Abstract
Background and purpose:
Recent experiments using non-selective 5-hydroxytryptamine (5-HT)2C receptor agonists including WAY 161503 suggested that midbrain 5-HT neurones are under the inhibitory control of 5-HT2C receptors, acting via neighbouring gamma-aminobutyric acid (GABA) neurones. The present study extended this pharmacological characterization by comparing the actions of WAY 161503 with the 5-HT2C receptor agonists, Ro 60-0275 and 1-(3-chlorophenyl) piperazine (mCPP), as well as the non-selective 5-HT agonist lysergic acid diethylamide (LSD) and the 5-HT releasing agent 3,4-methylenedioxymethamphetamine (MDMA).
Experimental approach:
5-HT neuronal activity was measured in the dorsal raphe nucleus (DRN) using extracellular recordings in anaesthetized rats. The activity of DRN GABA neurones was assessed using double-label immunohistochemical measurements of Fos and glutamate decarboxylase (GAD).
Key results:
Ro 60-0175, like WAY 161503, inhibited 5-HT neurone firing, and the 5-HT2C antagonist SB 242084 reversed this effect. mCPP also inhibited 5-HT neurone firing (∼60% neurones) in a SB 242084-reversible manner. LSD inhibited 5-HT neurone firing; however, this effect was not altered by either SB 242084 or the 5-HT2A/C receptor antagonist ritanserin but was reversed by the 5-HT1A receptor antagonist WAY 100635. Similarly, MDMA inhibited 5-HT neurone firing in a manner reversible by WAY 100635, but not SB 242084 or ritanserin. Finally, both Ro 60-0275 and mCPP, like WAY 161503, increased Fos expression in GAD-positive DRN neurones.
Conclusions and implications:
These data strengthen the hypothesis that midbrain 5-HT neurones are under the inhibitory control of 5-HT2C receptors, and suggest that the 5-HT2C agonists Ro 60-0175, mCPP and WAY 161503, but not LSD or MDMA, are useful probes of the mechanism(s) involved.
Keywords: 5-hydroxytryptamine, 5-HT2C receptor, dorsal raphe nucleus, WAY 161503, Ro 60-0175, mCPP, SB 242084
Introduction
Negative feedback regulation is considered to be an important mechanism controlling brain 5-hydroxytryptamine (5-HT) neurones. The role of inhibitory 5-HT receptors located on 5-HT neurones in feedback regulation is well recognized; somatodendritic 5-HT1A autoreceptors (nomenclature follows Alexander et al., 2008) inhibit the firing of 5-HT neurones, whereas nerve terminal 5-HT1B autoreceptors inhibit 5-HT release (see Barnes and Sharp, 1999). However, emerging data suggest that 5-HT neuronal activity is also regulated by 5-HT receptors located on non-5-HT neurones (i.e. post-synaptic; Sharp et al., 2007). These post-synaptic feedback mechanisms are postulated to inhibit 5-HT neuronal activity like 5-HT autoreceptors, but utilize additional 5-HT receptor subtypes, and operate via neuroanatomical circuits based on polysynaptic inputs to 5-HT neurones (Sharp et al., 2007).
One of these post-synaptic feedback mechanisms may involve 5-HT2C receptors. Earlier in vitro studies of 5-HT neurones in the dorsal raphe nucleus (DRN) showed that 5-HT application evoked inhibitory [gamma-aminobutyric acid (GABA)-mediated] post-synaptic currents, which were partially attenuated by the 5-HT2C receptor antagonist SB 242084 (Liu et al., 2000). Subsequent in vivo studies found that the non-selective 5-HT2 receptor agonists, 1–2,5-dimethoxy-4-iodoamphetamine (DOI) and (±)-2,5-dimethoxy-4-bromoamphetamine (DOB) inhibited 5-HT neuronal activity, and that this effect was blocked (partially) by the 5-HT2B/C receptor antagonist SB 206553 (Boothman et al., 2003). More recently, WAY 161503, an agonist with sixfold selectivity for 5-HT2C versus 5-HT2A receptors, was reported to inhibit 5-HT neuronal activity (Boothman et al., 2006). This effect was reversed by the 5-HT2A/C receptor antagonist ritanserin and also (in the few cells tested) by the more selective 5-HT2C receptor antagonist SB 242084. A complicating factor in all these experiments is that there is unequivocal evidence based on electrophysiological recordings and the use of non-selective 5-HT2 agonists (DOI, DOB) and a highly selective 5-HT2A receptor antagonist (MDL 100907), that activation of 5-HT2A receptors also results in inhibition of 5-HT neuronal activity (Liu et al., 2000; Martin-Ruiz et al., 2001; Boothman et al., 2003).
In addition to WAY 161503, a number of other agents demonstrate 5-HT2C receptor agonist activity, although selectivity for 5-HT2C versus other 5-HT receptor subtypes varies. These agents include the indoline derivative Ro 60-0175, which reportedly has up to 10-fold selectivity for 5-HT2C versus 5-HT2A receptors (Martin et al., 1998; Porter et al., 1999; Kimura et al., 2004). Additionally, the trazodone metabolite 1-(3-chlorophenyl) piperazine (mCPP) is marginally selective for 5-HT2C versus 5-HT2A and 5-HT1A/B receptors (Martin et al., 1998; Porter et al., 1999; Kimura et al., 2004), and this drug has been used as the benchmark 5-HT2C receptor agonist in both preclinical and clinical studies. The 5-HT ligand lysergic acid diethylamide (LSD) also has high affinity and an agonist action at 5-HT2C receptors but does not discriminate between 5-HT2C and several other 5-HT receptor subtypes (e.g. Porter et al., 1999; Nichols et al., 2002). Interestingly, both mCPP and LSD were shown to inhibit 5-HT cell firing in earlier experiments (Sprouse and Aghajanian, 1987; Blier et al., 1989) but a role for the 5-HT2C receptor has not been considered. This is also the case for the inhibition of 5-HT neuronal activity by 5-HT releasing agents such as 3,4-methylenedioxymethamphetamine (MDMA; Gartside et al., 1997).
Overall, the evidence for the control of 5-HT neurones by 5-HT2C receptors currently rests on experiments using a small number of non-selective 5-HT2C agonists particularly WAY 161503 (see earlier discussion), and a range of other such agents have yet to be included in the pharmacological analysis. The present study investigated the effect of Ro 60-0175, mCPP, LSD and MDMA, in comparison with WAY 161503, on the firing of 5-HT neurones in the DRN. The hypothesis was that 5-HT2C receptors contribute to the inhibition of 5-HT cell firing induced by each agent. Given evidence that WAY 161503 may inhibit DRN 5-HT neurones by activating neighbouring GABA neurones (Boothman et al., 2006), experiments also tested the effect of some of these agents on DRN GABA neurones. To this end immunohistochemistry was used to measure the effect of 5-HT2C receptor agonist administration on expression of the activity-dependent gene, c-fos, in DRN neurones labelled with the GABA neuronal marker, glutamate decarboxylase (GAD). The results strengthen the idea that midbrain 5-HT neurones are under the inhibitory control of 5-HT2C receptors, and suggest that Ro 60-0175, mCPP and WAY 161503 (but not LSD or MDMA) are likely to be useful probes of the mechanism(s) involved.
Methods
Animals
All animal care and procedures were carried out in accordance with the Animals (Scientific Procedures) Act (1986) and a local ethical review process. Male Sprague-Dawley rats (270–320 g; Harlan Olac, Bicester, UK) were group-housed under a 12:12 h light–dark cycle (lights on 0800–2000 h) and stable temperature (21 ± 1°C) and humidity, with free access to food and water. Animals arrived in the animal facility 5–7 days before commencing experiments and, for the immunohistochemistry study only, were handled daily for a minimum of 1 week prior to the start of each experiment.
Electrophysiological recording of 5-HT neuronal activity
Rats were anaesthetized with chloral hydrate (460 mg·kg−1 i.p. with additional doses as required), supplemented during surgery with saffan (1.2 mg·kg−1 i.v.), and maintained at 36°C using a thermoregulated blanket. Extracellular single-unit recordings were made as described previously (Boothman et al., 2003; 2006;). Single-barrel glass electrodes (2 M NaCl, 2% pontamine sky blue; 6–20 MΩ) were stereotaxically implanted into the DRN (coordinates relative to Bregma and the dura surface of A/P −7.5 mm, L/M 0.0 mm D/V −4.5 mm to −5.5 mm; Paxinos and Watson, 1998). Single-unit potentials were amplified and filtered (gain 1 k; 500 Hz to 1.5 kHz band pass; Neurolog system, Digitimer Ltd., Welwyn Garden City, UK), captured using a 1401plus interface (Cambridge Electronic Design Ltd, Cambridge, UK), and analysed offline using Spike2 software (version 4.01, Cambridge Electronic Design Ltd). Drugs and vehicle were injected via a cannulated lateral tail vein.
Neurones were selected for inclusion in the study on the basis that they fulfilled at least three of the following criteria, which are characteristic of 5-HT-containing neurones (Allers and Sharp, 2003): localization in the DRN; slow firing rate (<2 Hz); regular firing pattern [coefficient of variation (COV): standard deviation of interspike interval divided by interspike interval mean <0.4]; and triphasic extracellular waveform with a wide action potential (>1.5 ms). Some neurones were also tested for an inhibitory response to the 5-HT1A receptor agonist 8-OH-DPAT (10 µg·kg−1). Waveforms were averaged over 60 s and width was determined as the time between a 5% positive deviation from baseline to the return to baseline following a negative phase. Following recording, a small quantity of dye was expelled from the electrode (−3.6 mA, 200 ms pulse duration, 21 ms interpulse interval, 45 min duration) to enable histological identification of the recording site.
Experimental design: electrophysiology
Drugs were administered following a 3–5 min baseline period during which electrophysiological parameters were constant. Agonists were administered in accumulating doses at 2 min intervals as follows: WAY 161503, Ro 60-0175 or mCPP each at 0.125, 0.25, 0.5 and 1 mg·kg−1; LSD at 1.25, 2.5, 5 and 10 µg·kg−1; and MDMA at 0.2, 0.4, 0.8 and 1.6 mg·kg−1.
Two minutes after the final dose of agonist (although 38 min in one case), injection of antagonists commenced. In the case of WAY 161503, Ro 60-0175 and mCPP, the only antagonist given was SB 242084 (1 mg·kg−1). In the case of LSD and MDMA, SB 242084 (1 mg·kg−1) was followed by ritanserin (5-HT2; 1 mg·kg−1) and then WAY100635 (5-HT1A; 0.1 mg·kg−1). Finally, in some experiments, an injection of 8-OH-DPAT (10 µg·kg−1) was given (see Results for details). Only one 5-HT neurone was tested per animal. Separate groups of animals were used to test the effect of vehicle and SB 242084 alone.
Immunohistochemistry
Immunohistochemical measurement of cells double-labelled with Fos and the GABA synthetic enzyme GAD67/65 were made in the DRN of 5-HT2C agonist-treated rats, as described previously (Boothman et al., 2006). In brief, rats received two drug injections; antagonist (or its vehicle) followed 30 min later by agonist (or its vehicle). Two hours after the second injection, rats were anaesthetized with sodium pentobarbitone (300 mg·kg−1 i.p.) and transcardially perfused with 200 mL 0.9% saline followed by 200 mL fixative (4% paraformaldehyde in 0.1 M sodium phosphate buffer with 0.4% picric acid). Brains were removed and postfixed overnight at 4°C prior to preparation of Vibratome-cut coronal sections (40 µm, corresponding to plates 48–50 of the stereotaxic atlas of Paxinos and Watson, 1998).
For immunohistochemistry, sections were washed in phosphate buffered saline (PBS; 3 × 10 min) and exposed to 0.3% hydrogen peroxide (H2O2) for 10 min, and then standard blocking serum (10% normal goat serum in PBS) for 30 min, prior to overnight incubation (4°C) in rabbit anti-GAD (GAD67/65) primary antibody (Chemicon, AB 5992, 1:1000 dilution in standard blocking serum with 0.3% Triton). Next sections were exposed to biotinylated goat anti-rabbit secondary antibody (1:500 dilution; Vector, Laboratories Ltd, Peterborough, UK) for 2 h. GAD67/65 positive cells were visualized using a Vectastain ABC/diaminobenzidine reaction (Vector). Sections were then exposed to standard blocking serum with 0.3% Tritonx100, and incubated (4°C) in rabbit anti-Fos primary antibody (Santa Cruz Biotechnology, sc-253, 1:2000) for 72 h and then biotinylated goat anti-rabbit secondary antibody (in standard blocking serum) for 2 h. Fos positive cells were visualized using by Vectastain ABC/slate gray reaction (Vector). Finally, mounted sections were dehydrated, delipidated and cover-slipped.
Sections were examined using a Leitz Diaplan light microscope and images were captured with a Xillix microimager digital camera connected to a Macintosh computer using Openlab acquisition software (version 3.0.2; Improvision, Coventry, UK). Fos and GAD67/65 double-labelled cells were counted in a fixed area of the DRN (250 × 170 µm) defined by an eye-piece graticule. Counts were made bilaterally on six sections (×40 magnification) and then averaged to provide a single count per animal. Counts were made by an operator unaware of the treatments. There was an absence of specific staining in control sections in which either of the primary antibodies was excluded.
Data analysis
For electrophysiological experiments, the effect of an agonist was tested statistically by comparing the firing rate during the final minute of each 2 min post-drug period with the pre-drug baseline firing rate. ED50 values (dose required to produce 50% of maximal effect) were estimated for each cell by interpolation of semi-log dose-response plots, and then averaged to provide an ED50 value (±standard error of the mean) for each agonist. The effect of an antagonist was tested by comparing the firing rate after the last dose of agonist with the firing rate after the antagonist. Pre-drug baseline firing was also compared with the firing rate after the antagonist. The pairwise comparisons mentioned above were made using the least significant difference post hoc test following one-way analysis of variance (anova). In the Results section, reported P values refer to post hoc pairwise comparisons.
For immunohistochemical experiments, the effect of each agonist on the number of Fos/GAD67/65 double-labelled cells was compared against matched vehicle and antagonist treated control groups (one-way anova and least significant difference post hoc tests).
Materials
Drugs used (with supplier) were: WAY 161503 [8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazino(1,2-a)quinoxalin-5(6H)-one; Tocris, Bristol, UK]; Ro 60-0175 (αS)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate; Tocris); mCPP [1-(3-chlorophenyl)piperazine; Tocris]; LSD (9,10-didehydro-N,N-diethyl-6-methylergoline-8β-carboxamide; Sigma-Aldrich, Gillingham, UK); MDMA (3,4-MDMA; National Institute on Drug Abuse, Bethesda, MD, USA), SB 242084 (6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy) pyridin-3-yl carbamoyl] indoline; Eli Lilly & Co., Hampshire, UK); ritanserin [6-(2-[4-[bis(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-7-methyl-5H-thiazolo(3,2-a)pyrimidin-5-one; Janssen Phamaceuticals, Beerse, Belgium]; 8-OH-DPAT [8-hydroxy-2-(di-npropylamino)-tetralin; Sigma-Aldrich]; and WAY 100635 (n-[2-[4-(2-methoxyphenyl)-1-piperazinylethyl]-n-(2-pyridinyl) cyclohexane carboxamide trihydrochloride; Wyeth Pharmaceuticals, Berkshire, UK).
mCPP, LSD, MDMA, 8-OH-DPAT and WAY 100635 were dissolved in 0.9% saline. WAY 161503 and Ro 60-0175 were dissolved in deionized water. SB 242084 was dissolved in 10% cyclodextrin in 25 mM citric acid. Ritanserin was dissolved first in a few drops of glacial acetic acid and then diluted in 5% glucose.
Results
Electrophysiological characteristics of 5-HT neurons in the DRN
Putative 5-HT neurones recorded in the DRN (n= 41) had broad triphasic spikes (waveform length 2.83 ± 0.15 ms) that were fired in a slow (1.13 ± 0.25 Hz) and regular (COV 0.36 ± 0.04) firing pattern. Compared with pretreatment baseline values, neither the selective 5-HT2C receptor antagonist SB 242084 (1 mg·kg−1 i.v.), nor any of the vehicles (deionized water, 10% cyclodextrin in 25 mM citric acid, dilute glacial acetic acid in 5% glucose solution) altered firing rate or regularity (n= 2 each treatment; data not shown).
Effect of WAY 161503 on 5-HT neuronal activity
Administration of the putative 5-HT2C receptor agonist, WAY 161503 (0.125–1 mg·kg−1 i.v.) caused a dose-related inhibition (ED50 0.15 ± 0.02 mg·kg−1) of 5-HT neurone firing rate compared with pre-drug values in all neurones tested (P < 0.001; Figure 1). This effect was statistically significant (P < 0.01) at 0.25 mg·kg−1 WAY 161503, and the highest dose tested (1 mg·kg−1 i.v.) reduced firing to 4% of pre-drug values. This effect of WAY 161503 was reversed by 1 mg·kg−1 i.v. SB 242084 (Figure 1). The firing rate after reversal by SB 242084 was slightly less than pre-drug baseline values but this difference was not statistically significant (Figure 1B; firing rate pre-drug vs. WAY 161503 plus SB 242084, P= 0.09). Subsequent administration of 8-OH-DPAT (10 µg·kg−1 i.v.) resulted in a complete inhibition of neuronal activity (two neurones). In one experiment a cell was recorded for over 45 min, and it was found that the inhibitory effect of WAY 161503 was long-lasting (>30 min), but still reversed with SB 242084 (Figure 1C). The latter experiment is important because it demonstrates that the reversal of the inhibitory effects of WAY 161503 by SB 242084 is not an artefact due to the agonist effect rapidly wearing off.
Figure 1.
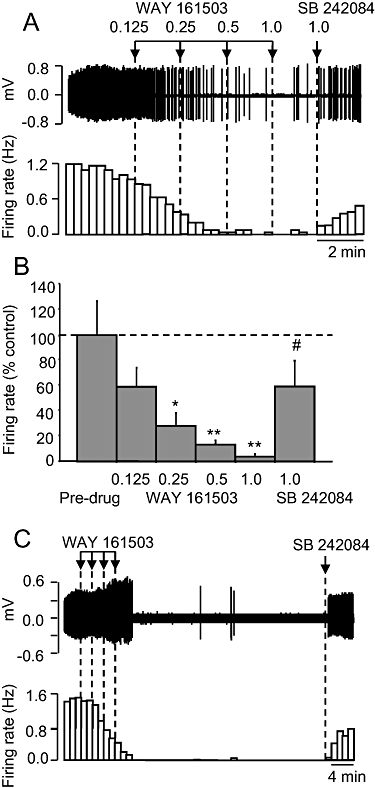
Effect of the putative 5-HT2C receptor agonist WAY 161503 on 5-hydroxytryptamine (5-HT) cell firing in the dorsal raphe nucleus of the anaesthetized rat. (A) Spike train (upper trace) and rate-meter recording of the inhibitory effect of WAY 161503 on the firing of a single 5-HT neurone and reversal by the 5-HT2C receptor antagonist SB 242084 administered 2 min after the final dose of WAY 161503. (B) Group data on effect of WAY 161503, followed by SB 242084. Data are mean + standard error of the mean values, n= 6 cells. *P < 0.01, **P < 0.001 versus pre-drug values. #P < 0.05 versus WAY 161503 (1.0 mg·kg−1 i.v.; one-way analysis of variance with least significant difference post hoc test). (C) Recordings of the inhibitory effect of WAY 161503 and reversal by SB 242084 administered 38 min later. Doses of WAY 161503 as indicated by arrows are 0.125, 0.25, 0.5 and 1.0 mg·kg−1 i.v.
Effect of Ro 60-0175 on 5-HT neuronal activity
The putative 5-HT2C receptor agonist, Ro 60-0175 (0.125–1 mg·kg−1 i.v.), also caused a dose-related inhibition of firing rate (ED50 0.55 ± 0.28 mg·kg−1) in all 5-HT neurones tested compared with pre-drug values (P < 0.01; Figure 2A and B). The grouped data revealed that the effects of Ro 60-0175 was statistically significant (P < 0.01) at the highest dose tested (1 mg·kg−1), which reduced firing rate to 20% of pre-drug values (Figure 2B). As with WAY 161503, the inhibitory effect of Ro 60-0175 was reversed by SB 242084 (1 mg·kg−1 i.v.) (Figure 2). The firing rate after reversal by SB 242084 was slightly less than pre-drug baseline values but this effect was not statistically significant (Figure 2B; P > 0.05). Subsequent administration of 8-OH-DPAT (10 µg·kg−1 i.v.) resulted in a complete inhibition of neuronal activity (two neurones).
Figure 2.
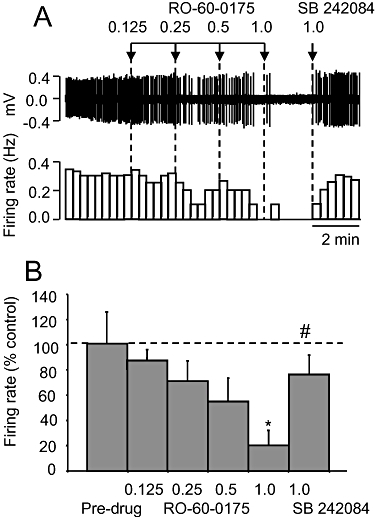
Effect of the putative 5-hydroxytryptamine (5-HT)2C receptor agonist RO-60-0175 on 5-HT cell firing. (A) Spike train (upper trace) and rate-meter recording of the inhibitory effect of RO-60-0175 on the firing of a single 5-HT neurone, and reversal by SB 242084. Doses in mg·kg−1. (B) Group data of the effect of RO-60-0175 followed by SB 242084. Data are mean + standard error of the mean values, n= 6 cells. *P < 0.01 versus pre-drug values. #P < 0.01 versus WAY 161503 (1.0 mg·kg−1 i.v.; one-way analysis of variance with least significant difference post hoc test).
Effect of mCPP on 5-HT neuronal activity
Administration of the non-selective 5-HT2C receptor agonist mCPP (0.125–1 mg·kg−1 i.v.) caused a dose-related inhibition of 5-HT neurone firing rate (ED50 0.44 ± 0.25 mg·kg−1) but only in four out of seven neurones tested (Figure 3). In responsive neurones, the inhibitory effect of mCPP was statistically significant (P < 0.01) at 0.5 mg·kg−1, and the highest dose tested (1 mg·kg−1 i.v.) reduced firing to 6% of pre-drug values (Figure 3). SB 242084 (1 mg·kg−1 i.v.) reversed this inhibitory effect of mCPP. The firing rate after reversal by SB 242084 was slightly less than pre-drug baseline values but this difference was of borderline statistical significance (P= 0.054; Figure 3B).
Figure 3.
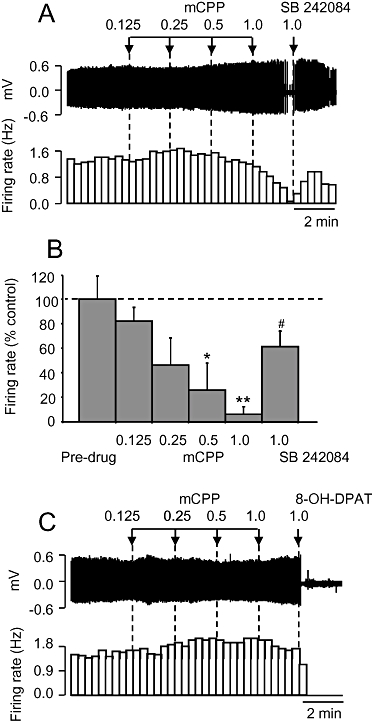
Effect of the putative 5-hydroxytryptamine (5-HT)2C receptor agonist mCPP on 5-HT cell firing. (A) Spike train (upper trace) and rate-meter recording of the inhibitory effect of 1-(3-chlorophenyl) piperazine (mCPP) on the firing of a single 5-HT neurone and reversal by SB 242084. Doses in mg·kg−1. (B) Group data of mCPP responsive 5-HT neurones. Data are mean + standard error of the mean values, n= 4 cells. *P < 0.05, **P < 0.01 compared with pre-drug values. #P < 0.05 versus mCPP (1.0 mg·kg−1 i.v.; one-way analysis of variance with least significant difference post hoc test). (C) Spike train (upper trace) and rate-meter recording of a single 5-HT neurone that was unresponsive to mCPP. Note inhibition induced by the 5-HT1A agonist, 8-OH-DPAT (10 µg·kg−1).
Neurones responsive to mCPP had a similar waveform width and baseline firing rate compared with those of unresponsive neurones (2.1 ± 0.15 vs. 2.0 ± 0.10 ms, and 1.5 ± 0.22 vs. 0.94 ± 0.16 Hz, respectively) but were slightly less regular (baseline COV 0.3 ± 0.02 vs. 0.43 ± 0.04; P < 0.05, Student's t-test). The three neurones unresponsive to mCPP were inhibited by 10 µg·kg−1 8-OH-DPAT (Figure 3C). The slight increase in firing rate at low doses of mCPP apparent in the two examples in Figure 3 was not common to either neurones inhibited by mCPP or those that were not.
Effect of LSD on 5-HT neuronal activity
Administration of LSD (1.25–10 µg·kg−1 i.v.) caused a dose-related inhibition of 5-HT neurone firing rate (ED50 0.25 ± 0.05 µg·kg−1) compared with the pre-drug values (P < 0.001; Figure 4A). This effect was statistically significant (P < 0.05) at 2.5 µg·kg−1 i.v. LSD, and the highest dose tested (10 µg·kg−1 i.v.) reduced firing to 1% of pre-drug values (Figure 4B). In contrast to WAY 161503, RO-60-0175 and mCPP, the inhibitory effect of LSD was not reversed by SB 242084 (Figure 4). Similarly, ritanserin (1 mg·kg−1 i.v.) did not reverse the effect of LSD whereas WAY 100635 (0.1 mg·kg−1 i.v.) evoked a striking recovery (Figure 4A and B).
Figure 4.
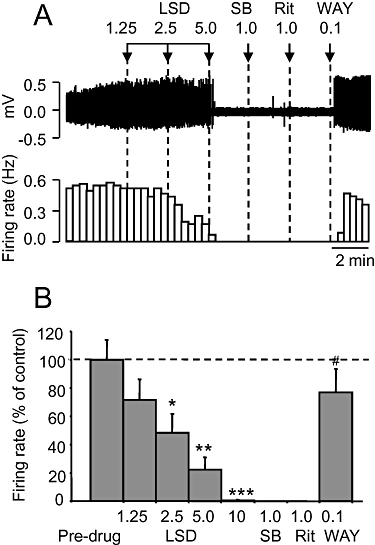
Effect of the 5-hydroxytryptamine (5-HT) agonist lysergic acid diethylamide (LSD) on 5-HT cell firing. (A) Spike train (upper trace) and rate-meter recording of the inhibitory effect of LSD on the firing of a single 5-HT neurone. The inhibition was not affected by SB 242084 or ritanserin, but reversed by WAY 100635. Doses in mg·kg−1 except LSD (µg·kg−1). (B) Group data of the effect of LSD. Data are mean + standard error of the mean values, n= 6 cells. *P < 0.05, **P < 0.01, ***P < 0.001 versus pre-drug values. #P < 0.001 versus LSD (10 µg·kg−1 i.v.; one-way analysis of variance with least significant difference post hoc test).
Effect of MDMA on 5-HT neuronal activity
MDMA (0.2–1.6 mg·kg−1 i.v.) caused a dose-related inhibition of 5-HT cell firing (ED50 0.78 ± 0.24 mg·kg−1) compared with the pre-drug values (P < 0.001; Figure 5A and B). This effect was statistically significant (P < 0.05) at 0.4 mg·kg−1, and the highest dose (1.6 mg·kg−1 i.v.) reduced firing to 5% of pre-drug values (Figure 5). As with LSD, this effect of MDMA was reversed by WAY 100635 (0.1 mg·kg−1 i.v.) but not SB 242084 (1 mg·kg−1 i.v.) or ritanserin (1 mg·kg−1 i.v.) (Figure 5A and B). After WAY 100635 firing rate appeared to overshoot pre-drug baseline values but this effect did not reach statistical significance (P= 0.21).
Figure 5.

Effect of the 5-hydroxytryptamine (5-HT) releasing agent 3,4-methylenedioxymethamphetamine (MDMA) on 5-HT cell firing. (A) Spike train (upper trace) and rate-meter recording of the inhibitory effect of MDMA on the firing of a single 5-HT neurone. The inhibition was not affected by SB 242084 or ritanserin, but reversed by WAY 100635. Doses in mg·kg−1. (B) Group data of the effect of MDMA. Data are mean + standard error of the mean values, n= 6. *P < 0.05, **P < 0.01, ***P < 0.001 versus pre-drug values. #P < 0.001 compared with MDMA (1.6 mg·kg−1 i.v.; one-way analysis of variance with least significant difference post hoc test).
Effect of WAY 161503, Ro 60-0175 and mCPP on DRN Fos/GAD65/67
As previously reported (Boothman et al., 2006), GAD67/65 immunoreactivity was most abundant in the lateral wings of the DRN. In vehicle-treated rats the number of Fos positive cells was low, and there were few Fos/GAD67/65 double-labelled cells.
WAY 161503 (3 mg·kg−1 i.p.) caused a fourfold increase in the number of Fos/GAD67/65 double-labelled cells in the DRN compared with vehicle controls (one-way anova: P < 0.001; least significant difference post hoc test: P < 0.001; Figure 6A). This effect of WAY 161503 was reduced by pretreatment with SB 242084 (1 mg·kg−1 i.p.), which alone was without effect (P < 0.01; Figure 6A).
Figure 6.
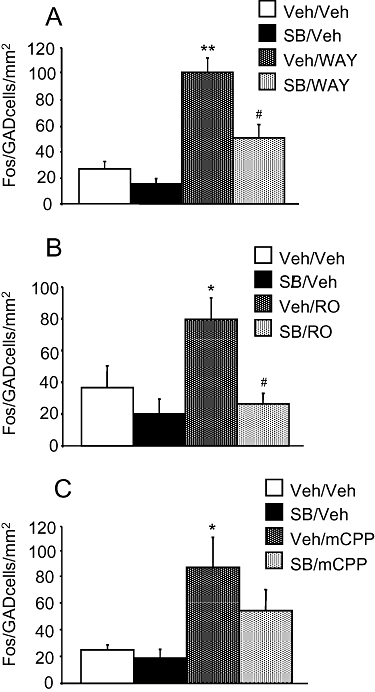
Effect of 5-HT2C receptor agonist administration on the number of Fos/GAD67/65 double-labelled cells in the dorsal raphe nucleus. Drug treatments were as follows: (A) WAY 161503 (3 mg·kg−1 i.p.), SB 242084 (1 mg·kg−1 i.p.), (B) RO-60-0175 (3 mg·kg−1 i.p.), SB 242084 (1 mg·kg−1 i.p.) and (C) 1-(3-chlorophenyl) piperazine (mCPP) (5 mg·kg−1 i.p.), SB 242084 (1 mg·kg−1 i.p.). Data (n= 6) are mean + standard error of the mean values. *P < 0.05 versus vehicle + vehicle (one-way analysis of variance with least significant difference post hoc test).
Importantly, as with WAY 161503, both Ro 60-0175 (3 mg·kg−1 i.p.) and mCPP (5 mg·kg−1 i.p.) increased (two- to threefold) the number of Fos/GAD67/65 double-labelled cells. In the case of Ro 60-0175 this effect was less in animals pretreated with SB 242084 (1 mg·kg−1 i.p.) and this difference was statistically significant (Ro 60-0175 vs. Ro 60-0175 plus SB 242084, P < 0.05; Figure 6B). In the case of mCPP this effect was also less in animals pretreated with SB 242084 (1 mg·kg−1 i.p.) but this difference was not statistically significant (mCPP vs. mCPP plus SB 242084, P > 0.05; Figure 6C). However, blockade of this response to mCPP by SB 242084 is suggested by the fact that Fos/GAD67/65 counts in animals treated with mCPP plus SB 242084 were not statistically different from vehicle controls (P > 0.05).
Discussion
Recent experiments using non-selective 5-HT2C receptor agonists including WAY 161503 have suggested that midbrain 5-HT neurones are inhibited by 5-HT2C receptor activation (see Introduction). Here we extend this pharmacological characterization by comparing the effect of WAY 161503 with a variety of additional 5-HT2C receptor ligands and a 5-HT releasing agent. Experiments showed that as with WAY 161503, the 5-HT2C receptor agonists Ro 60-0175 and mCPP inhibited the firing of 5-HT neurones in the rat DRN. Moreover, in each case this effect was reversed by the selective 5-HT2C receptor antagonist SB 242084. The 5-HT receptor agonist LSD and 5-HT releasing agent MDMA also inhibited 5-HT neuronal activity. However, in both cases the effect was unchanged by either SB 242084 or the 5-HT2A/C receptor antagonist ritanserin, but reversed by the selective 5-HT1A antagonist WAY 100635. Collectively, these data suggest that, although a 5-HT2C receptor mechanism contributed to the inhibition of 5-HT cell firing induced by some of the 5-HT agents tested, this was not the case for all.
Although Ro 60-0175, mCPP and WAY 161503 have limited (five- to 10-fold) selectivity for the 5-HT2C over the 5-HT2A receptor (Martin et al., 1998; Porter et al., 1999; Rosenzweig-Lipson et al., 2000; 2006; Kimura et al., 2004), evidence suggests that the inhibition of 5-HT neuronal activity induced by these agents is more likely to be mediated by 5-HT2C receptors. Firstly, Ro 60-0175, mCPP and WAY 161503 have 5-HT2C receptor agonist properties in vitro, and exert 5-HT2C receptor-mediated behavioural effects in animal models in vivo at doses in the range of those used here (Martin et al., 1998; Porter et al., 1999; Kimura et al., 2004; Rosenzweig-Lipson et al., 2006). We are not aware of previous experiments comparing side by side the in vivo potency of these three agonists, but the current ED50 data suggest that these agents are similarly potent, which is consistent with previous comparisons of mCPP and Ro 60-0175 in other in vivo models of 5-HT2C receptor function (Millan et al., 1997; Dekeyne et al., 1999). Secondly, and importantly, each agonist inhibited 5-HT neuronal activity in an SB 242084-reversible manner, as shown previously for WAY 161503 in a small number of neurones (Boothman et al., 2006). Previous positron emission tomography studies demonstrating that SB 242084, at the dose used here (1 mg·kg−1 i.v.), has no detectable occupancy at 5-HT2A receptors (Boothman et al., 2006) emphasize the importance of 5-HT2C receptors in the effects reported herein. The reversal by SB 242084 was not simply due to a spontaneous recovery in 5-HT cell firing as the effect always occurred immediately following injection and crucially, in one cell for which the duration of the inhibition induced by WAY 161503 continued for almost 40 min. SB 242084 did not reverse the inhibition induced by either LSD or MDMA (see further discussion), thereby ruling out ‘physiological antagonism’.
Not withstanding the arguments mentioned earlier, it is noteworthy that after SB 242084 reversal, there was a tendency for firing rate not to return fully to pre-drug baseline values although this difference did not reach statistical significance. Given much previous evidence that 5-HT2A receptor activation also inhibits 5-HT neuronal activity (Liu et al., 2000; Martin-Ruiz et al., 2001; Boothman et al., 2003), it is plausible that any residual inhibitory effect in the presence of SB 242084 is mediated by 5-HT2A receptors. This would be consistent with evidence that both mCPP and Ro 60-0175 evoked 5-HT2A receptor-mediated head twitches in rats treated with SB 242084 (Vickers et al., 2001).
Although mCPP inhibited 5-HT neuronal activity (via a SB 242084-sensitive mechanism), the drug inhibited only a proportion of the neurones tested (4/7). The latter observation has also been made by others (Sprouse and Aghajanian, 1987). One explanation for this observation is a contribution of actions of mCPP at other 5-HT receptors. For example, mCPP has moderate affinity at 5-HT1B receptors (Martin et al., 1998) and 5-HT1B receptor agonist administration increased 5-HT neuronal activity in the DRN (Adell et al., 2001). Another possibility is that neurones not inhibited by mCPP were by chance, not 5-HT-containing. Thus, although these neurones were 5-HT-like (i.e. slow firing, inhibition by 8-OH-DPAT), their somewhat irregular firing pattern is reminiscent of non-5-HT neurones in the DRN (Allers and Sharp, 2003).
Both LSD and MDMA inhibited 5-HT neuronal activity as seen previously (Blier et al., 1989; Gartside et al., 1997), but in neither case was the inhibition reversed by SB 242084 or ritanserin. Rather, the inhibitory effect of both LSD and MDMA was reversed by the selective 5-HT1A receptor antagonist WAY 100635. The latter finding is in agreement with previous work (Gartside et al., 1997; El Mansari and Blier, 2007). Overall, these findings suggest that the inhibition of 5-HT neuronal activity by LSD and MDMA is mediated by 5-HT1A receptors and that any contribution from 5-HT2C (or indeed 5-HT2A) receptors was not detectable under the present conditions.
The latter result is somewhat surprising given that both LSD and 5-HT (released by MDMA) have similar affinity for 5-HT2C and 5-HT1A receptors (LSD Ki 5 nM vs. 4 nM, and 5-HT Ki 11 nM vs. 2 nM, respectively, for agonist binding sites) (Hall et al., 1986; Peroutka, 1986; Egan et al., 2000; Nichols et al., 2002; NIMH Psychoactive Drug Screening Program, http://pdsp.med.unc.edu). This suggests that LSD and 5-HT may have a lower efficacy at 5-HT2C versus 5-HT1A receptors (at least in terms of ability to inhibit 5-HT neurones). Alternatively, the coupling efficiency of 5-HT1A autoreceptors may be greater than that of 5-HT2C receptor mediated feedback mechanisms. In relation to the latter it may be relevant that inhibitory 5-HT1A autoreceptors are located on 5-HT neurones, whereas 5-HT2C receptor feedback is likely to involve polysynaptic connections with 5-HT neurones that (by implication) are less reliable. An additional point is that as both LSD and 5-HT interact with multiple 5-HT receptor subtypes, it is also possible that ‘receptor cross talk’ leads to a diminution of the 5-HT2C receptor response.
The apparent lack of involvement of 5-HT2C receptors in the inhibitory effect of MDMA suggests that 5-HT reuptake inhibitors are also unlikely to inhibit 5-HT neurones via 5-HT2C receptor feedback. However, in microdialysis experiments 5-HT2C receptor antagonists such as SB 242084 augmented the effect of 5-HT reuptake inhibitors (Cremers et al., 2004; Boothman et al., 2006), and we observed a small but significant attenuation of the inhibitory effect of fluoxetine on 5-HT neurones in animals pretreated with either ritanserin or SB 206553 (L.J. Boothman, unpubl. obs.). It is possible that disparities between MDMA and 5-HT reuptake inhibitors reflect the actions of the former on other neurotransmitter systems including dopamine (see Gudelsky and Yamamoto, 2008).
In addition to electrophysiological findings, the current study used immunohistochemical experiments to demonstrate that Ro60-0175, mCPP and WAY 161503 increased Fos expression in DRN GAD positive neurones. Moreover, in each case SB 242082 antagonized this effect, thereby confirming the involvement of 5-HT2C receptors. Similar findings for WAY 161503 were reported previously (Boothman et al., 2006). These data add to recent suggestions that GABA neurones in the DRN are part of the neuroanatomical substrates underlying the inhibition of 5-HT neurones by 5-HT2C agonist administration. Specifically, it is thought that stimulation of inhibitory GABA neurones in the DRN, by 5-HT2C receptors located on these neurones and/or in more distant locations, triggers the inhibition of firing of neighbouring 5-HT neurones (Boothman and Sharp, 2005; Serrats et al., 2005; Boothman et al., 2006; Sharp et al., 2007).
In summary, the present data suggest that Ro 60-0175 and mCPP like WAY 161503 inhibited 5-HT cell firing via activation of 5-HT2C receptors, but that 5-HT1A and not 5-HT2C receptors mediated the inhibitory effect of LSD and MDMA. Although mCPP is not a selective tool to probe 5-HT2C receptors, in the present models, this drug had effects comparable with those of more selective agents. In this respect, the clinical availability of mCPP provides a probe of 5-HT2C feedback mechanisms in humans, should suitable models become available. Overall, these findings strengthen the hypothesis that 5-HT2C receptors are involved in mediating negative feedback on central 5-HT neurons (Boothman et al., 2003; 2006; Sharp et al., 2007), and identify a number of pharmacological agents to further dissect the mechanism(s) involved.
Acknowledgments
This work was supported by a grant from the European Community (Integrated Network, NEWMOOD; LSHM-CT-2004-503474; T.S.) and a PhD scholarship from the Government of Jersey (P Quérée).
Glossary
Abbreviations:
- DRN
dorsal raphe nucleus
- LSD
lysergic acid diethylamide
- mCPP
1-(3-chlorophenyl) piperazine
Conflict of interest
None.
References
- Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1, S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Blier P, Steinberg S, Chaput Y, de Montigny C. Electrophysiological assessment of putative antagonists of 5-hydroxytryptamine receptors: a single-cell study in the rat dorsal raphe nucleus. Can J Physiol Pharmacol. 1989;67:98–105. doi: 10.1139/y89-017. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman L, Sharp T. A role for midbrain raphe GABA neurones in 5-HT feedback control. Neuroreport. 2005;16:891–896. doi: 10.1097/00001756-200506210-00004. [DOI] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol. 2006;149:861–869. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mørk A, et al. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60-0175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, et al. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Blier P. In vivo electrophysiological assessment of the putative antidepressant Wf-516 in the rat raphe dorsalis, locus coeruleus and hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 2007;132:44–56. doi: 10.1007/s00210-007-0210-6. [DOI] [PubMed] [Google Scholar]
- Gartside SE, McQuade R, Sharp T. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on 5-HT cell firing and release: comparison between dorsal and median raphe 5-HT systems. Neuropharmacology. 1997;36:1697–1703. doi: 10.1016/s0028-3908(97)00171-8. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Gozlan H, Emerit MB, el Mestikawy S, Pichat L, Hamon M. Differentiation of pre- and post-synaptic high affinity serotonin receptor binding sites using physico-chemical parameters and modifying agents. Neurochem Res. 1986;11:891–912. doi: 10.1007/BF00965212. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Hatanaka K, Naitou Y, Maeno K, Shimada I, Koakutsu A, et al. Pharmacological profile of YM348, a novel, potent and orally active 5-HT2C receptor agonist. Eur J Pharmacol. 2004;483:37–43. doi: 10.1016/j.ejphar.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, et al. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, et al. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Peglion JL, Lavielle G, Perrin-Monneyron S. 5-HT2C receptors mediate penile erections in rats: actions of novel and selective agonists and antagonists. Eur J Pharmacol. 1997;325:9–12. doi: 10.1016/s0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD) J Med Chem. 2002;45:4344–4349. doi: 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxinc Coordinates. London: Academic Press Ltd; 1998. [Google Scholar]
- Peroutka SJ. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, and 5-HT1C binding sites in rat frontal cortex. J Neurochem. 1986;47:529–540. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Coupet J, Dunlop J, PM Antiobesity-like effects of the selective 5-HT2C agonist WAY 161503. FASEB. 2000;14:A1321. [Google Scholar]
- Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, et al. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res. 2006;16:1073–1074. doi: 10.1016/j.brainres.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Serrats J, Mengod G, Cortes R. Expression of serotonin 5-HT(2C) receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat. 2005;29:83–91. doi: 10.1016/j.jchemneu.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, et al. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]


