Abstract
The incidence of hepatocellular carcinoma (HCC) is increasing in the United States, and 50%-75% of patients with HCC will develop metastatic disease. Orbital metastases from HCC are extremely rare. We report the case of a 52-year-old male with known metastatic HCC, who presented with severe proptosis and diplopia. An orbital mass was identified on magnetic resonance imaging (MRI) and confirmed to have hypermetabolic activity on positron emission tomography/computed tomography. He received a palliative course of external beam radiation therapy to the right orbit. Intensity modulated radiation therapy (IMRT) was used to allow sparing of critical normal tissues in close proximity to the tumor. One month after completion of IMRT to 58 Gray in 30 fractions delivered over 6 wk, the patient had a complete clinical, radiologic (MRI) and symptomatic response. The patient continues to have local control in the orbit 1.7 years after therapy completion. All critical normal structures were kept below the tolerance dose using IMRT, and no toxicities were observed.
Keywords: Hepatocellular carcinoma, Eye neoplasms, Metastasis, Intensity modulated radiation therapy, Palliative therapy
INTRODUCTION
According to Surveillance, Epidemiology, and End Results data, the incidence of hepatocellular carcinoma (HCC) has been steadily increasing since the mid-1980s and thus presents an increasing health problem in the United States. The average, age-adjusted incidence rates for liver and intrahepatic bile duct cancer, two-thirds of which are HCC, rose from 3.2 per 100 000 persons in 1985 to 6.4 per 100 000 persons in 2005[1,2]. The incidence is 3-4 times higher in men than in women and is highest in the Asian population[2]. The prognosis for patients diagnosed with HCC is dismal with 5-year survival rates of 3%-5%[3].
Approximately 50%-75% of patients with HCC will develop metastases during the course of their disease[4,5]. The most common sites of metastatic disease are the regional lymph nodes and lung. Less common sites of metastases include bone, brain, adrenal glands, and skin[4,6-9]. The orbit has been reported as a site of metastasis from HCC only 14 times in the literature, and information on the palliative response of this highly symptomatic condition with radiation therapy has been very sparse. We report a case of a patient with an orbital metastasis from HCC, who achieved a complete clinical and radiographic response to intensity modulated radiation therapy (IMRT).
CASE REPORT
A 52-year-old Asian male with a history of hepatitis C but no known cirrhosis presented with elevated liver enzymes while being treated for polycythemia. High resolution triphasic spiral computed tomography (CT) scan of the abdomen demonstrated a well encapsulated, heterogeneous mass in the right lobe of the liver measuring 8.2 cm × 8.8 cm. Imaging characteristics were typical for HCC and α-fetoprotein was 30 ng/mL. Percutaneous biopsy confirmed HCC in a background of cirrhosis. Exploratory laparotomy with intraoperative ultrasound suggested the need for extended hepatectomy for a curative approach but the volume of the future liver remnant was less than 30%. As such, surgical resection was not completed at that time. Selective right transarterial chemoembolization, followed by portal vein embolization (PVE) of segments 4-8 was undertaken to promote atrophy of the tumor-bearing liver and hypertrophy of the future liver remnant. Four weeks following PVE, resection was again attempted but metastasis was identified in the future liver remnant. No extrahepatic disease was noted. He was subsequently treated with radioembolization using Yttrium-90.
Shortly after the Yttrium-90 therapy, he developed rapidly progressive diplopia and proptosis of the right eye. Physical examination showed severe proptosis, conjunctival hyperemia, excessive tearing, impaired vision, and limited extraocular muscle movement of the right eye. Magnetic resonance imaging (MRI) of the brain and orbits identified an extraconal 3.7 cm × 3.3 cm × 3.7 cm enhancing mass arising from the floor of the right anterior cranial fossa with extension to the right orbit, resulting in mass effect on the superior and lateral rectus muscles and globe (Figure 1). Positron emission tomography (PET)/CT confirmed a right orbital mass with a peak standardized uptake value (SUV) of 2.8.
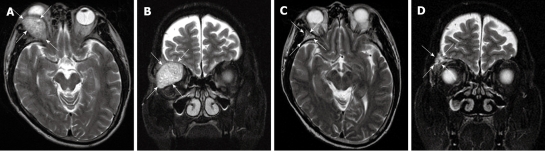
Figure 1.
Axial (A) and coronal (B) T2 weighted MRI of the brain demonstrating the large soft tissue mass in the superior and lateral aspect of the right orbit (white arrows) prior to radiation treatment and resolution of mass on follow-up MRI 12 mo after treatment on axial (C) and coronal images (D).
He received a course of palliative radiation therapy to a dose of 40 Gray (Gy) in 20 fractions to the right orbit using 6 MV photons and IMRT (Figure 2A). The total gross tumor volume (GTV) was 15.8 cm3. A CT scan 10 d after treatment completion showed no response. MRI 1 mo after treatment completion showed the tumor to be decreased from the initial 3.7 cm × 3.3 cm × 3.7 cm to 3.7 cm × 2.8 cm × 3.7 cm. Clinical improvement in the proptosis was also observed. An IMRT boost to the right orbit of an additional 18 Gy in 10 fractions was then delivered (Figure 2B). The total volume of the GTV had decreased to 8.8 cm3. The total dose delivered was 58 Gy in 30 fractions over 86 d. The dose to the critical normal structures was kept below the tolerance dose and is presented in Table 1.
Figure 2.
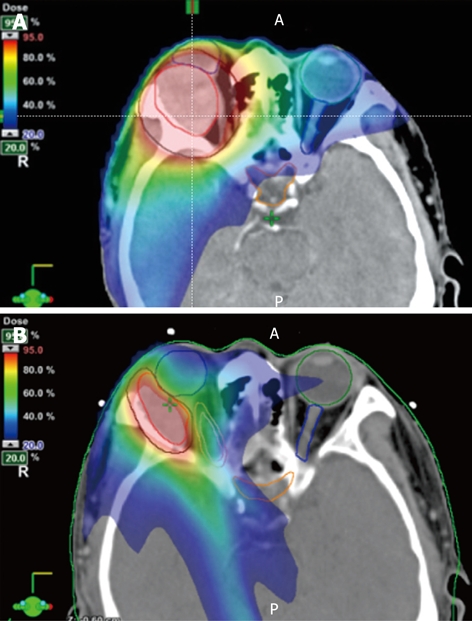
Three-dimensional contours for treatment planning demonstrate the gross target volume (GTV) encompassing the tumor (red contour) and the planning target volume (PTV, black contour) on initial (A) and boost (B) CT images. Doses from the initial 7 field intensity modulated radiation (IMRT) plan (A) are represented as color wash according to the scale shown in the figures, representing the range of 95% through 20% of dose coverage. The 7 field IMRT plan for the coned-down field (B) shows even tighter dose coverage and greater sparing of the right optic nerve (pink contour).
Table 1.
Critical structures mean dose for original and boost IMRT plans
| Structure | Original mean dose (Gy) (2 Gy/fx) | Boost mean dose (Gy) (1.8 Gy/fx) | Total dose (Gy) |
| Chiasm | 6.98 | 3.55 | 10.52 |
| Left eye | 15.93 | 1.42 | 17.34 |
| Left optic nerve | 13.3 | 1.96 | 15.26 |
| Right eye | 30.7 | 3.96 | 34.65 |
| Right optic nerve | 34.09 | 6.46 | 40.55 |
IMRT: Intensity modulated radiation therapy; Gy: Gray.
One month after receiving 58 Gy to the orbit, the patient reported complete resolution of the diplopia. On examination, his right extraocular muscles functioned normally and proptosis was no longer present. Follow-up PET/CT 1 mo after treatment completion showed decreased fluoro-deoxyglucose activity (peak SUV = 2.2) within the superolateral aspect of the right orbit. MRI of the brain 3, 12 and 17 mo after treatment demonstrated complete resolution of the lesion (Figure 1). The patient is alive with controlled orbital disease 20 mo after the initial diagnosis of the orbital metastasis and 26 mo after his original HCC diagnosis. As a result of progression in the liver following Yttrium-90 therapy, he is currently receiving Sorafenib with effective systemic disease control.
DISCUSSION
Orbital metastases are uncommon and account for 3%-7% of all orbital neoplasms[7,10,11]. The most common symptoms of orbital metastases include pain, proptosis, decreased vision or blindness, diplopia, displacement of the globe, exophthalmos, and occasionally enophthalmos. HCC very rarely metastasizes to the orbits, and only a total of 14 case reports of orbital metastases from HCC have been described (Table 2)[4-15]. Orbital metastases are usually associated with advanced disease and early mortality. The average survival after occurrence of the orbital metastases is approximately 10 mo[11], although the prognosis ultimately depends on the systemic tumor burden[5].
Table 2.
Case reports of orbital metastases from hepatocellular carcinoma
| Author | Gender | Age (yr) | Treatment | Survival |
| Gupta et al[10] | M | 45 | None | NA |
| Loo et al[8] | F | 71 | Transcranial orbitotomy | 3 mo |
| Schwab et al[12] | M | 19 | Anterior orbitotomy with biopsy | 2 wk (moribund state) |
| Wakisaka et al[9] | M | 58 | Left frontotemporal craniotomy | 11 mo |
| Lubin et al[22] | M | 69 | 3000 cGy in 2 wk | NA |
| Zubler et al[5] | M | 64 | 4000 cGy over 8 wk + chemotherapy | 3 mo |
| Srinivasan et al[4] | F | 76 | None | NA |
| Scolyer et al[23] | M | 77 | None | NA |
| Font et al[13] | F | 79 | Palliative RT | 3 yr |
| Kim et al[6] | F | 56 | None | 2 mo |
| Machado-Netto et al[11] | M | 57 | Megestrol acetate and Gemcitabine | 15 mo |
| Hirunwiwatkul et al[7] | F | 74 | NA | 2 mo |
| Tranfa et al[14] | M | 85 | Anterior orbitotomy with excisional biopsy | NA |
| Phanthumchinda et al[15] | F | 29 | 5400 cGy in 4 wk | NA |
NA: Not available; RT: Radiation therapy; F: Female; M: Male.
Among the 14 reported cases of orbital metastases from HCC (Table 2)[4-15], only 4 received primary radiation as the palliative treatment in doses ranging from 30 to 54 Gy, and all showed a response. However, specific details of the radiation planning and treatment delivery in this challenging location are not available. Long-term effects of treatment are poorly understood because only one of the patients survived longer than 1 year after treatment[11,13].
Our patient received a higher dose of radiation than used in the above case reports, and was the first to use IMRT for treatment of orbital HCC metastasis. The orbit is among the most challenging regions for radiation therapy because of the close proximity of dose-limiting critical normal structures, including the brain, ocular structures, and optic chiasm, that can all develop significant complications. IMRT enabled highly conformal treatment delivery and resulted in a durable complete radiologic response.
Our findings are in contrast to the common belief that HCC is a radioresistant tumor. Such reports frequently had to rely on older, less targeted radiation therapy techniques that were unable to spare normal tissues, thus severely limiting the deliverable radiation dose to the tumor to avoid serious toxicity to normal structures. This low tumor dose was not adequate to achieve a significant tumor response[16]. Our case shows that dose escalation to 58 Gy, enabled by IMRT, can afford effective local control in HCC. Our observations are supported by studies showing a dose-response relationship for treatment of metastases from HCC. In a retrospective review by Park et al[17], 91% of patients with intraabdominal lymph node metastases from HCC treated to ≥ 50 Gy10 had an objective response compared to 65% of patients treated to lesser doses. Recent studies have also shown excellent local control and improved survival with the use of higher doses for primary HCC, delivered with conformal radiation therapy to the partial liver, that were previously intolerable[18,19].
The tumor response in our patient is also characterized by a protracted time course. Symptoms improved slowly, and not until a treatment break and re-imaging 1 mo after a dose of 40 Gy, was the radiologic response evident. Such a slow response pattern may also have led to the conclusion that HCC is not a radio-sensitive tumor. However, the GTV reduction after a 5 wk break following 40 Gy allowed us to further escalate the dose to the orbital tumor, while effectively sparing the sensitive normal structures, especially the right optic nerve (Figure 2B). In conjunction with the highly conformal IMRT delivery, this interval tumor reduction and dose escalation resulted in a durable complete clinical and radiologic response.
Our case also illustrates the importance of 3-dimensional (3D) volumetric analysis of tumor imaging instead of diameter-based measurements for the assessment of tumor response. In the repeat CT for boost planning after 40 Gy, 3D tumor volumetry, determined by tumor delineation on each imaging slice and computation of the volume, demonstrated a reduced tumor volume from 15.8 to 8.8 cm3, a change of 44%. However, the diagnostic brain MRI 1 wk previously showed a decrease from 23.7 to 20.1 cm3 in MRI diameter-based tumor volume calculation, a change of 15%. Other studies have also shown that diameter-based measurements overestimate tumor size during and after radiation therapy compared to 3D volumetry. This is likely related to the irregular tumor configurations and non-linear tumor shrinkage, that are not adequately assessed by current gold standard diameter-based measurements[20]. The high precision of refined 3D volumetry-based measurements, which are easily obtained from treatment planning systems, can overcome the challenge of irregular tumor configuration[21].
In conclusion, because of the increasing incidence and improvement in systemic treatment of primary HCC, the prevalence of symptomatic metastases from HCC will likely increase. Radiation therapy is an excellent treatment option for palliation of challenging metastatic sites, including the orbit, but higher doses than the typical 30 Gy in 10 fractions may be required. With targeted radiation techniques, such as IMRT, that enable sparing of normal critical structures, and 3D volumetric assessment of the response, tumor volume-adapted dose escalation to optimal tumoricidal dose levels can provide durable effective palliation of debilitating symptoms.
Footnotes
Supported by NIH/NCI K12 CA133250-01
Peer reviewer: Dr. Chao-Hung Hung, Division of Hepatogastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, 123 Ta Pei Road, Niao Sung, Kaohsiung 833, Taiwan, China
S- Editor Wang JL L- Editor Cant MR E- Editor Zheng XM
References
- 1.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2005, National Cancer Institute. Bethesda, MD, based on November 2007 SEER data submission, posted to the SEER web site, 2008. Available from: http://seer.cancer.gov/csr/1975_2005/
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan R, Krishnanand G. Cytologic diagnosis of metastatic hepatocellular carcinoma presenting as an orbital mass. A case report. Acta Cytol. 2007;51:83–85. doi: 10.1159/000325689. [DOI] [PubMed] [Google Scholar]
- 5.Zubler MA, Rivera R, Lane M. Hepatoma presenting as a retro-orbital metastasis. Cancer. 1981;48:1883–1885. doi: 10.1002/1097-0142(19811015)48:8<1883::aid-cncr2820480828>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Kim IT, Na SC, Jung BY. Hepatocellular carcinoma metastatic to the orbit. Korean J Ophthalmol. 2000;14:97–102. doi: 10.3341/kjo.2000.14.2.97. [DOI] [PubMed] [Google Scholar]
- 7.Hirunwiwatkul P, Tirakunwichcha S, Meesuaypong P, Shuangshoti S. Orbital metastasis of hepatocellular carcinoma. J Neuroophthalmol. 2008;28:47–50. doi: 10.1097/WNO.0b013e31816754e7. [DOI] [PubMed] [Google Scholar]
- 8.Loo KT, Tsui WM, Chung KH, Ho LC, Tang SK, Tse CH. Hepatocellular carcinoma metastasizing to the brain and orbit: report of three cases. Pathology. 1994;26:119–122. doi: 10.1080/00313029400169321. [DOI] [PubMed] [Google Scholar]
- 9.Wakisaka S, Tashiro M, Nakano S, Kita T, Kisanuki H, Kinoshita K. Intracranial and orbital metastasis of hepatocellular carcinoma: report of two cases. Neurosurgery. 1990;26:863–866. doi: 10.1097/00006123-199005000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Honavar SG, Vemuganti GK. Orbital metastasis from hepatocellular carcinoma. Surv Ophthalmol. 2005;50:485–489. doi: 10.1016/j.survophthal.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Machado-Netto MC, Lacerda EC, Heinke T, Maia DC, Lowen MS, Saad ED. Massive orbital metastasis of hepatocellular carcinoma. Clinics (Sao Paulo) 2006;61:359–362. doi: 10.1590/s1807-59322006000400015. [DOI] [PubMed] [Google Scholar]
- 12.Schwab L, Doshi H, Shields JA, Kagame K, Chana H. Hepatocellular carcinoma metastatic to the orbit in an African patient. Ophthalmic Surg. 1994;25:105–106. [PubMed] [Google Scholar]
- 13.Font RL, Maturi RK, Small RG, Garcia-Rojas M. Hepatocellular carcinoma metastatic to the orbit. Arch Ophthalmol. 1998;116:942–945. doi: 10.1001/archopht.116.7.942. [DOI] [PubMed] [Google Scholar]
- 14.Tranfa F, Cennamo G, Rosa N, De Rosa G, Boscaino A, Bonavolontà G. An unusual orbital lesion: hepatoma metastatic to the orbit. Ophthalmologica. 1994;208:329–332. doi: 10.1159/000310532. [DOI] [PubMed] [Google Scholar]
- 15.Phanthumchinda K, Hemachuda T. Superior orbital fissure syndrome as a presenting symptom in hepatocellular carcinoma. J Med Assoc Thai. 1991;74:679–682. [PubMed] [Google Scholar]
- 16.Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer. 2006;106:1653–1663. doi: 10.1002/cncr.21811. [DOI] [PubMed] [Google Scholar]
- 17.Park YJ, Lim do H, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC, Nam HR, Oh DR, Park W, et al. Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol. 2006;41:1099–1106. doi: 10.1007/s00535-006-1895-x. [DOI] [PubMed] [Google Scholar]
- 18.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, Knol J, Dawson LA, Pan C, Lawrence TS. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 20.James K, Eisenhauer E, Christian M, Terenziani M, Vena D, Muldal A, Therasse P. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523–528. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 21.Mayr NA, Yuh WT, Oberley LW, Spitz D, Sorosky JI, Buatti JM. Serial changes in tumor oxygenation during the early phase of radiation therapy in cervical cancer-are we quantitating hypoxia change? Re: Lying et al., IJROBP 2000; 46:935-946. Int J Radiat Oncol Biol Phys. 2001;49:282–289. doi: 10.1016/s0360-3016(00)00794-x. [DOI] [PubMed] [Google Scholar]
- 22.Lubin JR, Grove AS Jr, Zakov ZN, Albert DM. Hepatoma metastatic to the orbit. Am J Ophthalmol. 1980;89:268–273. doi: 10.1016/0002-9394(80)90123-3. [DOI] [PubMed] [Google Scholar]
- 23.Scolyer RA, Painter DM, Harper CG, Lee CS. Hepatocellular carcinoma metastasizing to the orbit diagnosed by fine needle aspiration cytology. Pathology. 1999;31:350–353. doi: 10.1080/003130299104710. [DOI] [PubMed] [Google Scholar]