Abstract
Superior mesenteric artery (SMA) syndrome is an uncommon disease resulting compression of the third portion of the duodenum from the superior mesenteric artery. This disease shares many common manifestations with diabetic gastroparesis, including postprandial fullness, nausea, vomiting, and bloating. Therefore, it is often overlooked in diabetic patients. Here, we report a 41-year-old man with poorly controlled diabetic mellitus who developed SMA syndrome due to rapid weight loss. The diagnosis was confirmed by computed tomography and an upper gastrointestinal series. His condition improved after parenteral nutrient, strict sugar control, and gradual weight gain.
Keywords: Diabetes mellitus, Superior mesenteric artery syndrome, Gastroparesis
INTRODUCTION
Superior mesenteric artery (SMA) syndrome, also known as Wilkie’s syndrome or cast syndrome, is an uncommon disease resulting from superior mesenteric artery compression of the third portion of the duodenum. The clinical manifestations include postprandial fullness or pain, nausea, vomiting, and anorexia due to duodenal obstruction[1]. However, the symptoms are similar to those of diabetic gastrointestinal complications. Therefore, SMA syndrome could be misdiagnosed as diabetic gastroparesis. The delayed diagnosis of SMA syndrome might result in malnutrition, electrolyte imbalance, dehydration, and even death.
CASE REPORT
This 41-year-old male was diagnosed type 2 diabetes mellitus four years ago, but the disease was poor controlled. He did not take any oral antidiabetic agent or insulin therapy for one year. He visited our emergency room complaining of abdominal discomfort and vomiting 30 min after meals for 1 wk. The associated symptoms included general weakness, bilateral lower leg numbness, and a gradual bodyweight loss of 26 kg over the last 3 mo. Physical examination showed a distended abdomen and positive succussion splash sign. His glycemic control was poor and glycosylated hemoglobin (HbA1c) was 11.4%. The abnormal hematologic and biochemical findings included mild anemia (Hgb: 11.7 g/dL) and hypokalemia (K: 3.3 mEq/L). The plain abdomen revealed dilated duodenal bulb and distended stomach with air-fluid level (Figure 1). The gastroduodenoscopy showed a distended stomach with much gastric residue. Therefore, a proximal small bowel obstruction was tentatively diagnosed. To achieve the final diagnosis, we arranged a series of examinations. The upper gastrointestinal series demonstrated a sharp cut-off at the 3rd portion of the duodenum (Figure 2). Compression of the third portion of the duodenum, an aortomesenteric distance of 4.1 mm (normal: 10-28 mm) and a reduction of the aortomesenteric angle 16.6° (normal: 25°-60°) were noted by computed tomography (CT) scan[2] (Figure 3). After inserting a nasogastric tube, it drained over 3000 mL turbid, green fluid. His symptoms improved after nasogastric tube drainage and kept the left lateral decubitus position. We gave him total parenteral nutrition as a nutrition supply and controlled his sugar with an insulin pump for 2 wk. After 2 mo, his bodyweight increased from 44 to 50 kg and he returned to oral intake without subsequent symptoms.
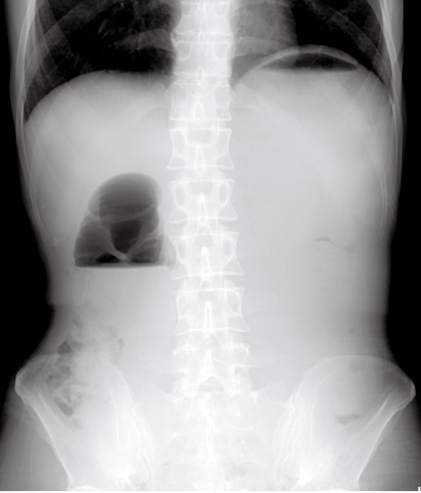
Figure 1.
Abdominal X-ray showing a distended stomach with air fluid level in the stomach and duodenal bulb. The “Double bubble sign” was consistent with high small bowel obstruction.
Figure 2.
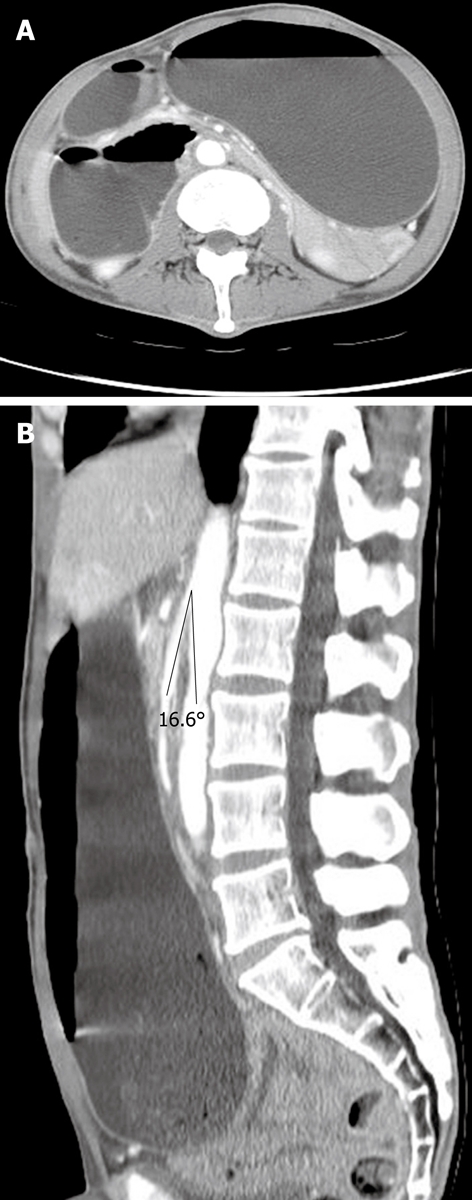
Computed tomography (CT) scan showing distended stomach and 2nd portion of duodenum (A); The angle between aorta and superior mesenteric artery (SMA) was 16.6° (B).
Figure 3.
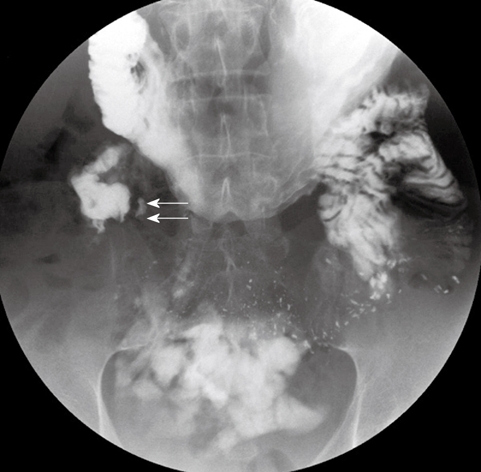
Upper gastrointestinal series showing an abrupt cut-off (short arrows) at the third portion of the duodenum.
DISCUSSION
Diabetes mellitus is one of the most common chronic disease of the world and the prevalence of diabetes mellitus is over 10% in Taiwan[3]. Gastroparesis is reported in 5% to 12% of diabetic patients. The cardinal symptoms include postprandial fullness, nausea, vomiting, and bloating. Treatment for gastroparesis is prokinetics including metoclopramide, domperidone, and erythromycin[4]. However, SMA syndrome can cause the same symptoms as diabetic gastroparesis.
To our knowledge, only two studies have reported diabetic patients with SMA syndrome and all of them had bodyweight loss[5,6]. The average bodyweight loss was 29.6 kg (16-50 kg). One of the reported cases received an exploratory laparotomy and the other received intravenous nutrition treatment. In our report, the patient also had a gradually weight loss of 26 kg and improved after medical treatment. The bodyweight loss is a manifestation of the new diagnosis or the poor control of the diabetic patient. It might result in the delayed or missed diagnosis of the SMA syndrome as diabetic gastroparesis.
SMA syndrome is a disease of duodenal obstruction. Weight loss results in loss of the mesenteric fat pad and the superior mesenteric artery compresses duodenum. Bodyweight loss with superior mesenteric artery syndrome, including eating disorders, cardiac cachexia, HIV patients, hereditary motor and sensory neuropathy, have been reported[7-10]. The radiographic studies used to establish diagnosis include an upper gastrointestinal series, computed tomography (CT), CT angiography, conventional angiography, abdominal sonography, and magnetic resonance angiography (MRA)[2,11-13]. The prone or left lateral decubitus position is effective in the acute status. Conservative treatment with adequate fluid and electrolyte supply is necessary after nasogastric tube placement. Enteral jejunal tube feeding and parenteral nutrition are useful to increase bodyweight. Surgery is indicated when the conservative treatment fails[1]. Laparoscopic duodenojejunostomy has been successful in the cases with SMA syndrome[14].
In conclusion, diabetic patients with gastrointestinal symptoms and bodyweight loss should be considered for SMA syndrome, despite the gastroparesis is the most common etiology. Computed tomography and upper gastrointestinal series are the reliable tools for diagnosis. Adequate nutrition supply is a useful treatment and the aim is bodyweight gain and symptom relief. Surgery is indicated when conservative treatment fails.
Footnotes
Supported by Grants From Kaohsiung Medical University Hospital, No. 94-KMUH-032 and No. M094015
Peer reviewer: Rabih M Salloum, MD, FACS, Associate professor of Surgery and Oncology, University of Rochester Medical center, 601 Elmwood Avenue Box SURG, Rochester, NY 14642, United State
S- Editor Wang YR L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Welsch T, Buchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007;24:149–156. doi: 10.1159/000102097. [DOI] [PubMed] [Google Scholar]
- 2.Konen E, Amitai M, Apter S, Garniek A, Gayer G, Nass S, Itzchak Y. CT angiography of superior mesenteric artery syndrome. AJR Am J Roentgenol. 1998;171:1279–1281. doi: 10.2214/ajr.171.5.9798861. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 5.Azami Y. Diabetes mellitus associated with superior mesenteric artery syndrome: report of two cases. Intern Med. 2001;40:736–739. doi: 10.2169/internalmedicine.40.736. [DOI] [PubMed] [Google Scholar]
- 6.Meneghini LF, Hogan AR, Selvaggi G. Superior mesenteric artery syndrome in type 1 diabetes masquerading as gastroparesis. Diabetes Care. 2008;31:1983–1984. doi: 10.2337/dc08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy A, Gisel JJ, Roy V, Bouras EP. Superior mesenteric artery (Wilkie's) syndrome as a result of cardiac cachexia. J Gen Intern Med. 2005;20:C3–C4. doi: 10.1111/j.1525-1497.2005.0201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stumpfle R, Wright AR, Walsh J. Superior mesenteric artery syndrome in an HIV positive patient. Sex Transm Infect. 2003;79:262–263. doi: 10.1136/sti.79.3.262-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin LC, Lee IH, Yang RC, Jong YJ. Superior mesenteric artery syndrome associated with hereditary motor and sensory neuropathy type II--a case report. Kaohsiung J Med Sci. 2001;17:484–488. [PubMed] [Google Scholar]
- 10.Adson DE, Mitchell JE, Trenkner SW. The superior mesenteric artery syndrome and acute gastric dilatation in eating disorders: a report of two cases and a review of the literature. Int J Eat Disord. 1997;21:103–114. doi: 10.1002/(sici)1098-108x(199703)21:2<103::aid-eat1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol. 2002;37:640–643. doi: 10.1007/s005350200101. [DOI] [PubMed] [Google Scholar]
- 12.Neri S, Signorelli SS, Mondati E, Pulvirenti D, Campanile E, Di Pino L, Scuderi M, Giustolisi N, Di Prima P, Mauceri B, et al. Ultrasound imaging in diagnosis of superior mesenteric artery syndrome. J Intern Med. 2005;257:346–351. doi: 10.1111/j.1365-2796.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 13.Unal B, Aktas A, Kemal G, Bilgili Y, Guliter S, Daphan C, Aydinuraz K. Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol. 2005;11:90–95. [PubMed] [Google Scholar]
- 14.Kim IY, Cho NC, Kim DS, Rhoe BS. Laparoscopic duodenojejunostomy for management of superior mesenteric artery syndrome: two cases report and a review of the literature. Yonsei Med J. 2003;44:526–529. doi: 10.3349/ymj.2003.44.3.526. [DOI] [PubMed] [Google Scholar]