Abstract
Background and purpose:
Previous studies have suggested a regulation of 5-hydroxytryptamine (5-HT) neurons by the endocannabinoid system. The aim of our work was to examine the effect of two CB1 receptor antagonists, SR141716A (rimonabant, Sanofi-Synthélabo Recherche, Montpellier, France) and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251, Tocris Cookson, Bristol, UK), on the firing rate of dorsal raphe nucleus (DRN) neurons.
Experimental approach:
Single-unit extracellular recordings were performed to study the effect of CB1 receptor antagonists in slices of the DRN from rat brain.
Key results:
Rimonabant (1 µM) and AM251 (1 µM) decreased the firing rate of about 50% of all the recorded DRN 5-HT cells. The GABAAreceptor antagonist picrotoxin (20 µM) (Sigma) prevented and also reversed the inhibitory effect of rimonabant (1 µM) and AM251 (1 µM), suggesting that CB1 receptors regulate 5-HT neurons through the GABAergic system. However, the CB1/CB2 receptor agonist R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)-methyl]pyrrolol[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt (10 µM) (WIN55212-2, Sigma, St. Louis, MO, USA) failed to change the firing activity of non-5-HT (presumably GABAergic) neurons in the DRN. The endocannabinoid N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (anandamide, Tocris Cookson) (10 µM) also inhibited the firing activity of a number of 5-HT neurons, but this inhibition was not blocked by rimonabant (1 µM) or AM251 (1 µM), and the stable analogue R-(+) N-(2-hydroxy-1methylethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (methanandamide, Tocris Cookson) (10 µM) did not mimic this effect. The selective CB1 receptor agonist arachidonoyl-2-chloroethylamide (ACEA) (1 µM) only slightly increased the firing rate of DRN 5-HT cells.
Conclusions and implications:
These results suggest a tonic/constitutive regulation of DRN 5-HT neurons by the endocannabinoid system, which may occur through a CB1 receptor-mediated inhibition of the GABAergic system. The inhibitory effect of anandamide may be mediated through a CB1 receptor-independent mechanism.
Keywords: dorsal raphe nucleus, rimonabant, AM251, single-unit extracellular recording, 5-HT, GABA
Introduction
The endocannabinoid system, a pharmacological target for the psychoactive components of marijuana, comprises specific Gi/o protein-coupled cannabinoid receptors and several endogenous cannabimimetic lipids. Endocannabinoids are involved in the regulation of common brain functions (nociception, cognition, reward and others) and include N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (anandamide) as one of the best characterized compounds (Fride and Mechoulam, 2003). In the CNS, the effects of endocannabinoids are mainly mediated by CB1 receptors (nomenclature follows Alexander et al., 2008) that are targeted by plant-derived and synthetic cannabinoids (Howlett et al., 2002). CB1 receptors are present in the basal ganglia, hippocampus, cerebellum and cerebral cortex, consistent with the main behavioural effects of cannabinoids (Tsou et al., 1998).
The dorsal raphe nucleus (DRN) expresses CB1 receptors, their mRNA and the catabolic enzyme for anandamide, fatty acid amide hydrolase (FAAH) (Martin et al., 1995; Tsou et al., 1998; Egertova et al., 2003; Häring et al., 2007). This nucleus not only constitutes the largest grouping of 5-hydroxytryptamine (5-HT) neurons in the rat brain, but also contains non-5-HT cells such as GABAergic interneurons (Piñeyro and Blier, 1999). It has been shown that the CB1/CB2 receptor agonist R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)-methyl]pyrrolol[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt (WIN55212-2, Sigma, St. Louis, MO, USA) and the FAAH inhibitor [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate (URB597) increase the firing activity of DRN 5-HT neurons in vivo (Gobbi et al., 2005; Bambico et al., 2007). Activation of the endocannabinoid system also increases the expression of c-Fos in the DRN (Murillo-Rodríguez et al., 2007). Projections arising from the DRN to all the neuroaxes can regulate various functions altered by cannabinoids, such as pain, learning/memory, attention and rewarding behaviour. In fact, several pharmacological effects of cannabinoids have been postulated to be linked to the regulation of the DRN. First, cannabinoid administration in the DRN induces antinociception (Martin et al., 1995) and, conversely, neuropathic pain increases endocannabinoid levels in the DRN (Palazzo et al., 2006). Second, raising the endocannabinoid tone in the rat elicits anxiolytic- and antidepressant-like effects through a modulation of the DRN (Gobbi et al., 2005; Braida et al., 2007). Finally, several studies have demonstrated that cannabinoids modulate the synthesis, release and turnover of 5-HT in the projection areas of the DRN (Nakazi et al., 2000; Egashira et al., 2002; Tzavara et al., 2003; Moranta et al., 2004; Sagredo et al., 2006).
Although various effects of cannabinoids on 5-HT neurotransmission have been reported, little direct evidence has been provided about the endogenous regulation of 5-HT neurons in the DRN by the endocannabinoid system. Therefore, the aim of our study was to characterize the effect of the CB1 receptor antagonists SR141716A (rimonabant, Sanofi-Synthélabo Recherche, Montpellier, France) and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251, Tocris Cookson, Bristol, UK), and the endocannabinoid anandamide (Tocris) on the firing activity of DRN 5-HT cells in vitro. For this purpose, single-unit extracellular recordings of 5-HT and non-5-HT neurons were performed in the DRN from rat brain slices.
This present work shows that the selective CB1 receptor antagonists rimonabant and AM251 inhibit a proportion of DRN 5-HT cells by a mechanism that is prevented by the GABAA receptor antagonist picrotoxin (Sigma).
Methods
Test systems used
Brain slice preparation
All animal care and experimental procedures reported in this manuscript were carried out in accordance with the European Community Council Directive on ‘Protection of Animals Used in Experimental and Other Scientific Purposes’ of 24 November 1986 (86/609/EEC), and all the efforts were made to minimize animal suffering and to reduce the number of animals used. Experiments used 202 male Sprague-Dawley (200–300 g) rats. The animals were housed under standard laboratory conditions (22°C, 12 h light/dark cycles) with free access to food and water.
The animals were anaesthetized with chloral hydrate (400 mg·kg−1 i.p) and then decapitated. The brain was rapidly removed after death and placed in an ice-cold artificial cerebrospinal fluid (ACSF) where NaCl was substituted by sucrose to improve neuronal viability. Coronal brainstem sections of 600 µm thickness containing the DRN were cut using a vibratome and incubated at 33°C in a modified Haas-type interface chamber continuously superfused with ACSF at a flow rate of 1.5 mL·min−1 (Mendiguren and Pineda, 2007). The ACSF contained (in mM) NaCl 129, KCl 3, NaH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 21 and D-glucose 10, and was equilibrated with 95% O2 plus 5% CO2 (pH 7.34). A recovery period of 2 h was allowed before starting electrophysiological recordings.
Measurements
Extracellular recordings
Single-unit extracellular recordings of DRN 5-HT neurons were made as previously described (Aghajanian and Lakoski, 1984). The recording electrode, which consisted of an Omegadot glass micropipette WPI, Sarasota, FL, USA, was pulled and filled with NaCl (0.05 M). The tip was broken back to a size of 2–5 µm (3–5 MΩ). The electrode was positioned in the DRN, which was identified visually as a dark area in the ventromedial part of the periaqueductal grey. The extracellular signal from the electrode was passed through a high-input impedance amplifier and monitored on an oscilloscope and also with an audio unit. Individual neuronal spikes were isolated from the background noise with a window discriminator, and the firing rate was analysed by means of a PC-based custom-made programme that generated histogram bars representing the cumulative number of spikes in consecutive 10 s bins (HFCP®, Cibertec SA, Madrid, Spain).
As the majority of 5-HT neurons in slice preparations is silent, due to an interruption of the noradrenergic excitatory input, the α1 adrenoceptor agonist phenylephrine (15 µM) (Research Biochemical International, Madrid, Spain) was superfused from the beginning of each experiment to drive the firing activity. DRN 5-HT cells were identified by their electrophysiological features: a regular discharging pattern, a slow firing rate and a long-lasting biphasic positive–negative waveform (2 ms). Non-5-HT neurons were identified by their electrophysiological properties, such as the fast firing rate, the irregular pattern of firing and the short-lived spike (<2 ms) (Aghajanian and Lakoski, 1984; Hajos and Sharp, 1996). Moreover, pharmacological criteria such as the inhibitory response to bath application of 5-HT (Sigma) (50–100 µM) or the 5-HT1A receptor agonist (±)-8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide (8-OH-DPAT, Sigma) (10 nM) were used to confirm the identity of 5-HT cells (Aghajanian and Lakoski, 1984; Hajos and Sharp, 1996). As both 5-HT and non-5-HT cells have been recently shown to be inhibited by 5-HT1A receptor activation (Kirby et al., 2003; Marinelli et al., 2004), we only considered, as 5-HT neurons, those cells that fulfilled both electrophysiological and pharmacological criteria.
Experimental design
To assess the regulation of the DRN by the endocannabinoid system, we first evaluated the effect of the CB1 receptor antagonists rimonabant (1 µM) and AM251 (1 µM) on the neuronal activity of 5-HT cells. To study the involvement of the GABAA receptor in the effect of rimonabant (1 µM) and AM251 (1 µM) on DRN 5-HT cells, the GABAA receptor antagonist picrotoxin (20 µM) was applied in the superfusing solution. To better explore the role of the endocannabinoid system in the regulation of DRN 5-HT cells, we tested the effect of the endocannabinoid anandamide (10 µM) and its metabolically stable analogue R-(+) N-(2-hydroxy-1methylethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (methanandamide, Tocris Cookson) (10 µM) on the firing activity of DRN 5-HT neurons. To further analyse the involvement of CB1 receptors in this effect, we superfused the selective CB1 receptor agonist arachidonoyl-2-chloroethylamide (ACEA, Tocris Cookson) (1 µM) and co-perfused anandamide with the CB1 receptor antagonists rimonabant (1 µM) or AM251 (1 µM). As the DRN is enriched with local non-5-HT (presumably GABAergic) neurons that regulate the firing activity of 5-HT neurons (Jolas and Aghajanian, 1997), we also studied the effect of the synthetic CB1/CB2 receptor agonist WIN55212-2 (10 µM) on DRN non-5-HT neurons. Control assays were carried out with the vehicles at their maximal concentrations.
Data analysis and statistical procedures
The effect of each cannabinoid administration on the phenylephrine-driven firing activity was calculated by subtracting the baseline firing rate before drug administration from the rate value at the time of the peak change (i.e. a positive value denotes excitation); this value of effect was then normalized as the percentage of baseline firing rate. To define the threshold for rate changes to be considered as ‘specific drug responses’, we randomly sampled a number of DRN 5-HT cells, then applied the vehicles in which drugs were dissolved [Tocrisolve (Tocris Cookson, Bristol, UK) or dimethyl sulphoxide (DMSO)] and finally calculated the 25th and the 75th percentile values of the control rate changes. Changes in the firing rate outside the range of 25th–75th percentiles of the control group were considered, and the cells were classified as sensitive (i.e. changes lower than the 25th percentile value of the control were considered as inhibitions, whereas those higher than the 75th percentile value were taken as excitations).
In order to analyse the effect of anandamide on 5-HT-induced inhibition, concentration-effect curves were constructed for 5-HT (1–200 µM) before and after anandamide (10 µM). Experimental data from each animal were analysed for the best simple non-linear fit to the logistical three parameters equation E=Emax/{1 + (EC50/A)n}, where E is the effect (change in the baseline firing rate expressed as percentages) induced by (A), Emax is the maximum effect, EC50 is the concentration of the drug that produced the 50% of the Emax, and n is the slope factor of the concentration-effect curve. EC50 and n were estimated using this analysis, and Emax was fixed to 100% as the maximal inhibition was always reached after 5-HT application.
Data are given as mean ± SEM. The firing rates before and after drug superfusions were compared by the paired Student's t-test. Comparisons between data from two independent groups were carried out by a two-sample (unpaired) Student's t-test. To statistically evaluate the sensitivity to cannabinoid drugs, we compared with control the number of sensitive cells in each group by the Fischer's exact test. The computer programmes GraphPad Prism v. 4.00 (GraphPad Software, San Diego, CA, USA) and SPSS v. 14.0. for windows (SPSS Inc, Chicago, IL, USA) were used for statistical evaluation. The level of significance was considered as P= 0.05.
Materials
ACEA, AM251, anandamide and methanandamide were purchased from Tocris Cookson. Rimonabant was generously provided by Sanofi-Synthélabo Recherche. Phenylephrine hydrochloride was obtained from Research Biochemical International. 5-HT, WIN55212-2, 8-OH-DPAT and picrotoxin were purchased from Sigma.
Stock solutions of WIN55212-2, AM251 and rimonabant were prepared in DMSO, those of ACEA in ethanol, and those of 5-HT and 8-OH-DPAT in distilled water. Stock solutions were diluted in ACSF to their final concentration, just before each application. WIN55212-2 dilutions were made in ACSF containing 1 g·L−1 bovine serum albumin to decrease cannabinoid adsorption to the superfusion system. The final concentrations of DMSO, ethanol and Tocrisolve in the superfusion fluid were 0.01, 0.01 and 0.07% respectively. Solutions of methanandamide and anandamide, as provided by Tocris Cookson (emulsion of Tocrisolve or anhydrous ethanol 5 mg·mL−1), were directly added to the ACSF. Picrotoxin and phenylephrine were also directly dissolved in ACSF.
Results
Effect of the cannabinoid receptor antagonists rimonabant and AM251 on the firing activity of DRN 5-HT cells
Previous electrophysiological studies have shown that the FAAH inhibitor URB597 and the CB1/CB2 cannabinoid receptor agonist WIN55212-2 stimulate DRN 5-HT cells in vivo (Gobbi et al., 2005; Bambico et al., 2007). To explore whether there is a role for the endocannabinoid system in the tonic regulation of the firing rate of DRN 5-HT cells, we superfused the selective CB1 receptor antagonists rimonabant and AM251, or the vehicle in which they were dissolved, for at least 5 min. In 18 recorded cells in which the vehicle was applied, we observed both excitatory and inhibitory responses, with a maximal excitation of 49% and a maximal inhibition of 5%. We used the 25th and 75th percentile values of the sampled changes caused by the vehicle (−0.5 and 8.5%, respectively) to establish the limits of the inhibitory and excitatory effects, respectively, and therefore to discriminate sensitive from non-sensitive cells (see Table 1 for the averages of excitation and inhibition in sensitive cells). Superfusion with rimonabant (1 µM) or AM251 (1 µM) induced an inhibitory response in 43 and 50%, respectively, of all the recorded DRN 5-HT neurons, and an excitatory effect in 43 and 42% of DRN 5-HT neurons (n= 21 and n= 12 respectively). However, only the inhibitory effects induced by these antagonists were statistically greater than those caused by the vehicle (rimonabant: 53 ± 13%, P < 0.005; AM251: 62 ± 15%, P < 0.05; vs. vehicle) (Table 1) (Figure 1A,B,D). DRN 5-HT cells responding with excitations, inhibitions or without a response did not differ in their anatomical location within the DRN. However, administration of the selective CB1 receptor agonist ACEA (1 µM, 10 min) induced a slight increase (11 ± 4%) in the firing rate of DRN 5-HT cells (in the presence of the vehicle, before ACEA: 1.02 ± 0.12 Hz; after ACEA: 1.14 ± 0.13 Hz; n= 11; P < 0.05) (Figure 1C). Therefore, these results indicate that CB1 receptor antagonism reduces the firing activity of a number of DRN 5-HT cells, whereas CB1 receptor activation only slightly increases the activity of these neurons, which suggests the existence of a tonic/constitutive excitatory regulation of DRN 5-HT cells by the endocannabinoid system.
Table 1.
Effects of the CB1 receptor antagonists rimonabant and AM251, or the endocannabinoid anandamide on the firing activity of DRN 5-HT cells
|
Inhibitory effect |
Excitatory effect |
|||
|---|---|---|---|---|
| Number of sensitive/total cells | Effect in sensitive cells (%) | Number of sensitive/total cells | Effect in sensitive cells (%) | |
| Antagonists | ||||
| Vehicle (DMSO 0.01%) | 5/18 | 3.1 ± 0.9 | 5/18 | 26.2 ± 7.2 |
| Rimonabant 1 µM | 9/21 | 53.1 ± 12.6** | 9/21 | 30.6 ± 5.2 |
| AM251 1 µM | 6/12 | 61.7 ± 15.1* | 5/12 | 16.6 ± 2.4 |
| Endogenous agonists | ||||
| Vehicle (Tocrisolve 0.07%) | 9/34 | 10.6 ± 2.2 | 9/34 | 37.5 ± 7.6 |
| Anandamide 10 µM | 24/47† | 43.3 ± 7.4** | 7/47 | 22.3 ± 0.7 |
Data are expressed as mean ± SEM of the percentage of inhibition or excitation induced by the drugs or vehicles in sensitive cells (limits: 25th and 75th percentiles of the vehicle effect).
P < 0.05,
P < 0.005 compared with the corresponding effect in the vehicle group by the two-sample Student's t-test.
P < 0.05 compared with the number of sensitive cells in the vehicle group by the Fischer's exact test.
5-HT, 5-hydroxytryptamine; AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; anandamide, N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide; DMSO, dimethyl sulphoxide; DRN, dorsal raphe nucleus; rimonabant, SR141716A.
Figure 1.
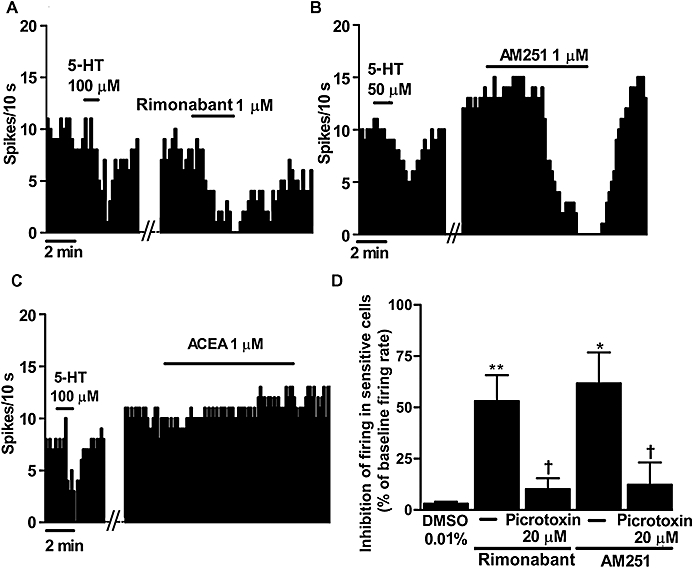
(A,B) Representative examples of firing rate recordings from three dorsal raphe nucleus (DRN) 5-hydroxytryptamine (5-HT) neurons, which show the inhibition of the firing activity of these neurons by the CB1 receptor antagonists SR141716A (rimonabant) (1 µM) (A) or N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) (1 µM) (B) and the slight increase of the 5-HT cell firing activity by the selective CB1 receptor agonist arachidonoyl-2-chloroethylamide (ACEA) (1 µM) (C). The vertical lines refer to the integrated firing rate values (spikes per 10 s), and the horizontal lines represent the time scale. Drugs were superfused at the concentration and for the time indicated by the horizontal bars; ACEA was applied in the continuous presence of the vehicle. (D) Bar histograms showing the inhibition of the firing activity of sensitive cells (mean ± SEM) from slices superfused with the vehicle (n= 5), rimonabant (1 µM) (n= 9), rimonabant (1 µM) + picrotoxin (20 µM) (n= 5), AM251 (1 µM) (n= 6) and AM251 (1 µM) + picrotoxin (20 µM) (n= 3). *P < 0.05, **P < 0.005 compared with the vehicle [dimethyl sulphoxide (DMSO)] group, and †P < 0.05 compared with the corresponding value in the absence of picrotoxin by Student's t-test. Note that both CB1 receptor antagonists decrease the firing activity of DRN 5-HT cells, which is blocked by picrotoxin.
Involvement of the GABAergic system in the inhibitory effect of rimonabant and AM251
GABA has been involved in the inhibitory regulation of 5-HT cells in the DRN (Jolas and Aghajanian, 1997). Therefore, we examined the involvement of GABA in rimonabant- and AM251-induced inhibition by superfusing the GABAA receptor antagonist picrotoxin from the beginning of the experiment. The firing rate of DRN 5-HT cells during picrotoxin (20 µM) application did not differ from that in the control (firing rate, control: 1.38 ± 0.18 Hz, n= 15; picrotoxin: 1.23 ± 0.21 Hz, n= 8). In the presence of picrotoxin (20 µM), bath application of rimonabant (1 µM) or AM251 (1 µM) decreased the firing activity in 36 and 23%, respectively, of the recorded DRN 5-HT cells, although the magnitudes of these responses were about 80% smaller (inhibition, rimonabant: 10 ± 5%, P < 0.05; AM251: 12 ± 11%, P < 0.05) than in the absence of picrotoxin (see above) (Figures 1D,2A,B). Furthermore, picrotoxin (20 µM) was able to increase the firing rate of DRN 5-HT neurons, and thus, restore their initial activity in two cells that had been previously inhibited by AM251 (1 µM) administration (n= 2 cells) (Figure 2C). These results indicate that the inhibitory effect of these two CB1 receptor antagonists is blocked by GABAA receptor antagonism.
Figure 2.
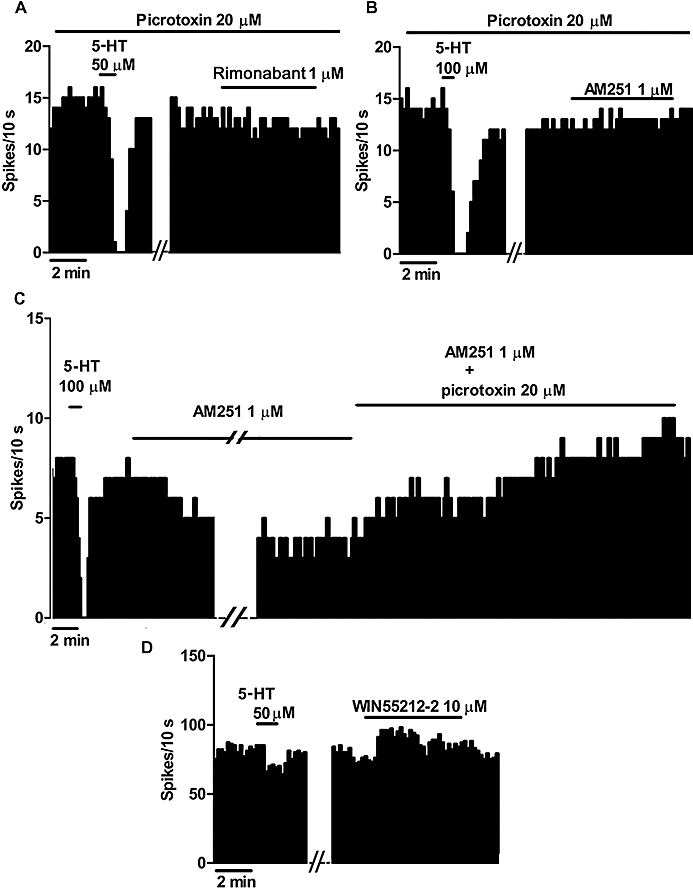
(A,B) Representative examples of firing rate recordings from two 5-hydroxytryptamine (5-HT) neurons in the dorsal raphe nucleus (DRN), which depict the effect of SR141716A (rimonabant) (1 µM) (A) and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) (1 µM) (B) in the presence of picrotoxin (20 µM). Note the lack of effect of both rimonabant and AM251 on the firing rate of DRN 5-HT cells in the presence of picrotoxin. (C) Representative example of a firing rate recording from a DRN 5-HT neuron, showing that picrotoxin (20 µM) reversed the inhibitory effect induced by AM251 (1 µM). (D) Representative example of a firing rate recording from a DRN non-5-HT neuron, showing the lack of effect of R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)-methyl]pyrrolol[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt (WIN55212-2) (10 µM). The vertical lines refer to the integrated firing rate values (spikes per 10 s), and the horizontal lines represent the time scale. Drugs were bath applied at the concentration and for the time indicated by the horizontal bars.
It is known that the DRN is enriched with local GABAergic neurons that regulate the firing activity of 5-HT neurons (Jolas and Aghajanian, 1997), and recently, the presence of CB1 receptors has been reported in non-5-HT cells of the DRN (Häring et al., 2007). Therefore, we examined the effect of the CB1/CB2 receptor agonist WIN55212-2 on the firing activity of these cells. As illustrated in Figure 2D, superfusion with WIN55212-2 (10 µM) for 20 min failed to modify the firing rate of non-5-HT cells (firing rate, before and after WIN55212-2: 5.65 ± 0.94 Hz and 5.06 ± 1.07 Hz, respectively; n= 7; paired Student's t-test, P= 0.22). These data do not support a modulation of the firing activity of non-5-HT (presumably GABAergic) neurons by cannabinoid agonists.
Effect of anandamide and methanandamide on the firing activity of DRN 5-HT cells
To further explore the role of the endocannabinoid system in the regulation of the firing activity of DRN 5-HT cells, we superfused the endocannabinoid anandamide or its vehicle (Tocrisolve) for 10 min. In these assays, the maximal inhibition of the firing activity induced by the vehicle was 11 ± 2%, whereas the maximal increase of the firing rate was 38 ± 8% (n= 34). As mentioned above, we used the 25th and 75th percentile values of the sampled changes by the vehicle (−1.5 and 18.5%, respectively) to set the limits of the inhibitory and excitatory effects respectively (Table 1). Superfusion with anandamide (10 µM) reduced the firing rate in 51% of the recorded 5-HT neurons in the DRN (n= 47) (Figure 3A), which was larger than the number of inhibited cells by the vehicle (P < 0.05) (Table 1). Accordingly, the magnitude of the inhibitory effect induced by anandamide in sensitive cells was stronger (43 ± 7%, P < 0.005) than the effect of its vehicle (Figure 3A,D) (Table 1). Anandamide increased the firing activity in 15% of all the recorded DRN 5-HT cells, but the percentage of cells excited and the magnitude of the excitatory effect were not different from those in the vehicle group (Table 1). However, in the continuous presence of rimonabant (1 µM), anandamide (10 µM) caused an inhibition in 33% of all the recorded DRN 5-HT cells; paradoxically, this effect was greater than that in the absence of the antagonist (anandamide inhibition during rimonabant superfusion: 76 ± 12%, n= 3; P < 0.05) (Figure 3C,D). Likewise, AM251 (1 µM) failed to modify the number of sensitive cells or the magnitude of the inhibitory effect of anandamide (10 µM) (data not shown). In order to further understand the mechanism of the inhibition induced by anandamide in the DRN, we applied methanandamide (10 µM), which is a more selective compound for CB1 receptors and is metabolically more stable than anandamide. Superfusion with methanandamide (10 µM) did not change the firing rate of DRN 5-HT neurons in any of the recorded cells (before methanandamide: 1.45 ± 0.15 Hz; after methanandamide: 1.48 ± 0.16 Hz; n= 12) (Figure 3B). Taken together, these results suggest that anandamide inhibits DRN 5-HT cells by a mechanism other than interaction with CB1 receptors.
Figure 3.
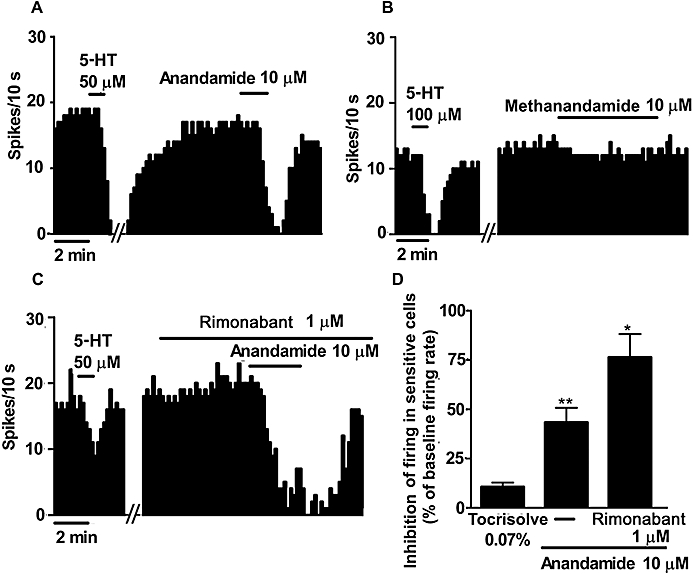
(A–C) Representative examples of firing rate recordings from three sensitive 5-hydroxytryptamine (5-HT) neurons in the dorsal raphe nucleus (DRN), illustrating the inhibitory effect of N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (anandamide) (10 µM) (A), the lack of effect of R-(+) N-(2-hydroxy-1methylethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (methanandamide) (10 µM) (B) and the effect of anandamide (10 µM) in the presence of SR141716A (rimonabant) (1 µM) (C) on the firing activity. The vertical lines refer to the integrated firing rate values (spikes per 10 s), and the horizontal lines represent the time scale. Drugs were superfused at the concentration and for the time shown by the horizontal bars. (D) Bar histograms showing the inhibition of the firing activity in sensitive 5-HT cells (mean ± SEM) in slices superfused with the vehicle (n= 9), anandamide (10 µM) (n= 24) and anandamide (10 µM) + rimonabant (1 µM) (n= 3). *P < 0.05, **P < 0.005 compared with the vehicle group by a two-sample (unpaired) Student's t-test. Note that anandamide decreased the firing activity of DRN 5-HT cells but methanandamide failed to change this activity (paired Student's t-test, P= 0.41). The inhibitory effect of anandamide was not blocked by rimonabant.
It has been shown that anandamide potentiates certain 5-HT1A receptor-mediated effects of 5-HT (Boger et al., 1998). Therefore, we explored the possible regulation of 5-HT1A receptors by constructing concentration-effect curves for 5-HT (1–200 µM) in the absence and in the presence of anandamide (10 µM). As expected, superfusion with several concentrations of 5-HT (1–200 µM) inhibited the firing activity of DRN 5-HT cells in a concentration-dependent manner (Figure 4A) (Emax= 100%; EC50= 46.4 ± 17.2 µM; n= 6). As shown in Figure 4B, superfusion with anandamide (10 µM) failed to change the potency (EC50) or the efficacy (Emax) of 5-HT to inhibit DRN 5-HT neurons (in the presence of anandamide, Emax= 100%; EC50= 36.8 ± 14.8 µM; n= 6).
Figure 4.
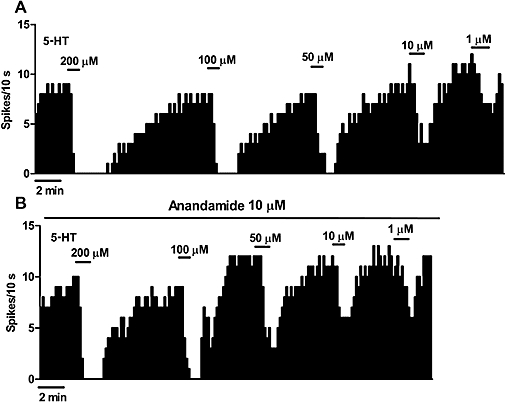
(A,B) Representative examples of firing rate recordings from two dorsal raphe nucleus 5-hydroxytryptamine (5-HT) neurons, which illustrate the inhibitory effect of several concentrations of 5-HT (1–200 µM) in the absence (A) and in the presence (B) of N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (anandamide) (10 µM). The vertical lines refer to the integrated firing rate values (spikes per 10 s), and the horizontal lines represent the time scale. Drugs were superfused at the concentration and for the time indicated by the horizontal bars. Note that anandamide did not change the inhibitory effect of 5-HT.
Discussion and conclusions
The present work was carried out to investigate the role of the endocannabinoid system in the tonic regulation of the firing activity of DRN neurons. Our results revealed that the selective CB1 receptor antagonists rimonabant and AM251 inhibited a proportion of DRN 5-HT cells by a mechanism that was prevented and also reversed by the GABAA receptor antagonist picrotoxin. The study also showed that anandamide inhibited a number of DRN 5-HT cells. However, this effect was not prevented by rimonabant or AM251, and methanandamide, a more stable CB1 selective compound, did not affect the firing activity of DRN 5-HT cells. The selective CB1 receptor agonist ACEA only slightly increased the firing rate of DRN 5-HT neurons.
We chose the synthetic CB1 receptor antagonists rimonabant and AM251 in view of their selectivity and affinity at CBl receptors (i.e. 150- to 300-fold more selective for CB1 than CB2 receptors) and their ability to block the effects mediated by CB1 receptors in vivo and in vitro (Howlett et al., 2002). In the present study, we found that rimonabant and AM251 inhibited the firing rate in about a half of all the recorded DRN 5-HT cells. The fact that two separate CB1 receptor antagonists inhibit the firing activity of DRN 5-HT cells argues against the involvement of non-specific mechanisms such as the blockade of voltage-sensitive Na+ channels or vanilloid receptor antagonism (Pertwee, 2005). Moreover, non-CB1 receptor mechanisms have been described to occur in vitro at higher concentrations than those used in our study (>1 µM) (De Petrocellis et al., 2001; Liao et al., 2004). Given that the CB1 receptor agonist ACEA slightly increased the firing rate of DRN 5-HT cells in vitro (see above) and the CB1/CB2 receptor agonist WIN55212-2 stimulates the firing activity of these neurons in vivo (Bambico et al., 2007), we can speculate that the inhibitory effect of rimonabant and AM251 on DRN 5-HT cells results from disruption of an excitatory endogenous/constitutive tonus of CB1 cannabinoid receptors on 5-HT cells.
Cannabinoids regulate GABA and glutamate release in various brain regions via presynaptic CB1 receptors (Howlett et al., 2002; Szabo and Schlicker, 2005). A major subpopulation of non-5-HT cells in the DRN is GABAergic (Piñeyro and Blier, 1999), which regulates the firing activity of 5-HT cells (Jolas and Aghajanian, 1997). A recent in situ hybridization study has reported the presence of CB1 receptor mRNA in DRN non-5-HT (purportedly GABAergic) cells (Häring et al., 2007). In our study, superfusion with the GABAA receptor antagonist picrotoxin prevented the inhibitory effect of rimonabant and AM251 on DRN 5-HT cells. Moreover, picrotoxin was able to fully reverse the inhibition of the firing activity induced by AM251 (1 µM) in DRN 5-HT neurons. These results suggest that the CB1 receptor antagonist-induced effect may be indirectly mediated by GABA release and GABAA receptors. However, in our study, the synthetic CB1/CB2 receptor agonist WIN55212-2 failed to alter the discharging activity of non-5-HT (presumably GABAergic) cells. This observation would not support a direct modulation of GABAergic neurons by cannabinoids at somatodendritic levels. One possibility that should be further explored is that cannabinoid antagonists inhibit 5-HT neurons by acting at GABAergic terminals, so that CB1 receptor antagonists would relieve the tonic inhibitory regulation exerted by presynaptic CB1 receptors on GABA release. In agreement, Egashira et al. (2002) have postulated that CB1 receptors affect 5-HT neurotransmission through regulation of the GABAergic system. However, our results do not rule out the possibility that somatodendritic CB1 receptors on GABAergic neurons could be occluded by high concentrations of endocannabinoids, which would prevent the effect of WIN55212-2 on these cells. Additionally, glutamate-related mechanisms may be involved in the stimulatory effect of cannabinoids on DRN 5-HT cells in vivo, as suggested by Bambico et al. (2007).
One of the best characterized endocannabinoids activating the CB1 receptor in the brain is anandamide (Fride and Mechoulam, 2003). The DRN has been shown to contain anandamide and the FAAH, the enzyme responsible for its degradation (Egertova et al., 2003; Palazzo et al., 2006). In our experiments, anandamide induced an inhibition of DRN 5-HT cells, although this effect was not dependent upon CB1 receptor activation because rimonabant and AM251 did not block it. The fact that the metabolically stable CB1 receptor agonist methanandamide (Thakur et al., 2005) failed to inhibit DRN 5-HT cells further suggests that the inhibitory effect of anandamide may be mediated by non-CB1 receptor mechanisms (e.g. cationic channels or non-CB1/CB2 receptors; Di Marzo et al., 2002; Nicholson et al., 2003) or by a degradation product (i.e. arachidonic acid or a further metabolite). Furthermore, the slight effect of ACEA on DRN 5-HT cells seen in our in vitro study (see above) and the lack of effect of the CB1/CB2 receptor agonist WIN55212-2 previously reported in slice preparations (Haj-Dahmane and Shen, 2005) point out that the endocannabinoid system may be maximally activated in the DRN in vitro. It has been shown that anandamide potentiates 5-HT1A receptor-mediated effects (Boger et al., 1998). However, we can rule out a direct enhancement of 5-HT1A receptors by anandamide in the DRN as it failed to change the concentration-effect curves for 5-HT.
In conclusion, the present work demonstrates that the CB1 receptor antagonists rimonabant and AM251 reduce the firing activity of a proportion of DRN 5-HT cells in vitro through a mechanism blocked by the GABAA receptor antagonist picrotoxin. The CB1 receptor agonist ACEA only slightly activates DRN 5-HT neurons. The endocannabinoid anandamide inhibits the firing activity of DRN 5-HT cells, but this effect is unlikely to be mediated by CB1 receptors or 5-HT1A receptors. Our data suggest that CB1 receptor antagonists may prevent the tonic/constitutive inhibition of the GABAergic transmission by an endocannabinoid system, thereby enhancing the inhibitory control of GABA on 5-HT neurons. The presence of an endocannabinoid system in the DRN may potentially explain certain behavioural effects elicited by cannabinoids, such as hypothermia, analgesia, impairment of memory, catalepsy and anxiety-related responses, which have been all shown to be mediated by the 5-HT system (Martin et al., 1995; Malone and Taylor, 2001; Egashira et al., 2002; 2006; Witkin et al., 2005). However, the endocannabinoid responsible for the regulation of DRN 5-HT cells remains to be identified. Indeed, as rimonabant and AM251 have been shown to be inverse agonists, we cannot state at the moment whether an endocannabinoid plays a role in the DRN or, alternatively, CB1 receptors are constitutively active in this nucleus. Finally, our study does not preclude the possibility that the endocannabinoid system could also modulate in vivo the 5-HT neurotransmission outside the DRN by a local activation of presynaptic inhibitory CB1 receptors onto 5-HT nerve terminals (Nakazi et al., 2000; Egashira et al., 2002; Moranta et al., 2004; Sagredo et al., 2006) or glutamatergic terminals (Bambico et al., 2007).
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia y Tecnología (SAF2008-03612), Ministerio de Salud y Consumo (MSC-FIS) (RTA G03/005 and PI05/0513), Basque Government (PE04UN12), University of the Basque Country (GIU07/46) and Plan Nacional sobre Drogas (PND-MSC 2005). A.M. was supported by predoctoral fellowships from the Ministerio de Educación y Cultura and UPV/EHU (Euskal Herriko Unibertsitatea). We thank Sanofi-Synthélabo for generously providing rimonabant.
Glossary
Abbreviations:
- 8-OH-DPAT
(±)-8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide
- ACEA
arachidonoyl-2-chloroethylamide
- ACSF
artificial cerebrospinal fluid
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- DMSO
dimethyl sulphoxide
- DRN
dorsal raphe nucleus
- FAAH
fatty acid amide hydrolase
- rimonabant
SR141716A
- URB597
[3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate
- WIN55212-2
R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)-methyl]pyrrolol[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt
Conflict of interest
None.
References
- Aghajanian GK, Lakoski JM. Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res. 1984;305:181–185. doi: 10.1016/0006-8993(84)91137-5. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Patterson JE, Jin Q. Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biologically active lipid oleamide. Proc Natl Acad Sci USA. 1998;95:4102–4107. doi: 10.1073/pnas.95.8.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Limonta V, Malabarba L, Zani A, Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur J Pharmacol. 2007;555:156–163. doi: 10.1016/j.ejphar.2006.10.038. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essent Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Katsurabayashi S, Yoshitake T, Matsumoto Y, Ishida J, et al. Involvement of 5-hydroxytryptamine neuronal system in Delta9-tetrahydrocannabinol-induced impairment of spatial memory. Eur J Pharmacol. 2002;445:221–229. doi: 10.1016/s0014-2999(02)01755-7. [DOI] [PubMed] [Google Scholar]
- Egashira N, Matsuda T, Koushi E, Mishima K, Iwasaki K, Shoyama Y, et al. Involvement of 5-hydroxytryptamine1A receptors in Delta9-tetrahydrocannabinol-induced catalepsy-like immobilization in mice. Eur J Pharmacol. 2006;550:117–122. doi: 10.1016/j.ejphar.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and CB1 cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Fride E, Mechoulam R. New advances in the identification and physiological roles of the components of the endogenous cannabinoid system. In: Maldonado R, editor. Molecular Biology of Drug Addiction. Totowa, NJ: Humana Express; 2003. pp. 173–197. [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Sharp T. Burst-firing activity of presumed 5-HT neurones of the rat dorsal raphe nucleus: electrophysiological analysis by antidromic stimulation. Brain Res. 1996;740:162–168. doi: 10.1016/s0006-8993(96)00869-4. [DOI] [PubMed] [Google Scholar]
- Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Zheng J, David LS, Nicholson RA. Inhibition of voltage-sensitive sodium channels by the cannabinoid 1 receptor antagonist AM 251 in mammalian brain. Basic Clin Pharmacol Toxicol. 2004;94:73–78. doi: 10.1111/j.1742-7843.2004.pto940204.x. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Involvement of somatodendritic 5-HT1A receptors in Delta9-tetrahydrocannabinol-induced hypothermia in the rat. Pharmacol Biochem Behav. 2001;69:595–601. doi: 10.1016/s0091-3057(01)00567-6. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Schnell SA, Hack SP, Christie MJ, Wessendorf MW, Vaughan CW. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol. 2004;92:3532–3537. doi: 10.1152/jn.00437.2004. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB1 cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–625. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Differential effects of acute cannabinoid drug treatment, mediated by CB1 receptors, on the in vivo activity of tyrosine and tryptophan hydroxylase in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:516–524. doi: 10.1007/s00210-004-0921-x. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodríguez E, Vázquez E, Millán-Aldaco D, Palomero-Rivero M, Drucker-Colin R. Effects of the fatty acid amide hydrolase inhibitor URB597 on the sleep-wake cycle, c-Fos expression and dopamine levels of the rat. Eur J Pharmacol. 2007;562:82–91. doi: 10.1016/j.ejphar.2007.01.076. [DOI] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, et al. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978:194–204. doi: 10.1016/s0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- Palazzo E, De Novellis V, Petrosino S, Marabese I, Vita D, Giordano C, et al. Neuropathic pain and the endocannabinoid system in the dorsal raphe: pharmacological treatment and interactions with the serotonergic system. Eur J Neurosci. 2006;24:2011–2020. doi: 10.1111/j.1460-9568.2006.05086.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- Sagredo O, Ramos JA, Fernandez-Ruiz J, Rodríguez ML, de Miguel R. Chronic Delta9-tetrahydrocannabinol administration affects serotonin levels in the rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:313–317. doi: 10.1007/s00210-005-0026-1. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. In: Pertwee RG, editor. Cannabinoids, Handbook of Experimental Pharmacology. Berlin: Springer; 2005. pp. 327–365. Vol. 168. [DOI] [PubMed] [Google Scholar]
- Thakur GA, Nikas SP, Li P, Makriyannis A. Structural requirements for cannabinoid receptor probes. In: Pertwee RG, editor. Cannabinoids, Handbook of Experimental Pharmacology. Berlin: Springer; 2005. pp. 209–246. Vol. 168. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GGA. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]


