absorbance was measured at 550 nm in a microtiter plate reader. A viability of 1 was defined as the absorbance of untreated cells.
Coating of microtiter plates
To ease ligand-receptor interaction with the crosslinking supplement IgG F(ab)’2, 96-well plates were coated with IgG F(ab)’2 before seeding cells. One hundred microliters of sterile filtered 100 nmol/L sodium bicarbonate buffer (pH 9.2) containing 5 μg/mL IgG F(ab)’2 was added to each well and incubated for 2 h at room temperature (RT). After replacement of F(ab)’2 buffer by cell culture media, plates were stored at 4°C. Coated plates were stable for at least 1 wk.
RNAi and transfection
To knock-down protein expression, we administered specific small interfering RNA (siRNA) against MCL-1 or BCL-xL. As a control we used siRNA specific for green fluorescent protein (GFP). The following siRNA sequences were applied (MWG Biotech, Ebersberg, Germany): BCL-xL, 5'-gcuuggauaaagaugcaaTT-3' (sense) and 5'-uugcaucuuuaucccaagcAG-3' (antisense), MCL-1, 5'-aaguaucacagacguucucTT-3' (sense) and 5'-gagaacgucugugauacuuTT-3' (antisense), GFP, 5'-ggcuacguccaggagcgcaccTT-3' (sense) and 5'-ggugcgcuccuggacguagccTT-3' (antisense). Here, capitals represent deoxyribonucleotides and lower case letters represent ribonucleotides. Huh7 cells were seeded onto 12-well plates and after 24 h transiently transfected in OPTIMEM with Lipofectamine RNAiMax (Invitrogen) according to the manufacturer’s protocol. Expression levels were analyzed 24, 48 and 72 h after transfection via Western blotting analysis.
Detection of apoptosis
HCC cells were seeded onto 12-well plates and treated as indicated 1 d after transfection. Forty eight hours after treatment, cells were washed with cold PBS, collected and resuspended in a hypotonic buffer containing 0.1% (w/v) sodium citrate, 0.1% (v/v) Triton X-100, and 50 μg/mL Propidium iodide (PI, Sigma). After 3 h incubation at 4°C, nuclei from apoptotic cells were quantified by fluorometric absorbance cell sorting according to the protocol of Nicoletti et al[24].
Cell lysis and Western blotting
Cell lysis, SDS-PAGE and Western blotting were performed as described previously[13]. Immunodetection of proteins was performed using the following antibodies: anti-BCL-xL (Labvison/NeoMarkers, Warm Springs Blvd. Fremont, Canada), anti-MCL-1 (Santa Cruz Biotechnology, Heidelberg, Germany) and anti-α-tubulin (Sigma) as loading control.
Detection of receptor expression
HCC cells were cultured as described and collected. Five hundred thousand cells for each receptor analysis were transferred to polystyrene tubes, washed twice with PBS and resuspended in PBS containing 0.5% BSA (Sigma). A specific monoclonal antibody to either TRAIL-R1, -R2, -R3, -R4 or unspecific mouse IgG1 as isotype control was applied at 5 μg/mL. Cells were incubated for 20 min with gentle rocking at RT. Cells were washed twice in PBS and secondary fluorescein isothiocyanate-conjugated polyclonal goat antibody to mouse IgG1 (1:200 in PBS containing 0.5% BSA) was added, followed by incubation protected from light for 30 min with gentle rocking at RT. Cells were then washed and resuspended in PBS containing 0.5% BSA. Analysis of receptor expression was performed via flow cytometry. All antibodies were purchased from Alexis.
Statistical analysis
All results are expressed as mean ± SD. Data were analyzed by students t-test (paired, two-sided) based on normal data distribution. P < 0.05 was considered significant.
RESULTS
TRAIL receptor expression in HCC cells upon treatment with TRAIL and chemotherapeutic agents
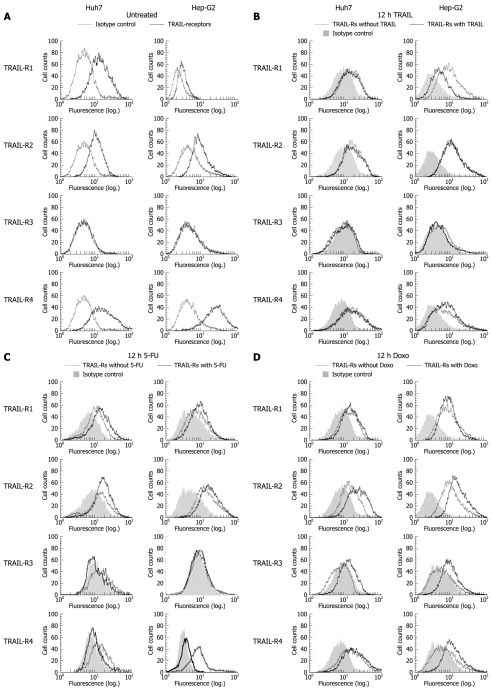
It is known that TRAIL resistance can be mediated at the receptor level, either by low expression of TRAIL-R1 and -R2 or by a comparably high expression of TRAIL-R3 and -R4[25]. Firstly, we analyzed surface receptor expression of the HCC cell lines Huh7 and Hep-G2. Except for TRAIL-R3, all receptors were found to be expressed: we detected high expression levels of TRAIL-R1, -R2 and -R4 in both cell lines (Figure 1A). Next, we analyzed the expression levels after treatment with TRAIL and consequently the possibility of TRAIL-induced regulation in a feedback manner. After 12 h-treatment with TRAIL, we observed downregulation of TRAIL-R1 and a moderate upregulation of TRAIL-R4 in Hep-G2 cells. In contrast, no changes in receptor expression were detected in Huh7 cells (Figure 1B).
Figure 1.
Surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptors on Huh7 and Hep-G2 cells. Flow cytometric analysis of TRAIL receptors was performed using monoclonal mouse IgG1, anti-TRAIL-R1, -R2, -R3, -R4 antibodies and secondary FITC-conjugated polyclonal goat anti mouse-IgG1 antibodies. Unspecific mouse IgG1 antibodies were used as isotype control. Receptor surface expression was analyzed in untreated Huh7 and Hep-G2 cells (A) and 12 h after treatment with 100 ng/mL rec. TRAIL + 1 μg/mL Enhancer (B), 50 μg/mL 5-fluorouracil (5-FU) (C) and 0.5 μmol/L doxorubicin (D). Diagrams are representative of at least two independent experiments. Doxo: Doxorubicin.
In order to study the effect of chemotherapeutics on TRAIL receptor expression, we treated HCC cells with 5-FU and Doxo, both applied for transarterial chemoembolization in patients with HCC[26]. 12 h-treatment with 5-FU resulted in upregulation of TRAIL-R1 and -R2 in both cell lines. In contrast, TRAIL-R3 was downregulated in Huh7 and unaffected in Hep-G2 cells. For TRAIL-R4, we observed a significant downregulation in both Hep-G2 and Huh7 cells (Figure 1C). 12 h-treatment with Doxo resulted in a slight upregulation of TRAIL-R1 in both cell lines. Remarkably, TRAIL-R2 was considerably upregulated. TRAIL-R3 surface expression was detectable in both cell lines after Doxo treatment. TRAIL-R4 was upregulated in Hep-G2 and unaffected in Huh7 cells (Figure 1D).
Sensitivity of HCC cells towards different TRAIL compounds
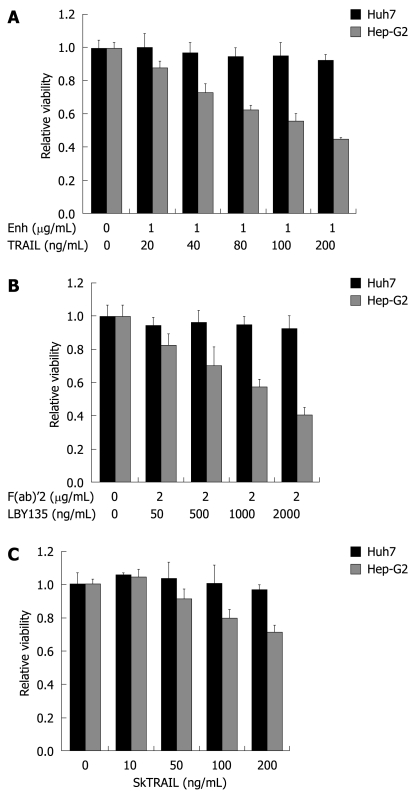
Next, we determined sensitivity of HCC cells towards TRAIL-induced apoptosis. Firstly, we analyzed the effect of recombinant TRAIL in concentrations from 20 up to 200 ng/mL (combined with Enhancer, 1 μg/mL). Hep-G2 cells were sensitive towards recombinant TRAIL in a dose-dependent manner, whereas Huh7 were resistant (Figure 2A). Next, we tested LBY135, a chimeric monoclonal antibody targeting TRAIL-R2, in concentrations from 50 to 2000 ng/mL, together with the cross linker F(ab)’2 (2 μg/mL). To optimize the interaction between LBY135 and F(ab)’2, we coated the plates with the F(ab)’2-fragment before seeding the cells. Hep-G2 showed moderate sensitivity towards LBY135-induced apoptosis in a dose-dependent manner, whereas Huh7 cells were resistant (Figure 2B). To discover whether resistance was due to impaired interactions between enhancer or F(ab)’2, the ligand and the receptor, we included SkTRAIL in our study. SkTRAIL interacts effectively with TRAIL receptors without additional supplements. Again, Hep-G2 cells revealed a dose-dependent sensitivity to SkTRAIL in concentrations from 10 to 200 ng/mL. In contrast, Huh7 cells were resistant to SkTRAIL (Figure 2C).
Figure 2.
TRAIL-induced apoptosis in hepatocellular carcinoma (HCC) cells. Huh7 and Hep-G2 cells were seeded onto 96-well plates and treated on day one after seeding with different TRAIL compounds. A: Cells were treated for 48 h with rec. TRAIL + 1 μg/mL Enhancer as indicated; B: Plates were coated with crosslinker IgG F(ab)’2 for 24 h before seeding of cells. Cells were then treated for 48 h with LBY135 + 1 μg/mL F(ab)’2 as indicated; C: Cells were treated for 48 h with SkTRAIL as indicated. Cell viability was analyzed by MTT assay. Viability is shown relative to untreated controls. Assays were performed in six-fold values and are representative of three independent experiments. Values are expressed as mean ± SD. Enh: Enhancer.
Treatment of HCC cells with TRAIL in combination with chemotherapy
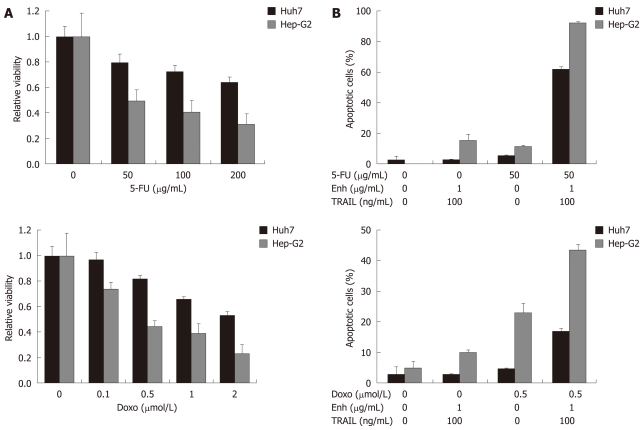
Next, we analyzed whether HCC cells were sensitized to TRAIL-induced apoptosis by co-treatment with the chemotherapeutic drugs 5-FU and Doxo. As a first step, we analyzed whether the chemotherapeutics induced loss of viability if applied alone: after 48 h treatment of Huh7 and Hep-G2 cells with 5-FU (50, 100 and 200 μg/mL) and Doxo (0.1, 0.5, 1 and 2 μmol/L), we observed a dose-dependent decrease of cell viability (Figure 3A). Next, we applied these agents in concentrations which exhibited less significant cytotoxic effects, in combination with recombinant TRAIL (+ Enhancer 1 μg/mL). 5-FU (50 μg/mL) or TRAIL (100 ng/mL) did not induce apoptosis in Huh7 cells when administered alone. However, combination of 5-FU and TRAIL induced apoptosis in 62% of Huh7 cells. In Hep-G2 cells, TRAIL (100 ng/mL) induced apoptosis in 15% of cells. 5-FU treatment alone triggered apoptosis of 12% of Hep-G2 cells. 5-FU and TRAIL co-treatment of Hep-G2 resulted in 93% apoptotic cells (Figure 3B, upper panel). Next, we tested the combination of Doxo (0.5 μmol/L) and TRAIL (100 ng/mL). Doxo induced apoptosis in less than 5% of Huh7 cells, whereas the combination of Doxo and TRAIL resulted in 17% apoptotic cells. Treatment of Hep-G2 with Doxo alone induced apoptosis in 24% of cells, whereas Doxo and TRAIL in combination led to apoptosis rates of 43% (Figure 3B, lower panel).
Figure 3.
Treatment of HCC cells with TRAIL in combination with 5-FU and doxorubicin. Values are expressed as mean ± SD. A: Huh7 and Hep-G2 cells were analyzed for cell viability after treatment with the chemotherapeutic agents 5-FU and doxorubicin alone. Cells were seeded onto 96-well plates and treated on day one after seeding. Cells were treated for 48 h with 5-FU (upper left panel) and doxorubicin (lower left panel) as indicated. Cell viability was analyzed by MTT assay. Viability is shown relative to untreated controls. Assays were performed in six-fold values; B: Apoptosis induction in Huh7 and Hep-G2 cells treated with 50 μg/mL 5-FU (upper right panel) and 0.5 μmol/L doxorubicin (lower right panel) either alone or in combination with 100 ng/mL TRAIL + 1 μg/mL Enhancer. Cells were seeded onto 12-well plates, harvested 48 h after treatment and analyzed for apoptosis induction by flow cytometry. Assays were performed in triplicate and are representative of at least two independent experiments.
Treatment of HCC cells with TRAIL in combination with specific kinase inhibitors
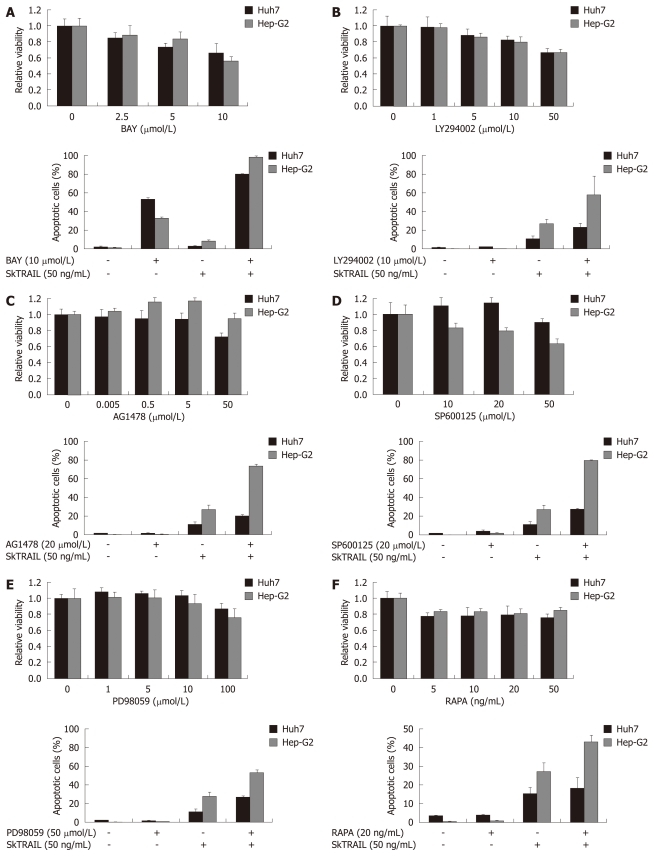
Antiapoptotic pathways such as PI3K/Akt, epidermal growth factor receptor (EGFR), [mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) kinase] (MEK)/ERK are well known to be activated in malignant cells, thus contributing to cell cycle progression and tumor growth. Therefore, we analyzed whether inhibition of kinases involved in these pathways could overcome resistance towards TRAIL-mediated apoptosis. Firstly, we applied the multi-kinase inhibitor Sorafenib to inhibit RAF/MEK/ERK signaling, in escalating concentrations (2.5, 5 and 10 μmol/L). A dose-dependent decrease of cell viability in Huh7 and Hep-G2 was observed (Figure 4A, upper panel). In a second step, we analyzed the impact of Sorafenib on TRAIL treatment. In Huh7 cells, Sorafenib (10 μmol/L) induced apoptosis rates of 50%. Strikingly, the combination of SkTRAIL (50 ng/mL) and Sorafenib (10 μmol/L) induced apoptosis in 80% of the cells. In Hep-G2 cells Sorafenib caused only minor apoptosis rates (33%). However, combination of TRAIL and Sorafenib led to 98% apoptotic cells (Figure 4A, lower panel).
Figure 4.
Treatment of HCC cells with TRAIL in combination with specific kinase inhibitors. Viability of HCC cells treated with kinase inhibitors alone (upper panels). On day one after seeding of Huh7 and Hep-G2 cells onto 96-well plates, cells were treated with multi-kinase inhibitor Sorafenib (A), PI3 kinase inhibitor LY294002 (B), EGFR kinase inhibitor AG1478 (C), JNK inhibitor SP600125 (+ 0.2% DMSO as vehicle) (D), MEK inhibitor PD98059 (E) and mTOR inhibitor rapamycin (RAPA) (F) at the indicated concentrations for 48 h. Cell viability was analyzed by MTT assay. Viability is shown relative to untreated or 0.2% DMSO treated controls, respectively. Assays were performed in six-fold values. Values are expressed as mean ± SD. Apoptosis induction in Huh7 and Hep-G2 cells treated with 10 μmol/L Sorafenib (A), 10 μmol/L LY294002 (B), 20 μmol/L AG1478(C), 20 μmol/L SP600125 (+ 0.2% DMSO as vehicle) (D), 50 μmol/L PD98059 (E) and 20 ng/mL rapamycin (F) in combination with 50 ng/mL SkTRAIL (lower panels). Cells were seeded 1 d before treatment onto 12-well plates, harvested 48 h after treatment and analyzed for apoptosis induction by flow cytometry. Assays were performed in triplicate and are representative of at least two independent experiments. Values are expressed as mean ± SD.
Next, we inhibited the PI3K/Akt pathway by application of the PI3K inhibitor LY294002. A slight decrease of cell viability was observed in Huh7 and Hep-G2 after 48 h treatment in concentrations lower than 50 μmol/L (Figure 4B, upper panel). However, combination of LY294002 (10 μmol/L) and SkTRAIL (50 ng/mL) doubled apoptosis rates in Hep-G2 cells to 59% compared to SkTRAIL treatment alone. In Huh7, we observed an increased rate of apoptosis after treatment with the combination of LY294002 and SkTRAIL compared to SkTRAIL alone (23% vs 11%, respectively. Figure 4B, lower panel). Furthermore, we used AG1478 to inhibit EGFR kinase. Interestingly, inhibition of EGFR kinase increased cell viability in Hep-G2 cells in concentrations up to 5 μmol/L. In Huh7 cells, AG1478 caused no significant changes in cell viability when applied in low concentrations (Figure 4C, upper panel). AG1478 (20 μmol/L) and SkTRAIL (50 ng/mL) co-treatment increased the rate of apoptotic cells to 27% in Huh7 and 74% in Hep-G2 cells (Figure 4C, lower panel). Next, we inhibited the c-Jun N-terminal kinases 1 and 2 (JNK1 and JNK2) with the anthrapyrazolone inhibitor SP600125. We observed increased cell viability in Huh7 cells and a slight, dose-dependent decrease of cell viability in Hep-G2 cells after 48 h of SP600125 treatment (Figure 4D, upper panel). Notably, a high percentage of cells were arrested in the G2 phase 48 h after treatment with the JNK inhibitor (data not shown). Combined treatment with SP600125 (20 μmol/L) and SkTRAIL (50 ng/mL) led to 28% apoptosis of Huh7 and to 80% apoptosis of Hep-G2 cells (Figure 4D, lower panel). Next, we included a specific inhibitor of MAP kinase kinase (MEK), PD98059, in our study. Again, a death inducing effect of MEK inhibition alone was only observed when applied in high concentrations of more than 50 μmol/L (Figure 4E, upper panel). However, in combination (50 μmol/L PD98059 and 50 ng/mL SkTRAIL), a two-fold increase of apoptosis, compared to monotherapy with SkTRAIL, was detectable in Huh7 and Hep-G2 cells (Figure 4E, lower panel).
Finally, we inhibited mammalian target of rapamycin (mTOR) with rapamycin (Sirolimus). Rapamycin alone only caused a moderate decrease of cell viability (20%) in Huh7 and Hep-G2 cells (Figure 4F, upper panel). Combination of 20 ng/mL rapamycin with 50 ng/mL SkTRAIL resulted in a slight increase of apoptosis rates in Huh7 cells (18% vs 15% SkTRAIL alone) and a profound increase of apoptosis in Hep-G2 cells (43% vs 27%, Figure 4F, lower panel).
Treatment of HCC cells with TRAIL after knock-down of MCL-1 and BCL-xL
The antiapoptotic BCL-2 proteins, MCL-1 and BCL-xL, are profoundly expressed in tissues of human HCC, thus contributing to apoptosis resistance of HCC cells[13,15,27]. To analyze the role of antiapoptotic BCL-2 proteins in TRAIL-induced apoptosis, we manipulated their expression in Huh7 cells via specific siRNA-mediated knock-down. An effective reduction of MCL-1 and BCL-xL expression levels was observed 24 h after transfection (Figure 5A).
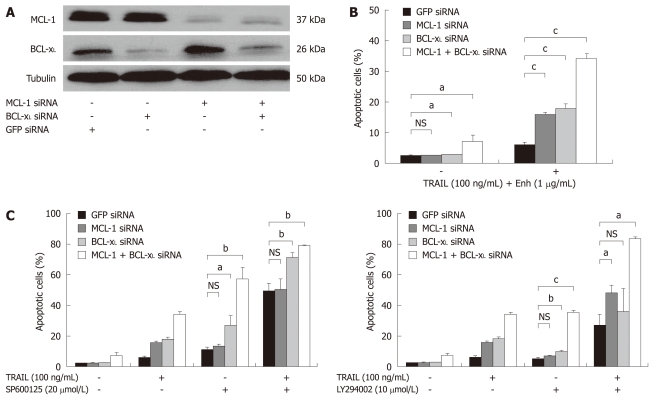
Figure 5.
TRAIL-induced apoptosis in Huh7 cells after targeted therapy approaches and knock-down of BCL-xL and MCL-1. A: Huh7 cells were transfected with siRNAs (40 nmol/L) specific for MCL-1 and BCL-xL either alone or in combination. SiGFP was used as control. Whole cell lysates were prepared 24 h after transfection. MCL-1 and BCL-xL expression was analyzed by Western blotting. α-Tubulin expression was used to control equal loading; B: 24 h after siRNA transfection, cells were treated for 48 h with 100 ng/mL TRAIL (+ 1 μg/mL Enhancer); C: 20 μmol/L SP600125 (left panel) or 10 μmol/L LY294002 (+ 0.2% DMSO as vehicle, right panel), either alone or in combination with 100 ng/mL TRAIL (+ 1 μg/mL Enhancer). Cells were harvested on day two after treatment and analyzed for apoptosis induction by flow cytometry. Assays were performed in triplicate and are representative of at least two independent experiments. Values are expressed as mean ± SD. NS: Not significant. aP < 0.05, bP < 0.01, cP < 0.001.
A knock-down of BCL-xL induced significant apoptosis in comparison to mock transfected cells (P < 0.05). Knock-down of MCL-1 did not induce significant apoptosis rates. Additionally, combined knock-down of MCL-1 and BCL-xL induced spontaneous apoptosis in 8% of Huh7 cells (P < 0.05, Figure 5B). Downregulation of either MCL-1 or BCL-xL significantly enhanced susceptibility towards TRAIL-induced apoptosis (17% vs 6% and 18% vs 6%, respectively, P < 0.001). Remarkably, we detected 34% apoptotic cells in Huh7 lacking BCL-xL and MCL-1 expression after treatment with TRAIL (P < 0.001, Figure 5B). Furthermore, we analyzed whether lack of MCL-1 and BCL-xL expression sensitized cells towards the JNK inhibitor SP600125 and the PI3K inhibitor LY294002. Inhibition of JNK and PI3K showed significantly enhanced anti-tumoral efficacy after knock-down of BCL-xL and MCL-1. In cells lacking BCL-xL expression, apoptosis was induced in 27% vs 11% of control cells after treatment with SP600125 (20 μmol/L) (P < 0.05, Figure 5C). In contrast, cells lacking MCL-1 did not show increased susceptibility to JNK inhibition (14% vs 11%, not significant, Figure 5C). Knock-down of MCL-1 and BCL-xL increased SP600125-induced apoptosis rates to 57% (P < 0.005, Figure 5C, left panel). Additionally, single knock-down of BCL-xL (P < 0.001) and double knock-down of MCL-1 and BCL-xL (P < 0.001) significantly increased apoptosis after combined treatment of SP600125 with recombinant TRAIL (100 ng/mL). Single knock-down of MCL-1 did not exhibit sensitizing effects (differences not significant, Figure 5C).
Next, we analyzed the effects of MCL-1 and BCL-xL knock-down in combination with the PI3K inhibitor LY294002. We observed a significant sensitizing effect of BCL-xL knock-down on LY294002-induced apoptosis in Huh7 cells (P < 0.005, Figure 5C, right panel). Knock-down of MCL-1 did not increase LY294002-induced apoptosis. However, in Huh7 cells lacking both MCL-1 and BCL-xL, apoptosis rates increased to 35% after LY294002 treatment (P < 0.001). Finally, we found an increased rate of apoptosis after combined treatment of LY294002 (10 μmol/L) with recombinant TRAIL (100 ng/mL) in cells lacking MCL-1 (48% vs 27% of mock transfected Huh7, P < 0.05). A moderate sensitizing effect in cells lacking BCL-xL was observed (not significant). Importantly, the combined knock-down of MCL-1 and BCL-xL caused apoptosis rates of 83%, if cells were treated with a combination of LY294002 and recombinant TRAIL (P < 0.05, Figure 5C, right panel).
DISCUSSION
Amongst the various approaches to induce apoptosis in tumor cells, application of the death receptor ligand TRAIL is very promising. Preclinical studies suggest that TRAIL induces apoptosis of tumor cells in vivo without lethal toxicities[28,29]. A major obstacle for the clinical use of TRAIL is its limited efficacy in monotherapeutic approaches in different tumor entities. Thus, it appears worthwhile to persist in investigating ways to enhance TRAIL’s capacity for apoptosis induction. Resistance towards TRAIL can be caused at receptor level by inhibitory proteins and at mitochondrial level by antiapoptotic proteins[17,18,21]. For example, a diminished membrane expression of TRAIL-R1 and -R2, as well as reduced caspase 8 levels, mediate TRAIL resistance in myeloma cells[19]. In this present study we analyzed different approaches in sensitizing HCC cells to TRAIL-induced apoptosis.
TRAIL receptor expression was similar in the HCC cell lines Huh7 and Hep-G2. After TRAIL treatment, expression patterns changed only slightly in Hep-G2 cells. Strikingly, chemotherapeutic drugs influenced the expression pattern in HCC cells. Upregulation of TRAIL-R1 and profound upregulation of TRAIL-R2 after Doxo and 5-FU treatment in both cell lines might represent a potential mechanism of chemotherapy-mediated TRAIL sensitization. Interestingly, TRAIL-R3 (DcR1) was also upregulated after chemotherapy. This could also represent a mechanism of TRAIL resistance upon chemotherapy, since TRAIL-R3 acts as a decoy receptor. Notably, upregulation of TRAIL receptors in Huh7 cells which express mutated p53 suggests that receptor regulation occurs independently from p53[30].
Several mAbs targeting TRAIL receptors and recombinant TRAIL agonists have already entered clinical trials[31-33]. In order to analyze the efficacy of different TRAIL compounds, we included LBY135, a chimeric antibody targeting TRAIL-R2, recombinant TRAIL and SkTRAIL in our study. We demonstrate that crosslinking elements IgG F(ab)’2 are mandatory for LBY135-induced apoptosis. Consistent findings were obtained for recombinant TRAIL, where combination with an enhancer is necessary to induce apoptosis. Comparing the death-inducing capacities of LBY135, TRAIL and SkTRAIL in HCC cells, we assume that TRAIL-R2 plays a major role, which would be in line with observations in colon and breast cancer[34]. In contrast, it has been shown that chronic leukemia cells are selectively sensitive to TRAIL-R1[35]. Taken together, it appears likely that a cell type dependency determines the efficiency of TRAIL-mediated apoptosis induction, even if both TRAIL-R1 and -R2 are expressed.
Chemotherapeutic drugs such as Doxo and 5-FU have shown limited efficacy for the treatment of HCC[26]. However, anti-tumoral effects have been described for Doxo if administered into the liver via chemoembolization[26,36]. In our study we aimed to discover whether the combination of TRAIL with chemotherapy exerts anti-tumoral effects in HCC. Importantly, chemotherapy with Doxo or 5-FU increased TRAIL susceptibility in Hep-G2 cells and sensitized Huh7 cells towards TRAIL, opening the possibility of a treatment regime including reduced doses of chemotherapeutic drugs in combination with TRAIL.
The multi-kinase inhibitor Sorafenib has recently been approved for the therapy of advanced HCC. Sorafenib acts by inhibition of the RAF/MEK/ERK pathway and downregulation of MCL-1, leading to a disruption of survival signals in HCC cells[4,37]. In combination with TRAIL, Sorafenib profoundly increased apoptosis induction advocating TRAIL as a potential and effective agent for HCC treatment along with Sorafenib.
There is evidence that constitutive activation of various antiapoptotic pathways is a basic principle of tumor growth, cell cycle progression and apoptosis resistance. A well described antiapoptotic pathway is the PI3K/Akt signaling pathway, found activated in several tumor entities, including HCC[38]. The PI3K inhibitor LY294002[39,40] has already been employed in preclinical studies in combination with TRAIL. Consistent with data for prostate cancer and leukemia cells, our results indicate that blockage of PI3K by LY294002 overcomes resistance towards TRAIL in HCC cells[22,23].
The mTOR, a protein with growing clinical relevance in oncology, is located downstream of PI3K[41]. The significant sensitization towards TRAIL in Hep-G2 cells by mTOR inhibition underlines a pivotal role of PI3K/Akt signaling for the resistance of HCC towards TRAIL.
In addition, the MAPK/ERK pathway exerts antiapoptotic effects in cancer cells. The MEK is a key component downstream of Raf serine/threonine kinases[42,43]. MEK inhibitors have been described as sensitizing human cancer cells to apoptosis, e.g. after treatment with chemotherapeutic agents[44,45]. In this study, we observed no apoptosis induction and only a slight decrease of cell viability after MEK inhibition in HCC cells. However, the combination of MEK inhibition and TRAIL caused a significant increase of TRAIL-induced apoptosis. This observation suggests that an abberantly activated Raf/MAPK/ERK pathway plays a crucial role for TRAIL resistance in HCC.
Furthermore, we focused on the EGFR, which is an upstream receptor in Ras-Raf-MEK-ERK signaling[46]. It has been shown that overexpression of EGFR represents a protective factor against apoptosis stimuli in HCC[47,48]. The combined treatment of TRAIL with the specific EGFR kinase inhibitor AG1478 caused a significant increase of TRAIL-induced apoptosis in HCC cells. Thus, EGFR blockage is another promising approach for TRAIL sensitization of HCC cells.
Recently, it has been shown that JNK inhibition sensitizes HCC cells, but not healthy hepatocytes, towards TRAIL-induced apoptosis[49]. In contrast, other results indicate that JNK activation is not relevant for TRAIL-induced apoptosis[50]. We found a significantly increased proapoptotic effect of TRAIL if combined with the JNK inhibitor SP600125.
Aberrant activity of survival signaling pathways exerts antiapoptotic effects at least in part via triggering of the expression of antiapoptotic proteins, such as antiapoptotic BCL-2 proteins. Importantly, antiapoptotic BCL-2 proteins, such as MCL-1 and BCL-xL, have been described as contributing to TRAIL resistance in cancer cells[51]. MCL-1 and BCL-xL mainly act by directly inhibiting their proapoptotic relatives BAX and BAK, thereby guarding the cell from various death stimuli. In addition, expression of antiapoptotic BCL-2 proteins is a prognostic factor for various tumor entities, e.g. expression of MCL-1 in breast and gastric cancer[52,53]. In the liver, MCL-1 has been found to be a key factor for apoptosis regulation[13,54,55]. A lack of MCL-1 causes increased rates of apoptosis and a significantly higher susceptibility towards chemotherapeutic treatment in HCC[54]. In addition, it has been shown that MCL-1 acts as a key factor for resistance towards TRAIL in leukemia cells[56]. In our study, we show that knock-down of MCL-1 or Bcl-xL increased TRAIL-induced apoptosis in HCC. Taking into consideration that there is a putative functional redundancy between these two proteins, we performed a double knock-down of MCL-1 and BCL-xL. Cells lacking both MCL-1 and BCL-xL expression showed profound spontaneous apoptosis, indicating that both proteins contribute to mitochondrial integrity in HCC cells. Importantly, HCC cells lacking MCL-1, BCL-xL or both showed an increased apoptosis rate after treatment with TRAIL. In summary, our data suggest a central role of MCL-1 and Bcl-xL for the resistance of HCC cells towards TRAIL-induced apoptosis.
Additionally, recent studies have revealed a synergistic effect of PI3K/Akt signaling with MCL-1 and BCL-xL, contributing to apoptosis resistance in cancer[57-59]. In our study, we treated HCC cells with TRAIL in combination with a PI3K inhibitor and with a knock-down of MCL-1 and BCL-xL via RNA interference. We found that cells lacking BCL-xL or lacking both MCL-1 and BCL-xL evolve a significantly increased sensitivity to apoptosis induced by PI3K inhibition. A knock-down of MCL-1 alone did not enhance LY294002-induced apoptosis. Importantly, we observed a profound increase of apoptosis in cells lacking MCL-1 and a rather low increase in cells lacking Bcl-xL after combined treatment with PI3K inhibitors and TRAIL. Strikingly, combined knock-down of MCL-1 and BCL-xL led to profound induction of apoptosis after treatment with PI3K inhibitors and TRAIL. In summary, our results suggest a major role of PI3K/Akt signaling in resistance towards TRAIL-mediated apoptosis and emphasize the role of antiapoptotic BCL-2 proteins for TRAIL resistance of HCC cells.
A recent study showed that JNK1 exerts antiapoptotic effects via stabilization of MCL-1 in hepatocytes[60]. Therefore, we analyzed the role of MCL-1 and BCL-xL expression for the apoptosis-inducing capacity of JNK inhibitors and TRAIL. We found profoundly increased apoptosis rates after JNK inhibition in cells lacking BCL-xL, whereas cells lacking MCL-1 did not exhibit sensitivity towards JNK inhibition. Strikingly, a combined treatment of a JNK inhibitor and TRAIL caused major rates of apoptosis in cells lacking MCL-1 and BCL-xL. Furthermore, we could show that inhibition of JNK is not capable of inducing apoptosis alone unless BCL-xL is effectively downregulated in HCC cells, revealing a major role of BCL-xL for the resistance of HCC cells towards apoptosis induced by JNK inhibitors.
In conclusion, the application of recombinant TRAIL, as well as that of monoclonal antibodies targeting TRAIL receptors, is an encouraging therapeutic approach in cancer patients. However, resistance to TRAIL treatment is a common phenomenon in many cancer entities. The aim of our study was to provide novel treatment options to overcome resistance of HCC cells towards TRAIL-induced apoptosis. Our results demonstrate that TRAIL is an effective treatment option in HCC if combined with the chemotherapeutic drugs Doxo and 5-FU or kinase inhibitors, such as LY294002, AG1478 and PD98059. In addition, we revealed a pivotal role of the antiapoptotic BCL-2 proteins MCL-1 and BCL-xL for HCC resistance towards TRAIL. Further studies are warranted to evaluate the potential of combined treatment approaches and clear the trail for clinical usage.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide. Numerous clinical trials have failed to establish an effective therapy regimen in patients with advanced or metastasized HCC. Thus, new strategies for these patients are mandatory. Defects in apoptosis signaling contribute to resistance of HCC cells towards the death receptor ligand tumor necrosis factor-related apoptosis inducing ligand (TRAIL), which is a promising anti-tumor agent since it is capable of killing tumor cells via receptor-mediated apoptosis. New combined treatment regimens have the aim of overcoming resistance towards TRAIL and to make TRAIL an effective treatment option in patients suffering from HCC.
Research frontiers
Hyperactivation of the PI3K/Akt, EGFR and [Mitogen-activated protein kinase/extracellular signal regulated kinase (ERK) kinase] (MEK)/ERK survival pathways and decreased mitochondrial sensitivity due to overexpression of the BCL-2 proteins MCL-1 and BCL-xL are key mechanisms of TRAIL resistance in HCC. Furthermore, resistance towards TRAIL can be located at receptor level, contributing to inefficient treatment of HCC cells with TRAIL compounds.
Innovations and breakthroughs
Previous articles have demonstrated that TRAIL is an effective treatment option in HCC in a combined setup with sensitizing agents. In this study the authors demonstrate new treatment options for the sensitization of HCC cells towards TRAIL-induced apoptosis by combination of chemotherapeutic drugs doxorubicin (Doxo) and 5-fluorouracil (5-FU) or kinase inhibitors, such as LY294002, AG1478, PD98059 and SP600125 with TRAIL. In addition, the authors reveal a pivotal role of the antiapoptotic BCL-2 proteins MCL-1 and BCL-xL for HCC resistance towards TRAIL. The importance of MCL-1 and Bcl-xL for mitochondrial integrity has been extensively studied in this study.
Applications
TRAIL is an effective treatment option in HCC if combined with the chemotherapeutic drugs Doxo and 5-FU or kinase inhibitors, such as LY294002, AG1478, PD98059 and SP600125. These results open the possibility of a treatment regime which includes reduced doses of chemotherapeutic drugs in combination with TRAIL. The authors also revealed a pivotal role of the antiapoptotic BCL-2 proteins MCL-1 and BCL-xL for HCC resistance towards TRAIL. Thus, downregulation of these anti-apoptotic proteins alone (e.g. by application of so-called “BH3-only mimetics”) is a promising approach for the treatment of HCC patients. “BH3-only mimetics” have already entered clinical trials in cancer patients.
Terminology
Apoptosis, also described as programmed cell death, is a genetically determined process of controlled cellular suicide characterized by typical morphological changes, e.g. fragmentation of DNA. TRAIL ligates two different types of receptors: (1) death receptors triggering TRAIL-induced apoptosis and (2) decoy receptors possibly inhibiting the TRAIL death-signaling pathway. MCL-1 and BCL-xL are antiapoptotic members of the BCL-2 family serving as protective factors against several death stimuli. Antiapoptotic pathways such as PI3K/Akt, EGFR, MEK/ERK are well known as being activated in malignant cells, thus contributing to cell cycle progression and tumor growth
Peer review
This is an interesting work, elegantly performed and with possible future clinical applications. Although the article contains a wealth of experimental details, making it a difficult reading for the uninitiated, I believe it will be of interest for both clinicians and basic science readers of the World Journal of Gastroenterology because of its possible clinical implications
Footnotes
Supported by Research grants from Merck KGaA, Darmstadt, Germany, to Schulze-Bergkamen H
Peer reviewers: Yuichiro Eguchi, MD, Saga Medical School, Department of Internal Medicine, 5-1-1 Nabeshima, 849-8501Saga, Japan; Emanuel K Manesis, MD, Professor of Medicine, Athens University School of Medicine, Liver Unit, Euroclinic, 19 Mavromateon Street 104 34, Athens, Greece; Chen Liu, MD, PhD, Associate Professor of Pathology, Director, Gastrointestinal and Liver Pathology, Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, 1600 SW Archer Rd, PO Box 100275, Gainesville, FL32610, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44 Suppl 19:96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 5.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008;134:379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Chaparro M, González Moreno L, Trapero-Marugán M, Medina J, Moreno-Otero R. Review article: pharmacological therapy for hepatocellular carcinoma with sorafenib and other oral agents. Aliment Pharmacol Ther. 2008;28:1269–1277. doi: 10.1111/j.1365-2036.2008.03857.x. [DOI] [PubMed] [Google Scholar]
- 7.Diamantis A, Magiorkinis E, Sakorafas GH, Androutsos G. A brief history of apoptosis: from ancient to modern times. Onkologie. 2008;31:702–706. doi: 10.1159/000165071. [DOI] [PubMed] [Google Scholar]
- 8.Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:513–520. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze-Bergkamen H, Krammer PH. Apoptosis in cancer--implications for therapy. Semin Oncol. 2004;31:90–119. doi: 10.1053/j.seminoncol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Mérino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets. 2007;11:1299–1314. doi: 10.1517/14728222.11.10.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 12.Zinkel SS. Investigation of the proapoptotic BCL-2 family member bid on the crossroad of the DNA damage response and apoptosis. Methods Enzymol. 2008;442:231–250. doi: 10.1016/S0076-6879(08)01412-2. [DOI] [PubMed] [Google Scholar]
- 13.Fleischer B, Schulze-Bergkamen H, Schuchmann M, Weber A, Biesterfeld S, Müller M, Krammer PH, Galle PR. Mcl-1 is an anti-apoptotic factor for human hepatocellular carcinoma. Int J Oncol. 2006;28:25–32. [PubMed] [Google Scholar]
- 14.Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 15.Schulze-Bergkamen H, Ehrenberg R, Hickmann L, Vick B, Urbanik T, Schimanski CC, Berger MR, Schad A, Weber A, Heeger S, et al. Bcl-x(L) and Myeloid cell leukaemia-1 contribute to apoptosis resistance of colorectal cancer cells. World J Gastroenterol. 2008;14:3829–3840. doi: 10.3748/wjg.14.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006;281:10153–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 17.Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 18.Schuchmann M, Schulze-Bergkamen H, Fleischer B, Schattenberg JM, Siebler J, Weinmann A, Teufel A, Wörns M, Fischer T, Strand S, et al. Histone deacetylase inhibition by valproic acid down-regulates c-FLIP/CASH and sensitizes hepatoma cells towards CD95- and TRAIL receptor-mediated apoptosis and chemotherapy. Oncol Rep. 2006;15:227–230. doi: 10.3892/or.15.1.227. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Benito M, Martinez-Lorenzo MJ, Anel A, Marzo I, Naval J. Membrane expression of DR4, DR5 and caspase-8 levels, but not Mcl-1, determine sensitivity of human myeloma cells to Apo2L/TRAIL. Exp Cell Res. 2007;313:2378–2388. doi: 10.1016/j.yexcr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, Krueger A, Weigand MA, Grosse-Wilde A, Stremmel W, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11 Suppl 1:S86–S96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- 21.He SQ, Rehman H, Gong MG, Zhao YZ, Huang ZY, Li CH, Zhang WG, Chen XP. Inhibiting survivin expression enhances TRAIL-induced tumoricidal activity in human hepatocellular carcinoma via cell cycle arrest. Cancer Biol Ther. 2007;6:1247–57. doi: 10.4161/cbt.6.8.4444. [DOI] [PubMed] [Google Scholar]
- 22.Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, Manzoli L, Cocco L, Martelli AM. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 25.Mahalingam D, Szegezdi E, Keane M, Jong S, Samali A. TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat Rev. 2009;35:280–288. doi: 10.1016/j.ctrv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz JD, Beutler AS. Therapy for unresectable hepatocellular carcinoma: review of the randomized clinical trials-II: systemic and local non-embolization-based therapies in unresectable and advanced hepatocellular carcinoma. Anticancer Drugs. 2004;15:439–452. doi: 10.1097/01.cad.0000131140.12228.bb. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe J, Kushihata F, Honda K, Mominoki K, Matsuda S, Kobayashi N. Bcl-xL overexpression in human hepatocellular carcinoma. Int J Oncol. 2002;21:515–519. [PubMed] [Google Scholar]
- 28.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 29.Koschny R, Walczak H, Ganten TM. The promise of TRAIL--potential and risks of a novel anticancer therapy. J Mol Med. 2007;85:923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 30.Hailfinger S, Jaworski M, Marx-Stoelting P, Wanke I, Schwarz M. Regulation of P53 stability in p53 mutated human and mouse hepatoma cells. Int J Cancer. 2007;120:1459–1464. doi: 10.1002/ijc.22519. [DOI] [PubMed] [Google Scholar]
- 31.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, Lo L, Gallant G, Klein J. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61:82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 33.Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- 34.Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 35.MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, Dyer MJ, Cohen GM. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005;12:773–782. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- 36.Hwu WJ, Salem RR, Pollak J, Rosenblatt M, D'Andrea E, Leffert JJ, Faraone S, Marsh JC, Pizzorno G. A clinical-pharmacological evaluation of percutaneous isolated hepatic infusion of doxorubicin in patients with unresectable liver tumors. Oncol Res. 1999;11:529–537. [PubMed] [Google Scholar]
- 37.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 38.He X, Zhu Z, Johnson C, Stoops J, Eaker AE, Bowen W, DeFrances MC. PIK3IP1, a negative regulator of PI3K, suppresses the development of hepatocellular carcinoma. Cancer Res. 2008;68:5591–5598. doi: 10.1158/0008-5472.CAN-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 40.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247–261. doi: 10.1586/14737140.9.2.247. [DOI] [PubMed] [Google Scholar]
- 42.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 43.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 44.Moon DO, Park C, Heo MS, Park YM, Choi YH, Kim GY. PD98059 triggers G1 arrest and apoptosis in human leukemic U937 cells through downregulation of Akt signal pathway. Int Immunopharmacol. 2007;7:36–45. doi: 10.1016/j.intimp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, Lee MS, Taylor R, Davis TL, Hauke R, et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478–485. doi: 10.1002/ijc.11413. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Jiang H, Zhou M, Xu Z, Liu S, Shi B, Yao X, Yao M, Gu J, Li Z. Epidermal growth factor receptor vIII enhances tumorigenicity and resistance to 5-fluorouracil in human hepatocellular carcinoma. Cancer Lett. 2009;279:30–38. doi: 10.1016/j.canlet.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Ellis AG, Nice EC, Weinstock J, Levitzki A, Burgess AW, Webster LK. High-performance liquid chromatographic analysis of the tyrphostin AG1478, a specific inhibitor of the epidermal growth factor receptor tyrosine kinase, in mouse plasma. J Chromatogr B Biomed Sci Appl. 2001;754:193–199. doi: 10.1016/s0378-4347(00)00606-x. [DOI] [PubMed] [Google Scholar]
- 48.Ellis AG, Doherty MM, Walker F, Weinstock J, Nerrie M, Vitali A, Murphy R, Johns TG, Scott AM, Levitzki A, et al. Preclinical analysis of the analinoquinazoline AG1478, a specific small molecule inhibitor of EGF receptor tyrosine kinase. Biochem Pharmacol. 2006;71:1422–1434. doi: 10.1016/j.bcp.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Mucha SR, Rizzani A, Gerbes AL, Camaj P, Thasler WE, Bruns CJ, Eichhorst ST, Gallmeier E, Kolligs FT, Göke B, et al. JNK inhibition sensitises hepatocellular carcinoma cells but not normal hepatocytes to the TNF-related apoptosis-inducing ligand. Gut. 2009;58:688–698. doi: 10.1136/gut.2008.154625. [DOI] [PubMed] [Google Scholar]
- 50.Puduvalli VK, Sampath D, Bruner JM, Nangia J, Xu R, Kyritsis AP. TRAIL-induced apoptosis in gliomas is enhanced by Akt-inhibition and is independent of JNK activation. Apoptosis. 2005;10:233–243. doi: 10.1007/s10495-005-6078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Likui W, Qun L, Wanqing Z, Haifeng S, Fangqiu L, Xiaojun L. Prognostic role of myeloid cell leukemia-1 protein (Mcl-1) expression in human gastric cancer. J Surg Oncol. 2009;100:396–400. doi: 10.1002/jso.21344. [DOI] [PubMed] [Google Scholar]
- 53.O'Driscoll L, Cronin D, Kennedy SM, Purcell R, Linehan R, Glynn S, Larkin A, Scanlon K, McDermott EW, Hill AD, et al. Expression and prognostic relevance of Mcl-1 in breast cancer. Anticancer Res. 2004;24:473–482. [PubMed] [Google Scholar]
- 54.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, Opferman JT, Schuchmann M, Galle PR, Schulze-Bergkamen H. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 57.Qian J, Zou Y, Rahman JS, Lu B, Massion PP. Synergy between phosphatidylinositol 3-kinase/Akt pathway and Bcl-xL in the control of apoptosis in adenocarcinoma cells of the lung. Mol Cancer Ther. 2009;8:101–109. doi: 10.1158/1535-7163.MCT-08-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Chen W, Zeng W, Bai L, Tesfaigzi Y, Belinsky SA, Lin Y. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther. 2008;7:1156–1163. doi: 10.1158/1535-7163.MCT-07-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang CC, Lin HP, Chen CS, Yang YT, Tseng PH, Rangnekar VM, Chen CS. Bcl-xL mediates a survival mechanism independent of the phosphoinositide 3-kinase/Akt pathway in prostate cancer cells. J Biol Chem. 2003;278:25872–25878. doi: 10.1074/jbc.M301744200. [DOI] [PubMed] [Google Scholar]
- 60.Kodama Y, Taura K, Miura K, Schnabl B, Osawa Y, Brenner DA. Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]