Abstract
Background and purpose:
The modulation by flavonoids of platelet responses induced by thrombin has been little investigated, and the antiplatelet activity, as well as possible inhibitory mechanisms of these compounds on thrombin signalling, has not yet been elucidated. We explored whether flavonoids affect platelet signalling pathways triggered by thrombin and by the selective activation of its protease-activated receptors (PARs) 1 and 4, and analysed the antagonism of these polyphenols at thrombin receptors.
Experimental approach:
We investigated the effect of a range of polyphenolic compounds on platelet aggregation, 5-HT secretion, intracellular calcium mobilization, protein kinase activity and tyrosine phosphorylation, triggered by thrombin and PAR agonist peptides (PAR-APs). The ability of these flavonoids to bind to thrombin receptors was investigated by competitive radioligand binding assays using 125I-thrombin.
Key results:
Quercetin, apigenin and genistein impaired platelet aggregation, as well as 5-HT release and calcium mobilization, induced by thrombin and PAR-APs. Quercetin and apigenin were inhibitors of protein kinases, but genistein exhibited a minimal ability to suppress platelet phosphorylation. Binding assays did not establish any kind of interaction between thrombin receptors and any of the flavonoids tested.
Conclusions and implications:
Quercetin, apigenin and genistein did not inhibit thrombin responses by interacting with thrombin receptors, but by interfering with intracellular signalling. While inhibition by genistein may be a consequence of affecting calcium mobilization, subsequent platelet secretion and aggregation, for quercetin and apigenin, inhibition of kinase activation may also be involved in the impairment of platelet responses.
Keywords: flavonoids, thrombin, protease-activated receptors, platelets
Introduction
Thrombin, the major protease in the coagulation cascade, is the most potent activator of platelets identified to date. Thrombin signalling occurs largely through the activation of protease-activated receptors (PARs) by proteolytic generation of a tethered ligand. Human platelets express two functional thrombin receptors, PAR1 and PAR4 (Kahn et al., 1998); (nomenclature follows Alexander et al. 2008), but their signalling pathways are not completely understood. Thus, how differential signalling through the thrombin receptors is regulated, and the potential physiological consequences of selective inactivation of PAR1 and PAR4 remain unclear.
It has been shown that stimulation of platelets with thrombin results in activation of phospholipase C β (PLCβ) and subsequent calcium mobilization and protein kinase C (PKC) activation, which in turn mediate granule secretion. Recent reports establish a key role of TxA2 generation and ADP secretion in the irreversible aggregation observed in response to low or medium concentrations of thrombin through signalling amplification mechanisms involving Src kinases and the mitogen-activated protein kinase ERK1/2, among other molecules (Falker et al., 2004; Shankar et al., 2006). The signalling events activated by these secondary mediators synergize with primary calcium-dependent and PKC-regulated pathways to induce Rap1b and αIIbβ3 integrin activation (Cho et al., 2002; Quinton et al., 2002; Kamae et al., 2006). In addition to PAR1 and PAR4, which are generally assumed to account for the moderate- and low-affinity binding sites for thrombin, respectively (Kahn et al., 1999), thrombin can also bind to platelet GPIb/IX/V. This represents a high-affinity receptor for the protease, and although the relative importance of such mechanisms is still a subject of controversy (Brass, 2003; Coughlin, 2005), there is a mounting body of evidence indicating that it may contribute to the activation of platelets (De Marco et al., 1991; Lova et al., 2004).
The limitations of existing antiplatelet drugs provide opportunities for new agents. Attention has focused on novel ADP receptor antagonists and on drugs that target PAR1. PAR1 is also found on endothelial cells, smooth muscle cells, fibroblasts and cardiac myocytes (Seiler and Bernatowicz, 2003), and thrombin-mediated activation of PAR1 on these cells may contribute to the proliferative and pro-inflammatory effects of thrombin. Therefore, it is possible that PAR1 antagonism will not only attenuate arterial thrombosis, but may also modulate other thrombin-mediated processes, including restenosis (Ramachandran and Hollenberg, 2008). At least two orally active PAR1 antagonists, SCH-530348 and E-5555, are undergoing phase III clinical evaluation, and preliminary results are encouraging (Hamilton, 2009).
Platelet activation may also be inhibited by flavonoids, natural dietary polyphenols used since ancient times in traditional Chinese medicine (Middleton et al., 2000). However, the modulation by flavonoids of platelet responses induced by thrombin has been little investigated (Pace-Asciak et al., 1995), and the antiplatelet activity, as well as possible inhibitory mechanisms of these compounds on thrombin signalling, has not yet been elucidated. Our previous data using high thrombin concentrations suggested possible inhibitory effects by certain flavonoids (Guerrero et al., 2007). In the present study, we have assessed such effects by analyzing platelet aggregation, 14C-5-HT secretion, calcium mobilization, protein kinase and tyrosine phosphorylation induced by a submaximal concentration of thrombin and selective PAR1 and PAR4 agonist peptides (APs), and investigated the ability of these flavonoids to bind to thrombin receptors by competitive radioligand binding assays using 125I-thrombin.
Our data suggest that quercetin, apigenin and genistein affect thrombin responses exclusively by affecting intracellular signalling. While the inhibitory effect of genistein may be essentially a consequence of affecting calcium mobilization, subsequent platelet secretion and aggregation, inhibition of kinase activation may also be partially implicated in the impairment of platelet responses mediated by quercetin and apigenin.
Methods
Isolation of platelets
Venous blood was obtained in 3.8% sodium citrate (final concentration 0.129 M) from healthy volunteers who had not taken any medication for at least 10 days and gave their informed consent prior to enrollment, according to the ethical standards at our institution. To study the specific effects of flavonoids on PAR1 and PAR4 signalling cascades without the interference of these compounds on TxA2 signalling (Guerrero et al., 2005; Navarro-Nuñez et al., 2009), blood was treated with 10 µM indomethacin before isolating platelet-rich plasma by centrifugation at 200×g for 15 min. Platelets were washed in the presence of 0.1 µg·mL−1 prostaglandin E1 with modified HEPES/Tyrode's buffer pH 6.5 (137 mM NaCl, 2.9 mM KCl, 0.42 mM NaH2PO4, 11.9 mM NaHCO3, 2 mM MgCl2, 5.5 mM d-glucose, 10 mM HEPES), resuspended in the specific assay buffer, adjusted to an appropriate count and left undisturbed for 30 min at room temperature prior to experiments.
Platelet aggregation studies
Washed platelets were resuspended in HEPES/Tyrode's buffer pH 7.35 containing 2 mM CaCl2 (300 × 109 L−1) and incubated for at least 15 min with different inhibitors, flavonoids or with ≤0.2% dimethylsulphoxide (DMSO) as control. They were then stimulated during 5 min with thrombin (0.2 U·mL−1), PAR1-AP (25 µM) or PAR4-AP (150 µM) in an Aggrecorder II aggregometer (Menarini Diagnostics, Florence, Italy), and the percentage of maximum aggregation was recorded as the percentage of light transmission, with washed platelets as the baseline and HEPES/Tyrode's buffer as 100% of light transmission. The concentration of flavonoids necessary to obtain half-maximal inhibition of platelet aggregation (IC50) was determined for each agonist by incubating washed platelets with increasing concentrations of flavonoids (0–100 µM) or DMSO (final concentration ≤0.2%). Dose-dependent inhibition curves were generated with a non-linear curve-fitting software (GraphPad Prism for Windows version 4.0, GraphPad Inc., San Diego, CA, USA).
5-HT release
Washed platelets in HEPES/Tyrode's buffer pH 7.35 containing 2 mM CaCl2 (300 × 109 L−1) were radiolabeled with 1 µM [14C]-5-HT for 45 min at 37°C, and then treated with 50 µM flavonoids or LJ-CP8 antibody (1 µM) plus 5 µM imipramine to prevent re-uptake of secreted 5-HT. The secretion of [14C]-5-HT was induced with 0.2 U·mL−1 thrombin, 25 µM PAR1-AP or 150 µM PAR4-AP for 5 min at 37°C with stirring (1000 rpm), and release was analysed as described previously (Guerrero et al., 2005).
Measurement of calcium mobilization
Washed platelets in calcium-free Krebs–HEPES buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 4.2 mM NaHCO3, 11.7 mM glucose, 10 mM HEPES, pH 7.4) were incubated in the dark with 3 µM Oregon green BAPTA-1 AM in the presence of 1% pluronic acid for 45 min at 37°C. The excess of fluorochrome was removed by two washing steps; platelets were then resuspended at 300 × 109 L−1 in Krebs–HEPES buffer containing 2 mM EGTA and incubated with different inhibitors, 50 µM flavonoids or with an equivalent volume of DMSO (final concentration ≤0.2%) before inducing internal calcium mobilization with 0.2 U·mL−1 thrombin, 25 µM PAR1-AP or 150 µM PAR4-AP. Changes in fluorescence were recorded as previously described (Guerrero et al., 2007).
In vitro kinase assays
Using a commercial kinase activity test (SelectScreen Kinase Profiling Service, Invitrogen, Paisley, UK), several intraplatelet kinases were assayed at their approximate ATP Km values with a fluorescence-based Z'-LYTE biochemical assay based on the differential sensitivity to proteolytic cleavage of phosphorylated and non-phosphorylated peptides labelled with two different fluorophores (Rodems et al., 2002) (see description on the Invitrogen Internet Website http://www.invitrogen.com/site/us/en/home/Products-and-Services/Services/Screening-and-Profiling-Services/SelectScreen-Profiling-Service/SelectScreen-Kinase-Profiling-Service.html). The biochemical assays were performed with a final concentration of 50 µM flavonoid (apigenin, genistein, quercetin or rutin) or 1% DMSO as control. The percentage of kinase activity inhibition in each case was calculated using XLfit (IDBS, Guildford, UK).
Analysis of protein phosphorylation
Washed platelets (800 × 109 L−1) resuspended in calcium-free HEPES/Tyrode's buffer pH 7.35 were incubated either with flavonoids or with specific protein inhibitors, and then stimulated with agonists at 37°C for 2 min under stirring. Reactions were stopped and lysates prepared by addition of one volume of 2× ice-cold 2% Nonidet P40 lysis buffer (2% NP-40, 300 mM NaCl, 20 mM Tris, 2 mM EDTA, 2 mM EGTA, 10 mM Na3VO4, 10 mM NaF, 4 mM PMSF, 4 mM benzamidine, 10 µM aprotinin and 2 µg·mL−1 pepstatin A, pH 7.4), and proteins were resolved by polyacrylamide gel electrophoresis under reducing conditions. Immunodetection of total tyrosine-phosphorylated proteins was performed by ECL Western blotting using 4G10 (1/1000 dilution) antibody followed by an anti-mouse peroxidase-labelled secondary antibody. Densitometric analyses were performed as previously described (Guerrero et al., 2007).
Selective inhibition of specific signalling elements involved in PAR1 and PAR4 functional responses
Platelet aggregation induced by thrombin, PAR1-AP or PAR4-AP was examined in the presence and absence of the PLC inhibitor U73122 (10 µM), PI3K inhibitor LY294002 (50 µM), PKC inhibitor Ro 31-8220 (10 µM), Src kinase inhibitor PP2 (10 µM), as well as with staurosporine as an inhibitor of multiple kinases (1 µM). As phosphatidylinositol 3-kinase (PI3K) and PLC are important for the thrombin-mediated increase in intraplatelet calcium concentration, in such experiments platelets were pre-incubated with LY294009 or U73122, for 15 min and then stimulated accordingly. In protein tyrosine phosphorylation studies, platelets were incubated with two agents known to strongly inhibit tyrosine phosphorylation, either PP2 or staurosporine, and phosphorylation was analysed after stimulation by immunoblotting after SDS–polyacrylamide gel electrophoresis.
125I-thrombin binding assays
Cold binding assays were performed by incubating washed platelets (240 × 109 L−1) with a single low concentration of 125I-thrombin (4 nM) and varying concentrations of unlabelled thrombin (0–1 µM) or flavonoids (0–1.25 mM) in a final volume of 250 µL of binding buffer [136 mM sodium acetate, 25 mM Tris–HCl, 0.6% polyethyleneglycol (average molecular weight 8000), 1% BSA, pH 7.3]. Non-specific binding was determined in the presence of 1 µM unlabelled thrombin. After incubation for 15 min at room temperature without stirring, platelet-bound and free ligands were separated by centrifugation through a layer of 20% sucrose (1200×g, 5 min), and their associated radioactivity was measured in a gamma counter (LKB, Multigamma Pharmacia, Uppsala, Sweden). Binding data were fitted using a non-weighted non-linear model with two sites using Prism for Windows 4.0. (GraphPad Inc.) to obtain the affinity constant of the ligand for these sites (KD) and the corresponding inhibition constant (Ki) for each flavonoid.
Statistical analysis
Unless stated specifically, data are expressed as means ± SEM from at least three experiments performed in different samples. Statistical comparisons were achieved by two-tailed Student's t-test using GraphPad Prism for Windows version 4.0. Differences were considered to be significant at P < 0.05.
Materials
All the polyphenolic compounds tested except genistein were kindly provided by Nutrafur-Furfural Español S.A. (Murcia, Spain) (see Supporting Information Figure S1). Genistein, the protease and phosphatase inhibitor cocktails and PAR1-AP, SFLLRN-OH, were from Sigma-Aldrich Química (Madrid, Spain). PAR4-AP (AYPGKF-amide) was obtained from Bachem (Weil am Rhein, Germany). Both PAR1- and PAR4-APs had a purity of >95%. Human thrombin, PLC inhibitor U73122, Src kinase inhibitor PP2, phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002, broad spectrum inhibitor of protein kinases staurosporine and PKC inhibitor Ro 31-8220 were obtained from Calbiochem-Novabiochem AG (Lucerne, Switzerland). Oregon Green 488 BAPTA-1 AM was from Molecular Probes (Eugene, OR, USA). Antiphosphotyrosine 4G10 mouse immunoglobulin was purchased from Millipore (Watford, UK). The anti αIIbβ3 monoclonal antibody LJ-CP8 was generously provided by Dr ZM Ruggeri (Scripps Research Institute, La Jolla, CA, USA). Antimouse IgG horseradish peroxidase antibody, ECL detection system and [14C]5-HT were from Amersham Biosciences Europe GmbH (Barcelona, Spain). Na125I, specific activity 17.4 Ci·mg−1, was obtained from Perkin Elmer (Boston, MA, USA), and the idodination reagent used for thrombin radiolabelling (Iodo-Gen) was from Pierce Chemical Co. (Rockford, IL, USA). All other chemicals were from Sigma-Aldrich Química.
Results
Effect of flavonoids on thrombin-, PAR1-AP- and PAR4-AP-induced platelet aggregation
In preliminary assays, we first tested the potential inhibitory effect of 13 different polyphenolic compounds (200 µM) belonging to distinct structural subgroups on platelet aggregation induced by 0.2 U·mL−1 thrombin under our experimental conditions (Figure 1). Among all the compounds tested, only quercetin, apigenin and genistein showed a significant inhibitory effect (Supporting Information Figure S2). These polyphenols, together with the glycosylated flavonoid rutin as a negative control, were selected for further experiments. Concentrations of PAR1-AP (25 µM) and PAR4-AP (150 µM) producing similar platelet aggregation responses to those obtained with 0.2 U·mL−1 thrombin (78 ± 1%, 85 ± 2% and 83 ± 2%, respectively) were next established throughout the study.
Figure 1.
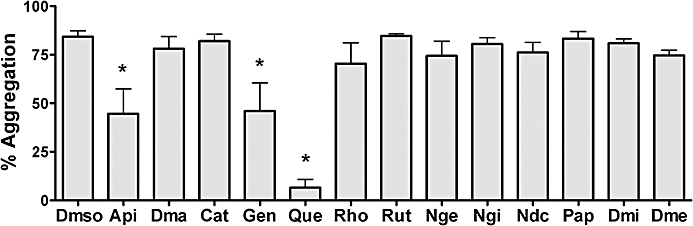
Effect of different flavonoids on platelet aggregation induced by 0.2 U·mL−1 thrombin. Washed platelets (300 × 109 L−1) were incubated in the absence or presence of different flavonoids (200 µM) for 15 min before the addition of the agonist. Results are expressed as the percentage of platelet aggregation, with washed platelets as the baseline and Tyrode's buffer as 100% of light transmission. Data shown are means ± SEM from five different experiments. *P < 0.05 compared with dimethylsulphoxide-treated platelets (control sample). Api, apigenin; Dma, dimethylapigenin; Cat, catechin; Gen, genistein; Que, quercetin; Rho, rhoifolin; Rut, rutin; Nge, naringenin; Ngi, naringin; Ndc, neohesperidin dihydrochalcone; Pap, phloroacetophenone; Dmi, diosmin; Dme, diosmetin.
To establish the relative potency of these compounds as inhibitors of platelet aggregation, dose–response assays were performed (Table 1). Quercetin and apigenin exhibited a similar ability to impair PAR4-AP-induced aggregation, while quercetin was slightly more effective on PAR1-AP- and thrombin-induced responses. When genistein was present, aggregation was similarly inhibited in response to both PAR1-AP and PAR4-AP, while thrombin-induced inhibition became less marked. As expected, rutin showed no inhibiting effects at any of the concentrations tested.
Table 1.
Inhibition of platelet aggregation by flavonoids (IC50± SEM in µM)
| Apigenin | Genistein | Quercetin | Rutin | |
|---|---|---|---|---|
| Thrombin 0.2 U·mL−1 | 56.4 ± 9.8 | >100 | 37.2 ± 5.8 | >100 |
| PAR1-AP 25 µM | 54.4 ± 5.7 | 56.0 ± 5.5 | 22.0 ± 4.2 | >100 |
| PAR4-AP 150 µM | 20.8 ± 3.5 | 47.6 ± 6.5 | 23.1 ± 1.5 | >100 |
Washed platelets were stimulated with thrombin, PAR1-AP or PAR4-AP in the absence or presence of flavonoids, and aggregation was recorded as described in Methods. Data were derived from four different experiments.
The signalling pathways downstream of thrombin require PLC, PI3K, PKC and Src activities; however, the functional importance of such specific elements for thrombin-induced platelet responses remains controversial. To investigate whether this signalling is influenced by quercetin, apigenin or genistein, selective and commonly used inhibitors at concentrations to fully inhibit their target in platelets were included in aggregation, calcium mobilization and protein phosphorylation studies, and their effects were compared to those of flavonoids. Hence, we evaluated the effect of hindering such intracellular elements on thrombin-, PAR1-AP- and PAR4-AP-induced platelet aggregation (Table 2): inhibition of PLC, PKC or of multiple kinases completely blocked platelet aggregation induced by thrombin and PAR-APs, while suppression of PI3K had a significant, but partial, effect on responses to thrombin and PAR-APs. Nevertheless, stimulation of Src-inhibited platelets resulted in slightly decreased aggregation only in thrombin and PAR1-AP-activated platelets.
Table 2.
Effect of several inhibitors on thrombin-, PAR1-AP- and PAR4-AP-induced platelet aggregation (% ± SEM)
| Control DMSO≤0.2% | U73122 10 µM | LY294002 50 µM | Ro31-8220 10 µM | PP2 10 µM | Staurosporine 1 µM | |
|---|---|---|---|---|---|---|
| Thrombin 0.2 U·mL−1 | 83.3 ± 1.7 | 1.2 ± 0.2* | 48.2 ± 7.9* | 5.6 ± 0.8* | 48.8 ± 6.7* | 13.8 ± 3.5* |
| PAR1-AP 25 µM | 78.3 ± 0.8 | 0.8 ± 0.1* | 37.9 ± 2.9* | 8.9 ± 2.4* | 45.6 ± 4.3* | 5.9 ± 0.5* |
| PAR4-AP 150 µM | 85.5 ± 2.1 | 0.9 ± 0.2* | 46.4 ± 8.3* | 1.0 ± 0.2* | 73.3 ± 5.0 | 5.8 ± 0.7* |
Washed platelets were stimulated with thrombin, PAR1-AP or PAR4-AP in the absence or presence of the phospholipase C inhibitor U73122, PI3K inhibitor LY294002, protein kinase C inhibitor Ro 31-8220, Src kinase inhibitor PP2 and the broad spectrum inhibitor of protein kinases staurosporine, and aggregation was recorded as described in Methods. Data were derived from four different experiments.
P < 0.05 versus control activated samples.
Effect of flavonoids on 5-HT release
5-HT is secreted from activated platelets during platelet aggregation. To determine whether these flavonoids are inhibiting secretion of dense granules, we measured 14C-5-HT release after agonist stimulation under stirring conditions. As seen in Figure 2, quercetin, apigenin and genistein significantly inhibited 5-HT secretion after stimulation with thrombin and PAR-APs. No significant differences were observed in 5-HT release in the presence of rutin under any of the conditions tested. Similarly, pretreatment of platelets with LJ-CP8, a monoclonal antibody that reacts with αIIbβ3 integrin and inhibits platelet aggregation, did not induce any significant change in 5-HT release.
Figure 2.
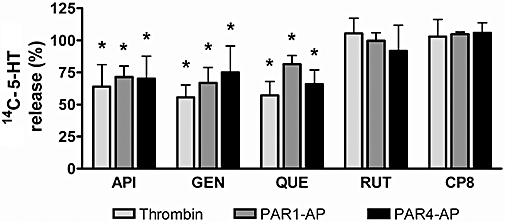
Effects of flavonoids on thrombin-, PAR1-AP- and PAR4-AP-induced 14C-5-HT release. Platelets loaded with 14C-5-HT were incubated with flavonoids (50 µM) or LJ-CP8 (1 µM) and then stimulated under stirring conditions. Released radioactivity was measured in supernatants by liquid scintillation counting. Data (n= 4) are percentage of secretion versus the release obtained in stimulated control samples without flavonoids (dimethylsulphoxide). 5-HT release values (% vs. total radioactivity incorporated) in the samples without flavonoids upon stimulation with 0.2 U·mL−1 thrombin, 25 µM PAR1-AP or 150 µM PAR4-AP were 88.5 ± 2.0, 65.7 ± 2.0 and 76.8 ± 11.7, respectively. *P < 0.05 versus the 14C-5-HT release induced by each agonist in untreated platelets.
Effect of flavonoids on intracellular calcium mobilization
We next investigated whether flavonoids interfere with intraplatelet calcium mobilization after activation of PARs. Stimulation of Oregon Green-loaded platelets with thrombin, PAR1-AP or PAR4-AP (in the presence of 2 mM EGTA to avoid the influx of extracellular calcium) raised intraplatelet calcium concentration, which was prevented by the PLC inhibitor, U73122 (Figure 3). As previously reported (Covic et al., 2000), PAR1 stimulation caused a rapid spike response, while a much more prolonged increase was observed after the addition of PAR4-AP or thrombin (data not shown). Pretreatment of platelets with 50 µM quercetin, apigenin, genistein or the PI3K inhibitor LY294002 significantly inhibited calcium mobilization induced by all agonists. As expected, rutin did not significantly affect thrombin-, PAR1-AP- nor PAR4-AP-mediated calcium increase.
Figure 3.

Effect of flavonoids on thrombin-, PAR1-AP- and PAR4-AP-induced [Ca2+]i mobilization. Oregon Green BAPTA-1 AM-loaded platelets were incubated with dimethylsulphoxide as control, flavonoids (50 µM), the PI3K inhibitor LY294002 (50 µM) or the phospholipase C inhibitor U73122 (10 µM) and stimulated with 0.2 U·mL−1 thrombin, 25 µM PAR1-AP or 150 µM PAR4-AP. Data (n= 4) represent mean percentage of peak calcium mobilization (values obtained in stimulated control samples = 100%). *P < 0.05 versus control activated samples. NA, non-activated; API, apigenin; GEN, genistein; QUE, quercetin; RUT, rutin; LY, LY294002.
Effect of flavonoids on in vitro kinase activity
Several flavonoids including genistein and quercetin have been classically defined as broad-spectrum kinase inhibitors (Akiyama et al., 1987; Hubbard et al., 2003; 2004;), and selective inhibition of protein kinases by quercetin, apigenin, genistein, but not rutin may explain our present observations. By using a precise, commercial fluorescence assay, we aimed to establish the effect of these flavonoids (50 µM) on several tyrosine and serine–threonine kinases reported as key signalling elements of the platelet activation process. As shown in Table 3, quercetin, and to a lesser extent apigenin, displayed a substantial direct inhibitory effect against Fyn, Lyn, Src and Syk. We also observed a major effect of quercetin against PI3K β, γ and δ isoforms, and a moderate effect on Akt 1 and 2. On the other hand, quercetin did not show a major inhibitory activity upon the serine/threonine kinases ERK1/2 and p38 α, members of the MAP kinase family, nor on the distinct PKC isoforms analysed. In all cases, apigenin exerted weaker effects than quercetin, and genistein proved not to have a particular potency against any of the kinases tested. The glycosylated flavonoid rutin was confirmed as a potent inhibitor of PI3Kδ with a mild effect on PI3Kγ, while it did not exert a significant inhibition of any of the other kinases examined.
Table 3.
Invitrogen SelectScreen Kinase Profiling data for apigenin, genistein, quercetin and rutin (% inhibition ± SEM at 50 µM)
| Apigenin | Genistein | Quercetin | Rutin | |
|---|---|---|---|---|
| Fyn | 54.5 ± 4.1 | 16.4 ± 2.2 | 75.4 ± 0.7 | 0.7 ± 0.5 |
| Lyn A | 53.9 ± 0.8 | 20.4 ± 0.6 | 54.0 ± 1.9 | 0.8 ± 0.6 |
| Src | 52.1 ± 1.4 | 19.3 ± 1.1 | 63.9 ± 2.2 | 13.7 ± 0.8 |
| Syk | 68.2 ± 5.3 | 18.6 ± 2.1 | 80.4 ± 0.1 | 8.5 ± 0.2 |
| MAPK1 (ERK2) | 0.9 ± 0.6 | 10.1 ± 2.7 | 20.7 ± 0.5 | 0.0 ± 0.0 |
| MAPK3 (ERK1) | 0.0 ± 0.0 | 5.9 ± 1.0 | 9.3 ± 1.4 | 0.0 ± 0.0 |
| MAPK14 (p38α) | 16.1 ± 3.7 | 16.8 ± 0.4 | 16.0 ± 1.4 | 0.0 ± 0.0 |
| AKT1 (PKBα) | 13.8 ± 1.6 | 16.1 ± 2.0 | 46.2 ± 1.0 | 0.0 ± 0.0 |
| AKT2 (PKBβ) | 13.9 ± 4.1 | 13.1 ± 1.3 | 51.2 ± 5.0 | 0.6 ± 0.5 |
| PRKCA (PKCα) | 0.0 ± 0.0 | 0.0 ± 0.0 | 9.8 ± 7.1 | 2.7 ± 1.9 |
| PRKCB1 (PKCβ1) | 10.7 ± 0.7 | 5.9 ± 0.9 | 24.0 ± 1.9 | 1.4 ± 1.0 |
| PRKCD (PKCδ) | 0.0 ± 0.0 | 2.7 ± 1.9 | 23.2 ± 2.3 | 0.0 ± 0.0 |
| PI3Kβ | 32.0 ± 2.8 | 4.0 ± 0.7 | 75.5 ± 2.5 | 12.5 ± 0.4 |
| PI3Kγ | 21.0 ± 2.1 | 13.5 ± 0.4 | 85.5 ± 1.8 | 37.0 ± 2.1 |
| PI3Kδ | 25.5 ± 3.2 | 7.5 ± 2.5 | 99.0 ± 0.7 | 99.0 ± 0.0 |
The activity of several flavonoids against a panel of protein kinases was assessed using a fluorescence resonance energy transfer method. The assays were performed in 1% dimethylsulphoxide and an ATP concentration equal to the Km[app] of each kinase. MAPK, mitogen-activated protein kinase; PKB, protein kinase B; PRKC, protein kinase C; PI3-kinase, phosphatidylinositol 3-kinase. Data were obtained from two different experiments.
Effect of flavonoids on platelet whole tyrosine phosphorylation induced by activation of PARs
Experiments were, therefore, performed to assess whether the deleterious effect of quercetin and apigenin, but not genistein nor rutin, on protein kinases was related to selective impairment of protein tyrosine phosphorylation upon thrombin- and PAR-APs-induced platelet stimulation. In order to ensure a sufficiently strong phosphorylation response, agonist concentrations were increased up to 0.5 U·mL−1 thrombin, 100 µM PAR1-AP and 500 µM PAR4-AP. Consequently, in these assays flavonoids were also tested at increased concentrations (100 µM). As shown in Figure 4, thrombin elevated phosphotyrosine protein content, and this increase was significantly impaired by the multiple kinase inhibitor staurosporine. Under these experimental conditions, quercetin and, to a lesser extent, apigenin behaved as efficient inhibitors of thrombin- and PAR-APs-induced whole protein tyrosine phosphorylation, while genistein and rutin displayed a minor inhibitory effect.
Figure 4.

Effect of flavonoids on thrombin-, PAR1-AP- and PAR4-AP-induced tyrosine phosphorylation. Washed platelets were incubated with vehicle (dimethylsulphoxide), flavonoids (100 µM), the Src kinase inhibitor PP2 (10 µM) or the multiple kinase inhibitor staurosporine (1 µM), and stimulated with 0.5 U·mL−1 thrombin, 100 µM PAR1-AP or 500 µM PAR4-AP for 2 min. Platelets were then lysed, and phosphotyrosine-containing proteins were identified by Western blot using the 4G10 antibody. A representative blot is shown in the upper panels. In each case, tyrosine phosphorylation was quantified by densitometry using Quantity One software. The lower panel shows comparative results of tyrosine phosphorylation achieved with platelets stimulated in the absence or presence of flavonoids or inhibitors. Data are mean ± SEM from three separate experiments. NA, non-activated; API, apigenin; GEN, genistein; QUE, quercetin; RUT, rutin; STA, staurosporine. *P < 0.05 compared with phosphotyrosine content in stimulated platelets in the absence of inhibitors.
Effects of flavonoids on 125I-thrombin binding
The fact that the inhibition by specific flavonoids of in vitro platelet responses seems to be related, at a great extent, to their ability to compete for binding to the TxA2 receptor (Guerrero et al., 2005; 2007; Navarro-Nuñez et al., 2009), prompted us to investigate if the potential interaction of flavonoids with thrombin receptors may also play a role in their effect on thrombin-induced activation. For that purpose, a cold saturation binding assay was carried out in washed platelets by examining the reduction of 125I-thrombin binding with increasing concentrations of unlabelled thrombin. Our binding data were best fitted to a two-site model, obtaining affinity constants of 12.3 and 145.2 nM for thrombin binding sites, probably corresponding to PAR1 and PAR4 receptors. As illustrated in Figure 5, under our experimental conditions none of the flavonoids tested competed for this binding.
Figure 5.
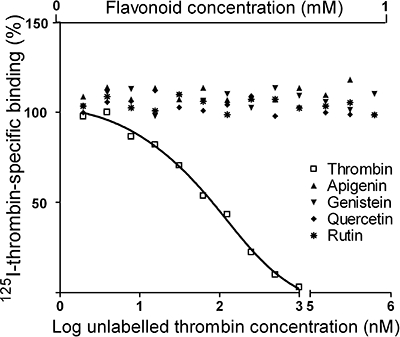
Effect of flavonoids on 125I-thrombin binding to platelets. Washed platelets were incubated with 125I-thrombin (4 nM) in the presence of increasing concentrations of unlabelled thrombin or flavonoids as competitors. The plot represents the resultant dose-dependent displacement curve of the specific binding obtained with unlabelled thrombin and the lack of interaction between tested flavonoids and thrombin receptors. Data were obtained from three different experiments.
Discussion
This study focused on the effects of different flavonoids on thrombin-induced platelet activation through the assessment of their effects on the specific signalling cascades downstream of PAR1 and PAR4 receptors. Here, we show that three flavonoids, quercetin, apigenin and genistein, inhibit both PAR1-AP- and PAR4-AP-mediated responses of platelets, via inhibition of calcium mobilization, granule release and aggregation. While the cellular mechanism for the antiplatelet activity of quercetin, and to a lesser extent of apigenin, may be partly due to the inhibition of multiple platelet kinases, this mechanism does not appear to be responsible for the genistein-induced effects. Furthermore, we show that flavonoids do not compete for thrombin binding, and thus, receptor antagonism does not seem to account for platelet inhibition with these compounds.
The mechanisms by which PARs signal platelet activation have not been well defined. Although there is evidence that PAR1 and PAR4 signalling pathways are not redundant (Steinhoff et al., 2005; Holinstat et al., 2006), outlining the precise signalling and downstream sequelae of each receptor is presently controversial and essentially unclear. Both ADP secreted from platelet dense granules, as well as TxA2 generation, are important mediators in thrombin-mediated responses (Cho et al., 2002; Falker et al., 2004; Begonja et al., 2007). Our previous data indicate that quercetin, apigenin and genistein inhibit platelet function by binding to TxA2 receptors with subsequent inhibition of its signalling pathways (Guerrero et al., 2005; 2007;). In the present study, we therefore investigated the inhibitory effect of these flavonoids on thrombin responses independently of TxA2 signalling pathways in the presence of indomethacin to avoid selective inhibition of the amplifying actions of TxA2 at the receptor level. Whereas inhibition of cyclooxygenase by aspirin or indomethacin inhibits the activation induced by low doses of thrombin, the activation promoted by higher doses, such as those used in the present study, seems not to be affected (Falker et al., 2004; Begonja et al., 2007).
Here, we show for the first time that quercetin, apigenin and genistein inhibit both PAR1- and PAR4-mediated platelet aggregation, whereas they appear to be somewhat weaker inhibitors of thrombin-induced aggregation. These results suggest that the lesser effects of these compounds on thrombin might be due to the contributing and adding effects of both receptors on the signalling by the protease. The inhibitory effect of these flavonoids on other thrombin-stimulated responses (calcium mobilization, serotonin release, tyrosine phosphorylation) does not support other potential explanations, such as flavonoids hindering the proteolytic activity of thrombin, nor reflecting the strengthening contribution of GPIb signalling to thrombin-induced platelet activation at the doses of the protease used in this study.
Aggregation and secretion appear to contribute to a mutually amplifying positive feedback loop upon thrombin activation. Thus, at low levels of thrombin, without secretion, aggregation does not occur; likewise, without aggregation, discernable dense granule secretion does not take place (Liu et al., 2008). Nevertheless, in our hands, pretreatment of platelets with LJ-CP8 did not prevent dense granule release, reflecting that under our experimental conditions inhibition of 5-HT secretion by quercetin, apigenin and genistein was not a consequence, but probably a direct cause of the impaired aggregation response. The initiation of secretion by thrombin is the result of the synergistic action of Ca2+ mobilization and PKC activation, as a consequence of PLC-mediated phosphoinositide hydrolysis (Walker and Watson, 1993). Thus, in this study, Ro 31-8220, a classical PKC inhibitor, inhibited thrombin- and PAR-AP-induced platelet aggregation. Phosphorylation studies (see below) do not suggest an inhibition by quercetin, apigenin nor genistein of PKC signalling pathways, implying that impaired calcium mobilization might account for the effect of these flavonoids on platelet dense granule secretion.
As expected, quercetin, apigenin and genistein, but not rutin, significantly reduced thrombin-, PAR1-AP- and PAR4-AP-induced release of Ca2+ from intracellular stores. Under our experimental conditions, and in contrast to previous studies (Holinstat et al., 2006), PLC blockade with U-73122 had a significant inhibitory effect on thrombin-, PAR1-AP- and PAR4-AP-induced aggregation. This discrepancy is probably due to the different experimental conditions employed: in fact, Holinstat et al. (2006) measured the platelet aggregation response using lower concentrations of the inhibitor and higher concentrations of peptides than those used here, and in the absence of indomethacin, possibly reflecting that TxA2 is a major requirement for platelet aggregation by PAR-APs and thrombin when PLC and subsequent calcium mobilization are inhibited. Data from this study, specifically those related to genistein, suggest that flavonoids might be modulating calcium levels through mechanisms other than inhibition of protein kinases, such as affecting polyphosphoinositide turn-over (Ozaki et al., 1993) or by blockade of calcium channels (Dobrydneva et al., 2002).
To shed further light on the signal transduction steps specifically affected by these flavonoids, we analysed protein phosphorylation by biochemical assays that had been validated or used by others (Gharbi et al., 2007; Kothe et al., 2007). Consistent with a previous study, quercetin (Hubbard et al., 2003), and to a lesser extent, apigenin, predominantly inhibited the non-lipid kinases Syk, Fyn, Src and Lyn, and the activity of the lipid PI3K. Genistein, however, only weakly attenuated protein phosphorylation, while rutin displayed a considerable effect only on PI3Kδ. Consistent with these data, quercetin and apigenin, in contrast to genistein and rutin, substantially inhibited thrombin- and PAR-AP-induced increases in total protein tyrosine phosphorylation, which validates the thoroughness of the previous assay. Other reports have established that Lyn (Cho et al., 2002) and Akt 1, a principal downstream effector of PI3K signalling (Chen et al., 2004), are required for thrombin induction of secretion-dependent platelet aggregation. These were the kinases substantially affected by quercetin and apigenin. However, this potential mode of action would be unlikely to account entirely for the impairment of thrombin- and PAR-AP-promoted aggregation by these two flavonoids, as PP2 and LY294002, specific Src and PI3K inhibitors, respectively, were only partially effective in reducing such responses. Some of these flavonoids, most notably genistein, have long been considered to act as non-selective inhibitors of tyrosine kinases (Akiyama et al., 1987). However, our results further support the concept that the isoflavone genistein is not a particularly good inhibitor of tyrosine kinases in human platelets following stimulation with thrombin (Nakashima et al., 1991; McNicol, 1993).
Previously, we reported an antagonistic activity of flavonoids against the platelet TxA2 receptor (Guerrero et al., 2005; 2007; Navarro-Nuñez et al., 2009). This result prompted us to examine whether the inhibitory effect that flavonoids exerted on PAR-induced responses was due to membrane receptor antagonism as well. However, our binding assays did not establish any kind of interaction between such receptors and any of the flavonoids tested, indicating that these compounds inhibit thrombin responses exclusively by affecting intracellular signalling.
Recent reports established that platelet Rap1b and integrin αIIbβ3 activation downstream of Gq occurs through sequential calcium-dependent, PKC-regulated and secretion-derived secondary mechanisms (Quinton et al., 2002; Cifuni et al., 2008). Calcium and diacylglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEFI) has been reported as the primary signalling molecule mediating calcium-dependent activation of Rap1b and αIIbβ3 (Crittenden et al., 2004). Our results showed that quercetin, apigenin and genistein partially prevented intracellular calcium mobilization in response to thrombin, PAR1-AP and PAR4-AP, and that apigenin and quercetin also impair kinase activation. Hence, the inhibitory effect of genistein on PAR1-AP- and PAR4-AP-induced aggregation may be essentially a consequence of diminished CalDAG-GEFI activation due to attenuated free intracellular calcium levels, while for quercetin and apigenin not only the above mechanism, but also inhibition of kinase activation may be partly implicated.
Flavonoids, by affecting signalling pathways involved in the release of secondary mediators needed to reinforce and stabilize PAR-mediated platelet activation, impair the response to submaximal stimulation of platelet thrombin receptors. Although these effects are seen with concentrations of flavonoids that exceed those obtained after standard consumption of flavonoid-rich foods, these data raise the question of the role of pharmacological supplementation with these compounds in situations such as the prevention of atherothrombosis.
Acknowledgments
L Navarro-Núñez is an FPI fellow from the Spanish Ministry of Education (BES-2005-7496). JA Guerrero holds a postdoctoral position funded by the Instituto de Salud Carlos III. C Martínez is an investigator from the Fundación para la Formación e Investigación Sanitarias de la Región de Murcia (FFIS). This work was partially financed by the Spanish Ministry of Education and FEDER (SAF 2004-07535, SAF 2006-06212), and Fundación Séneca (04515/GERM/06, 03116/PI/05).
Glossary
Abbreviations:
- BSA
bovine serum albumin
- CalDAG-GEFI
calcium and diacylglycerol-regulated guanine nucleotide exchange factor I
- DAG
diacylglycerol
- DMSO
dimethylsulphoxide
- ERK
extracellular signal-regulated kinase
- GP
glycoprotein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- MAP kinase
mitogen-activated protein kinase
- PAR
protease-activated receptor
- PAR1-AP
protease-activated receptor 1 agonist peptide
- PAR4-AP
protease-activated receptor 4 agonist peptide
- PKC
protein kinase C
- PI3K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- TxA2
thromboxane A2
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Structures of the different polyphenolic compounds used in this study. Only the flavonoids apigenin, genistein and quercetin were found to exert significant inhibitory effects on platelet responsiveness to thrombin. These flavonoids share the same benzo-y-pyrone structure consisting of two aromatic rings (named A and B) linked by a three-carbon chain that forms an oxygenated heterocyclic ring (C ring). The single difference between the chemical structure of apigenin and genistein lies in the site of B-ring insertion (carbon 3 for genistein, carbon 2 for apigenin), while quercetin differs from apigenin due to inclusion of a hydroxyl group in carbon 3. Rutin, the glycosylated counterpart of quercetin, did not show any effect on platelet function. Two synthetic compounds were also included in the preliminary assays: neohesperidin dihydrochalcone, a synthetic sweetening agent produced by alkaline hydrogenation of the Citrus flavonoid neohesperidin, and phloroacetophenone, a key intermediate in flavonoid synthesis.
Figure S2 Representative platelet aggregation curves. Washed platelet suspensions treated with 50 μM flavonoids or 0.1% DMSO as control were stimulated with thrombin (0.2 U·mL−1), PAR1-AP (25 μM) or PAR4-AP (150 μM) in an Aggrecorder II aggregometer. Changes in light transmission of platelet suspensions over baseline were monitored for 5 min, and the percentages of maximal aggregation were recorded. API, apigenin; GEN, genistein; QUE, quercetin; RUT, rutin.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begonja AJ, Geiger J, Rukoyatkina N, Rauchfuss S, Gambaryan S, Walter U. Thrombin stimulation of p38 MAP kinase in human platelets is mediated by ADP and thromboxane A2 and inhibited by cGMP/cGMP-dependent protein kinase. Blood. 2007;109:616–618. doi: 10.1182/blood-2006-07-038158. [DOI] [PubMed] [Google Scholar]
- Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104:1703–1710. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gamma-thrombin. Blood. 2002;99:2442–2447. doi: 10.1182/blood.v99.7.2442. [DOI] [PubMed] [Google Scholar]
- Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008;112:1696–1703. doi: 10.1182/blood-2008-02-139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry. 2000;39:5458–5467. doi: 10.1021/bi9927078. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- De Marco L, Mazzucato M, Masotti A, Fenton JW, Ruggeri ZM. Function of glycoprotein Ib alpha in platelet activation induced by alpha-thrombin. J Biol Chem. 1991;266:23776–23783. [PubMed] [Google Scholar]
- Dobrydneva Y, Williams RL, Morris GZ, Blackmore PF. Dietary phytoestrogens and their synthetic structural analogues as calcium channel blockers in human platelets. J Cardiovasc Pharmacol. 2002;40:399–410. doi: 10.1097/00005344-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Falker K, Lange D, Presek P. ADP secretion and subsequent P2Y12 receptor signalling play a crucial role in thrombin-induced ERK2 activation in human platelets. Thromb Haemost. 2004;92:114–123. doi: 10.1160/TH03-12-0729. [DOI] [PubMed] [Google Scholar]
- Gharbi S, Zvelebil M, Shuttleworth S, Hancox T, Saghir N, Timms J, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero JA, Lozano ML, Castillo J, Benavente-Garcia O, Vicente V, Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- Guerrero JA, Navarro-Nuñez L, Lozano ML, Martinez C, Vicente V, Gibbins JM, et al. Flavonoids inhibit the platelet TxA(2) signalling pathway and antagonize TxA(2) receptors (TP) in platelets and smooth muscle cells. Br J Clin Pharmacol. 2007;64:133–144. doi: 10.1111/j.1365-2125.2007.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JR. Protease-activated receptors as targets for antiplatelet therapy. Blood Rev. 2009;23:61–65. doi: 10.1016/j.blre.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Voss B, Bilodeau ML, McLaughlin JN, Cleator J, Hamm HE. PAR4, but not PAR1, signals human platelet aggregation via Ca2+ mobilization and synergistic P2Y12 receptor activation. J Biol Chem. 2006;281:26665–26674. doi: 10.1074/jbc.M602174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard GP, Stevens JM, Cicmil M, Sage T, Jordan PA, Williams CM, et al. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J Thromb Haemost. 2003;1:1079–1088. doi: 10.1046/j.1538-7836.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–2145. doi: 10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, et al. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamae T, Shiraga M, Kashiwagi H, Kato H, Tadokoro S, Kurata Y, et al. Critical role of ADP interaction with P2Y12 receptor in the maintenance of alpha(IIb)beta3 activation: association with Rap1B activation. J Thromb Haemost. 2006;4:1379–1387. doi: 10.1111/j.1538-7836.2006.01941.x. [DOI] [PubMed] [Google Scholar]
- Kothe M, Kohls D, Low S, Coli R, Rennie GR, Feru F, et al. Selectivity-determining residues in Plk1. Chem Biol Drug Des. 2007;70:540–546. doi: 10.1111/j.1747-0285.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Jackson CW, Gartner TK. The Src requirement for washed platelet aggregation and dense granule secretion in response to stimulation by a low level gamma-thrombin. J Thromb Haemost. 2008;6:1035–1037. doi: 10.1111/j.1538-7836.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- Lova P, Campus F, Lombardi R, Cattaneo M, Sinigaglia F, Balduini C, et al. Contribution of protease-activated receptors 1 and 4 and glycoprotein Ib-IX-V in the Gi-independent activation of platelet Rap1B by thrombin. J Biol Chem. 2004;279:25299–25306. doi: 10.1074/jbc.M313199200. [DOI] [PubMed] [Google Scholar]
- McNicol A. The effects of genistein on platelet function are due to thromboxane receptor antagonism rather than inhibition of tyrosine kinase. Prostaglandins Leukot Essent Fatty Acids. 1993;48:379–384. doi: 10.1016/0952-3278(93)90118-g. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Nakashima S, Koike T, Nozawa Y. Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses. Mol Pharmacol. 1991;39:475–480. [PubMed] [Google Scholar]
- Navarro-Nuñez L, Castillo J, Lozano ML, Martinez C, Benavente-Garcia O, Vicente V, et al. Thromboxane A2 receptor antagonism by flavonoids: structure–activity relationships. J Agric Food Chem. 2009;57:1589–1594. doi: 10.1021/jf803041k. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Yatomi Y, Jinnai Y, Kume S. Effects of genistein, a tyrosine kinase inhibitor, on platelet functions: genistein attenuates thrombin-induced Ca2+ mobilization in human platelets by affecting polyphosphoinositide turnover. Biochem Pharmacol. 1993;46:395–403. doi: 10.1016/0006-2952(93)90515-x. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- Quinton TM, Kim S, Dangelmaier C, Dorsam RT, Jin J, Daniel JL, et al. Protein kinase C- and calcium-regulated pathways independently synergize with Gi pathways in agonist-induced fibrinogen receptor activation. Biochem J. 2002;368:535–543. doi: 10.1042/BJ20020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153:S263–S282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodems SM, Hamman BD, Lin C, Zhao J, Shah S, Heidary D, et al. A FRET-based assay platform for ultra-high density drug screening of protein kinases and phosphatases. Assay Drug Dev Technol. 2002;1:9–19. doi: 10.1089/154065802761001266. [DOI] [PubMed] [Google Scholar]
- Seiler SM, Bernatowicz MS. Peptide-derived protease-activated receptor-1 (PAR-1) antagonists. Curr Med Chem Cardiovasc Hematol Agents. 2003;1:1–11. doi: 10.2174/1568016033356689. [DOI] [PubMed] [Google Scholar]
- Shankar H, Garcia A, Prabhakar J, Kim S, Kunapuli SP. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of ERK1/2 activation. J Thromb Haemost. 2006;4:638–647. doi: 10.1111/j.1538-7836.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Walker TR, Watson SP. Synergy between Ca2+ and protein kinase C is the major factor in determining the level of secretion from human platelets. Biochem J. 1993;289:277–282. doi: 10.1042/bj2890277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


