Abstract
Background and purpose:
The transient receptor potential ankyrin receptor 1 (TRPA1) is a cation channel, co-expressed with the pro-tussive transient receptor potential vanilloid type 1 (TRPV1) channel in primary sensory neurons. TRPA1 is activated by a series of irritant exogenous and endogenous α,β-unsaturated aldehydes which seem to play a role in airway diseases. We investigated whether TRPA1 agonists provoke cough in guinea pigs and whether TRPA1 antagonists inhibit this response.
Experimental approach:
Animals were placed in a Perspex box, and cough sounds were recorded and counted by observers unaware of the treatment used.
Key results:
Inhalation of two selective TRPA1 agonists, allyl isothiocyanate and cinnamaldehyde, dose-dependently caused cough in control guinea pigs, but not in those with airway sensory nerves desensitized by capsaicin. Coughs elicited by TRPA1 agonists were reduced by non-selective (camphor and gentamicin) and selective (HC-030031) TRPA1 antagonists, whereas they were unaffected by the TRPV1 antagonist, capsazepine. Acrolein and crotonaldehyde, two α,β-unsaturated aldehydes recently identified as TRPA1 stimulants and contained in cigarette smoke, air pollution or produced endogenously by oxidative stress, caused a remarkable tussive effect, a response that was selectively inhibited by HC-030031. Part of the cough response induced by cigarette smoke inhalation was inhibited by HC-030031, suggesting the involvement of TRPA1.
Conclusions and implications:
A novel pro-tussive pathway involves the TRPA1 channel, expressed by capsaicin-sensitive airway sensory nerves and is activated by a series of exogenous (cigarette smoke) and endogenous irritants. These results suggest TRPA1 may be a novel target for anti-tussive medicines.
Keywords: cough, TRP ankyrin 1 (TRPA1) channel, cigarette smoke, allyl isothiocyanate, cinnamaldehyde, acrolein, crotonaldehyde
Introduction
Cough, activated by a variety of potentially harmful stimuli, is initiated by activation of myelinated Aδ-fibres with a contribution of non-myelinated C-fibres. Tussive stimuli act via multiple mechanisms, as different irritants may target various membrane receptors or ionic channels expressed in the plasma membrane of sensory neurons innervating both the upper and lower airways. These include the transient receptor potential vanilloid type 1 (TRPV1) (Anderson, 2004; Adcock, 2009; Materazzi et al., 2009); nomenclature follows Alexander et al., 2008), which is a non-selective cation channel belonging to a larger mammalian superfamily of 28 TRP ion channels (Caterina et al., 1999; Nilius, 2007). TRPV1 is activated by capsaicin, the hot principle contained in the plants of the genus Capsicum, and by the ultrapotent agonist, resiniferatoxin (RTX) (Szallasi and Blumberg, 1989), noxious heat, low extracellular pH and certain lipid derivatives (Bevan and Geppetti, 1994; Hwang et al., 2000). Capsaicin is one of the commonest stimuli for cough provocation test in experimental animals (Lalloo et al., 1995; Trevisani et al., 2004) and in humans (Collier and Fuller, 1984; Laude et al., 1993), and sensitivity to capsaicin-evoked cough is increased in a variety of respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD) (Wong and Morice, 1999; Doherty et al., 2000). Thus, TRPV1 has been suggested to contribute to cough generation in different pathological conditions, and is, thereby, considered as a major target for novel anti-tussive drugs.
In addition to TRPV1, other excitatory TRP channels are expressed on terminals of primary sensory neurons, including TRPV2, TRPV3 and TRPV4 channels, which are gated by warm, non-noxious and noxious temperatures, and small reductions in tonicity, respectively (Nilius, 2007). More recently, the TRP ankyrin 1 (TRPA1) channel which seems to act as a sensor of oxidative stress (Trevisani et al., 2007; Andersson et al., 2008; Bessac et al., 2008; Materazzi et al., 2008; Taylor-Clark et al., 2009a) has been identified as being co-expressed with TRPV1 in sensory neurons (Story et al., 2003; Nagata et al., 2005). All these TRP channels could, in principle, trigger the cough reflex, and this hypothesis may be tested by using selective channel agonists and antagonists.
A series of isothiocyanates and thiosulphinate compounds and cinnamaldehyde, which are the pungent ingredients found in mustard and cinnamon, respectively, have recently been recognized as selective TRPA1 stimulators (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2006). In addition, acrolein (Bautista et al., 2006) and crotonaldehyde (Andre et al., 2008), two α,β-unsaturated aldehydes that are contained in cigarette smoke (Facchinetti et al., 2007) and contribute to environmental pollution, or are produced by lipid peroxidation that follows oxidative stress (Aldini et al., 2007) have been shown to selectively stimulate TRPA1. Recently, the first selective TRPA1 antagonist has been reported (McNamara et al., 2007).
Because TRPA1 receptors are exclusively expressed in a subpopulation of TRPV1-expressing neurons (Story et al., 2003; Nagata et al., 2005), we reasoned that activation of these neurons by TRPA1 activators might evoke cough in a manner similar to that which follows TRPV1 stimulation. To test this hypothesis, guinea pigs were challenged with a series of TRPA1 agonists and antagonists.
Methods
Animals
All animal care and experimental procedures complied with the national guidelines and were approved by the regional ethics committee. Male Dunkin–Hartley guinea pigs (250–350 g, Pampaloni, Pisa, Italy) were acclimatized in cages (24 ± 0.5°C) for 1 week before the beginning of the experiments, and were allowed free access to water and standard rodent diet (Morini, Reggio Emilia, Italy).
Experimental set-up
After a period of acclimatization to laboratory conditions, the animals were individually placed in a transparent Perspex box (20 × 10 × 10 cm, Vetrotecnica, Padova, Italy) ventilated with a constant airflow of 400 mL·min−1. All compounds tested were nebulized via a mini-ultrasonic nebulizer (model 2511; PulmoSonic, DeVilbiss, Somerset, PA, USA). The particle size produced had an aerodynamic mass median diameter of 0.9 µM, and the output of the nebulizer was 0.4 mL·min−1. Waves of cough sounds were monitored and recorded through a microphone (Sony, Tokyo, Japan) connected to a personal computer and analysed by an appropriate software during the 10 min exposure to the various stimuli. By recording the waves of cough sounds, we could differentiate true cough from sneezing. The number of coughs were also confirmed by the characteristic posture of the animal, and counted by observers unaware of the treatment used.
Study protocols
All experiments were carried out at the same time: 0900 h.
All the agonists and antagonists used in the present study were administered by aerosol. The ability of TRPA1 agonists to elicit cough was determined by exposing guinea pigs to an aerosol of allyl isothiocyanate (1–30 mM), cinnamaldehyde (10–30 mM), acrolein (10 mM), crotonaldehyde (10 mM) or their vehicles for 10 min (see Figures 1A–3). Non-selective or selective TRPA1 or TRPV1 antagonists were used to prevent cough elicited by the agonists. These included: ruthenium red (3 mM), (+)camphor (1 mM), gentamicin (100 µM), HC-030031 (0.3 mM) and capsazepine (10 µM).
Figure 1.
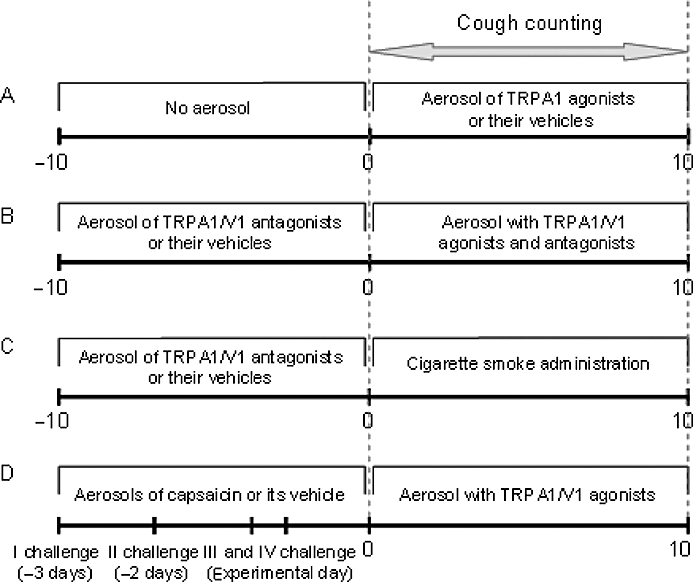
Schematic diagram of the different cough protocols. (A) Dose–response curve with allyl isothiocyanate, cinnamaldehyde or their vehicles. (B) Effect of ruthenium red, gentamicin, (+)camphor, HC-030031 and capsazepine on TRPA1/V1 agonist-induced cough. (C) Effect of ruthenium red, (+)camphor, HC-030031 and capsazepine on cigarette smoke-induced cough. (D) Effect of capsaicin desensitization on TRPA1/V1 agonist-induced cough.
Figure 3.

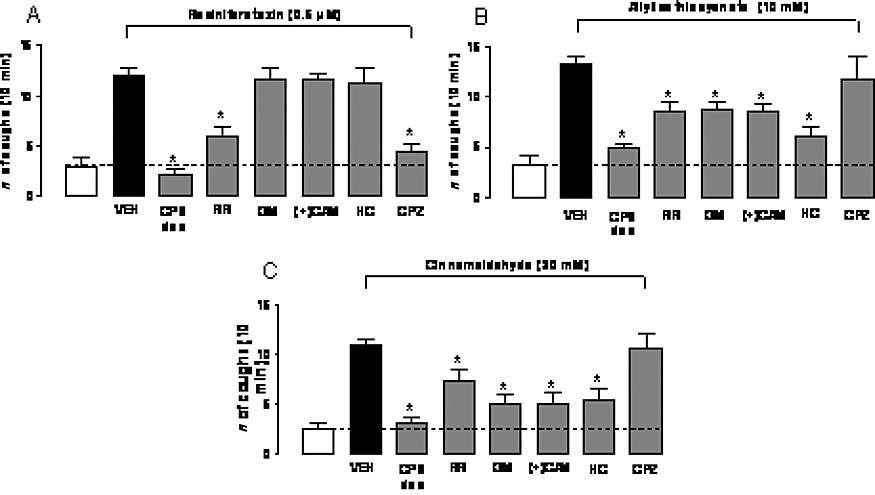
Effect of aerosolized ruthenium red (RR, 3 mM), gentamicin (GM, 100 µM), (+)camphor [(+)CAM, 1 mM], HC-030031 (HC, 0.3 mM), capsazepine (CPZ, 10 µM) or desensitization of guinea pig airways by repeated capsaicin administration (CPS des, 30 µM) on (A) allyl isothiocyanate- (10 mM), (B) cinnamaldehyde- (30 mM) or (C) resiniferatoxin-induced cough in guinea pig. Open bars represent the response induced by the vehicle of the tussive stimulus (5% ethanol and 3.5% Tween-80 in isotonic saline). VEH indicates results obtained by the stimuli after pretreatment with 6% dimethyl sulphoxide and 3.5% Tween-80 in isotonic saline. *P < 0.05, analysis of variance and Bonferroni's test versus vehicle (VEH). Each column is presented as mean ± SE of at least six experiments.
In preliminary experiments, we found that 10 µM capsazepine aerosolized 10 min before the initiation of the agonist challenge and aerosolized for additional 10 min together with the agonist, selectively reduced the cough response evoked by TRPV1 stimulation. Thus, this same procedure was adopted in all the experiments in which we used capsazepine and all the other antagonists. Practically, in the present study, guinea pigs were exposed to the aerosolized antagonist for 10 min, and immediately after (∼1 min) an aerosol containing the antagonist and the agonist was delivered for an additional 10 min (Figure 1B). We would like to emphasize that the antagonist administration procedure described was different from that used in our previous publication (Trevisani et al., 2004). In that study, capsazepine was administered only once to guinea pigs, 10 min prior to the cough challenge with capsaicin. The present procedure allowed us to use lower concentrations of all tested antagonists, and appeared to be more selective.
The aerosolized concentrations of capsazepine and ruthenium red [10 µM and 3 mM (∼0.3%), respectively] were chosen on the basis of their activity as reported in two previous studies (Bolser et al., 1991; Gatti et al., 2006). Aerosolized concentrations of gentamicin, (+)camphor and HC-030031 were selected on the basis of their solubility in isotonic saline, dimethyl sulphoxide (DMSO) and DMSO plus Tween-80 respectively. Thus, the highest concentrations (100 µM for gentamicin, 1 mM for (+)camphor and 0.3 mM for HC-030031, respectively) obtainable in these solvents were used. To assess selectivity of (+)camphor and gentamicin, these antagonists were also employed against cough induced by RTX (0.5 µM), an ultrapotent and selective agonist of the TRPV1 receptor (Szallasi and Blumberg, 1989) (treatment schedule in Figure 1B). In another set of experiments, guinea pig airways were desensitized to capsaicin according to the following protocol: the animals were exposed for 10 min to aerosolized capsaicin (30 µM) once a day, for three consecutive days. Two hours after the last capsaicin administration (at day 3), an additional capsaicin challenge (30 µM for 10 min) was performed (see schedule in Figure 1D). Absence of cough response to this last challenge indicated desensitization to capsaicin. In order to assess whether allyl isothiocyanate (10 mM), cinnamaldehyde (30 mM) or RTX (0.5 µM) were able to elicit cough in capsaicin-desensitized animals, these agonists were aerosolized for 10 min to animals 30 min after the fourth capsaicin treatment. In preliminary experiments, we confirmed that ruthenium red, (+)camphor, gentamicin, HC-030031 and capsazepine, at the doses used in the present study, were not able to cause any significant cough response (not shown).
In a different set of experiments, room air or cigarette smoke (obtained from the filter end of Marlboro Red cigarettes, 12 mg tar, 0.9 mg nicotine each) was delivered into a Perspex box (20 × 10 × 10 cm, Vetrotecnica) following a method reported previously, with modifications (Matsumoto et al., 1998) (see Figure 1C). Briefly, an exhaust vent was connected to a vacuum pump in order to maintain a constant airflow of 400 mL·min−1 and, in this manner, the smoke was dispersed throughout the chamber. Cigarettes were attached to an inlet hole on the front panel, and lit. One cigarette took 5 min to burn and was then immediately detached from the chamber and substituted with another cigarette. Two cigarettes were lit and ‘smoked’ by each animal. The aerosolized agonists were administered for 10 min, and the number of coughs was counted solely during the smoke administration (Figure 1C). In preliminary experiments, we verified that cigarette smoke, delivered according to the present protocol, produced a number of coughs comparable to that of the various TRPV1 and TRPA1 agonists used in the present study.
Data analysis
Values are presented as mean ± SE. Data are compared using one-way analysis of variance following by Bonferroni's post hoc test. A P value <0.05 was considered significant. A minimum of six guinea pigs were used to test the effect of vehicle or of each single dose of the test compounds.
Materials
Capsaicin, allyl isothiocyanate, trans-cinnamaldehyde, acrolein, crotonaldehyde, RTX, capsazepine, (+)camphor, gentamicin and ruthenium red were all from Sigma-Aldrich, Milan, Italy. HC-030031 [2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-purin-7-yl)-N-(4-isopropylphenyl)-acetamide] was synthesized at the Department of Pharmaceutical Chemistry, University of Ferrara, by reacting theophylline-7-acetic acid with an equimolar amount of 4-isopropylaniline in the presence of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride and N-hydroxybenzotriazole. The structure of the compound was confirmed by H-NMR and mass spectroscopy.
Stock solutions of allyl isothiocyanate (5 M) and cinnamaldehyde (3.5 M) were prepared in 50% ethanol and 50% Tween-80, and maximal tested concentrations (30 mM) contained 5% ethanol and 3.5% Tween-80 in isotonic saline. (+)Camphor (100 mM), capsazepine (1 mM), capsaicin (10 mM) and RTX (1 mM) were prepared in 100% DMSO, and the final tested concentration did not exceed 6% DMSO. HC-030031 (10 mM) was prepared in 100% DMSO, then, 1 mM HC-030031 was prepared in 20% DMSO and 10% Tween-80. Maximal HC-030031 concentration tested (0.3 mM) contained 6% DMSO and 3.5% Tween-80. All the other compounds were prepared in isotonic saline. In preliminary experiments, we did not observe any difference in the cough response induced by TRPA1 and TRPV1 agonists in the absence or presence of a combination of all the vehicles (6% DMSO, 3.5% Tween-80 and isotonic saline) used for delivering the various antagonists. To provide the best control for non-specific effects of the vehicles, in experiments with antagonists, the combination of all the antagonist vehicles was used and labelled as ‘vehicle’.
Results
TRPA1 and TRPV1 agonists evoke cough in guinea pigs
Aerosolized allyl isothiocyanate (1–30 mM) or cinnamaldehyde (10–30 mM) elicited a dose-dependent cough response, whereas their vehicles elicited a minor pro-tussive effect (Figure 2). The ultrapotent TRPV1 agonist RTX (0.5 µM) caused a significant tussive response in guinea pigs, significantly (P < 0.05) exceeding the effect evoked by its vehicle (Figure 4A).
Figure 2.
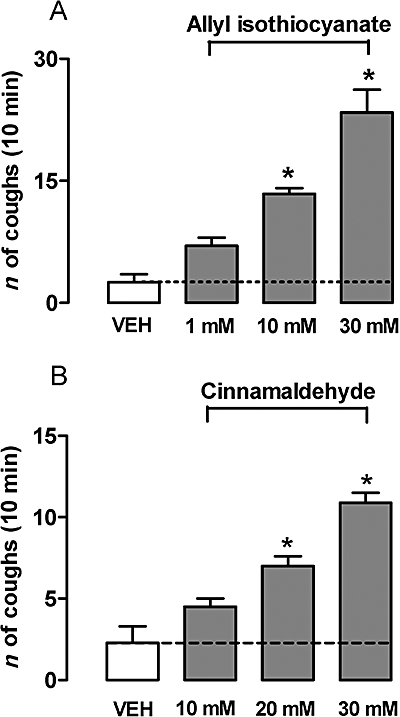
Aerosolized allyl isothiocyanate- (1–30 mM) or cinnamaldehyde- (10–30 mM) induced cough in a concentration-dependent manner. *P < 0.05, analysis of variance and Bonferroni's test versus vehicle (VEH, 5% ethanol and 3.5% Tween-80 in isotonic saline). Each column is presented as mean ± SE of at least six experiments.
Figure 4.
Effect of aerosolized HC-030031 (HC, 0.3 mM) or capsazepine (CPZ, 10 µM) on cough induced by acrolein (10 mM) (A) or crotonaldehyde (10 mM) (B) in guinea pig. Open bars represent the response induced by the vehicle of the tussive stimulus (isotonic saline). VEH indicates results obtained by the stimuli after pretreatment with 6% dimethyl sulphoxide and 3.5% Tween-80. *P < 0.05, analysis of variance and Bonferroni's test versus VEH. Each column is presented as mean ± SE of at least six experiments.
Subsequently, we investigated whether TRPA1 agonists induce cough via stimulation of capsaicin-sensitive sensory neurons. Capsaicin has the unique property to first excite and then desensitize sensory neurons by stimulating TRPV1. Importantly, desensitization to capsaicin results in neuronal insensitivity to capsaicin and to other irritant stimuli (Szallasi and Blumberg, 1999). In animals rendered unresponsive to capsaicin by repeated administration of capsaicin aerosols (see Methods), the response to the selective TRPV1 agonist, RTX, was practically abolished, compared to the response observed in control animals pretreated for three consecutive days with capsaicin vehicle, as expected for a TRPV1 receptor activator (Figure 4A). Because TRPA1 receptors were found to be exclusively present in a subpopulation of TRPV1-expressing neurons (Story et al., 2003), we anticipated that capsaicin desensitization would also produce a lack of response to TRPA1 agonists. Indeed, either allyl isothiocyanate or cinnamaldehyde was totally unable to produce cough responses after capsaicin desensitization (Figure 4B,C).
Secondly, to identify TRPA1 more specifically as the mediator of the tussive action of allyl isothiocyanate and cinnamaldehyde, the animals were pretreated with the non-selective TRP channel blocker, ruthenium red (3 mM), with the poorly selective TRPA1 antagonists (+)camphor (1 mM) and gentamicin (100 µM) or with the selective TRPA1 antagonist, HC-030031 (0.3 mM) before exposing them to the agonists. Another group of animals was pretreated with the TRPV1 selective antagonist, capsazepine. Ruthenium red, gentamicin, (+)camphor and HC-030031 were all able to significantly reduce the number of coughs provoked by aerosols of allyl isothiocyanate (47, 45, 57 and 63% inhibition, respectively, P < 0.05 vs. vehicle) (Figure 4B) and cinnamaldehyde (43, 69, 70 and 67% inhibition, respectively, P < 0.05 vs. vehicle) (Figure 4C). As expected, capsazepine (10 µM) did not affect the cough response evoked by either allyl isothiocyanate or cinnamaldehyde (Figure 4B,C). In contrast, the cough response induced by RTX was significantly inhibited by ruthenium red and capsazepine pretreatments (66 and 84% inhibition, respectively, P < 0.05 vs. vehicle, Figure 4A), but not by (+)camphor, gentamicin or HC-030031 (P > 0.05 vs. vehicle, Figure 4A). Responses evoked by allyl isothiocyanate, cinnamaldehyde, acrolein or crotonaldehyde were not completely inhibited by selective or non-selective TRPA1 blockers (Figures 3 and 4). This could be due to an insufficient dosing of the antagonists, which, however, for solubility limits could not be augmented in our present experimental conditions.
Effect of cigarette smoke
Because acrolein and crotonaldehyde are abundantly contained in cigarette smoke (Facchinetti et al., 2007), we reasoned that cough caused by smoke inhalation, at least in part, could be caused by these two α,β-unsaturated aldehydes via TRPA1 receptor stimulation. Indeed, inhalation of cigarette smoke for 10 min caused a significant increase in the number of coughs, compared to air inhalation (Figure 5). Ruthenium red (3 mM), (+)camphor (1 mM) and HC-030031 (0.3 mM) all significantly inhibited the cough response elicited by cigarette smoke (Figure 5). Also, the selective TRPV1 antagonist, capsazepine (10 µM), produced a significant inhibition of the tussive response evoked by cigarette smoke (Figure 5).
Figure 5.
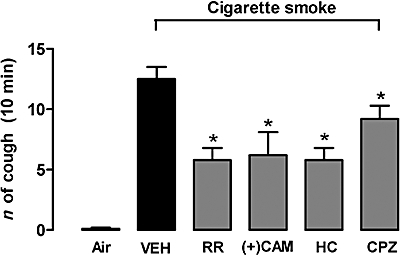
Effect of aerosolized ruthenium red (RR, 3 mM), (+)camphor [(+)CAM, 1 mM], HC-030031 (0.3 mM) or capsazepine (CPZ, 10 µM) on cigarette smoke-induced cough in guinea pig. VEH indicates results obtained by cigarette smoke after pretreatment with 6% dimethyl sulphoxide and 3.5% Tween-80. *P < 0.05, analysis of variance and Bonferroni's test versus VEH. Each column is presented as mean ± SE of at least six experiments.
Discussion and conclusions
In the present study, we have shown that inhalation of different TRPA1 stimulants, including the selective agonists allyl isothiocyanate and cinnamaldehyde (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2005), provoked a dose-dependent and robust tussive response in guinea pigs. We also observed that the cough elicited by TRPA1 agonists required the functional integrity of capsaicin-sensitive sensory neurons. Desensitization to the selective TRPV1 agonist, capsaicin, disables TRPV1-expressing neurons to other irritant stimuli (Szallasi and Blumberg, 1999). Thus, the inability of guinea pigs desensitized to capsaicin to respond to allyl isothiocyanate or cinnamaldehyde indicates that TRPA1 agonists act on a target expressed by TRPV1-expressing sensory neurons. This conclusion is fully consistent with the more general neuroanatomical finding that TRPA1 is substantially co-expressed by TRPV1-positive neurons (Story et al., 2003; Nagata et al., 2005). More recent findings that TRPA1 mRNA is exclusively expressed in TRPV1-positive vagal sensory neurons innervating mouse airways, and that TRPA1 agonists generate action potentials from bronchopulmonary C-fibres, thus eliciting nocifensor reflex responses (Nassenstein et al., 2008), further corroborate our present results.
The involvement of a TRP channel in the cough response by allyl isothiocyanate and cinnamaldehyde is suggested by the observation that the tussive effects by these two agents were significantly inhibited by the non-selective TRP channel blocker, ruthenium red (Lalloo et al., 1995; Trevisani et al., 2004). The role of the TRPA1 channel in mediating the responses to both allyl isothiocyanate and cinnamaldehyde is underlined by the inhibitory effect of (+)camphor and gentamicin, two compounds which have been reported to show some selectivity for this channel subtype (Nagata et al., 2005; Xu et al., 2005). More importantly, the observation that the selective TRPA1 antagonist, HC-030031 (McNamara et al., 2007), inhibited allyl isothiocyanate- and cinnamaldehyde-evoked cough, strongly argues in favour of the role of TRPA1 in the tussive response. It is worth noting that (+)camphor, gentamicin or HC-030031 was unable to affect cough evoked by the TRPV1 agonist, RTX, whereas capsazepine, a selective TRPV1 receptor antagonist (Bevan et al., 1992), failed to reduce the cough response elicited by any TRPA1 agonist. Selectivity of capsazepine on one side and that of (+)camphor, gentamicin or HC-030031 on the other side strongly argues in favour of the specific and independent role of TRPA1 as an initiator of the cough reflex.
Several chronic and degenerative diseases, including asthma and COPD, are characterized by oxidative stress, a condition in which an uncontrolled production of reactive oxygen species (ROS) contributes to tissue damage and inflammation. Oxidative decomposition of polyunsaturated fatty acids of cell membranes, caused by ROS, results in the formation of various reactive carbonyl species including the most reactive α,β-unsaturated aldehyde, acrolein (Aldini et al., 2007). Increased levels of ROS in the airways and lung result in increased amounts of lipid peroxidation products, including acrolein and 4-hydroxy-2-nonenal, that have been detected in the airspaces, breath, sputum, lungs and blood from patients with both asthma and COPD (Kirkham and Rahman, 2006). Acrolein is also a ubiquitous pollutant in the environment, produced by incomplete combustion of organic matter (Aldini et al., 2007), and, with crotonaldehyde, is a main component of cigarette smoke (Facchinetti et al., 2007).
Both acrolein and crotonaldehyde have been found to selectively stimulate TRPA1 channels, and by this mechanism they were shown to cause irritation and pain (Bautista et al., 2006; Andre et al., 2008). In particular, early inflammation produced by cigarette smoke inhalation in rodents, a neurogenic inflammatory process that requires activation of capsaicin-sensitive sensory fibres (Lundberg et al., 1983) and is blocked by ruthenium red (Geppetti et al., 1993), has been recently reported to be entirely mediated by acrolein and crotonaldehyde via TRPA1 activation (Andre et al., 2008). The ability of cigarette smoke to produce cough has been poorly investigated in the past. In a previous study (Lou et al., 1991), guinea pigs were exposed to cigarette smoke for only 3 min, and during this short period of time, a slight tussive effect was recorded (about three coughs), a response which was partially, but not significantly, reduced by ruthenium red. In our present study, guinea pigs were exposed to smoke for a longer time (10 min), and a more consistent response (more than 10 coughs) was recorded. Under these circumstances, a large and significant proportion (about 50%) of the response was inhibited by ruthenium red, (+)camphor and HC-030031. Because ROS, which are contained in cigarette smoke, have been recently reported to activate TRPA1 (Andersson et al., 2008), it is possible that these species may also contribute to the cough caused by cigarette smoke and mediated by TRPA1. Thus, it may be that TRPA1 plays a significant role in the pro-tussive effect of cigarette smoke, and that acrolein and crotonaldehyde contained in smoke are likely to contribute to this response. However, the observation that after TRPA1 receptor inhibition, the reduction in cigarette smoke-evoked cough was only partial, suggests that the tussive response to cigarette smoke was mediated by multiple mechanisms. Stimulation of nicotinic receptors has been previously suggested to play a role (Lee et al., 1989), and our present results with capsazepine suggested that a TRPV1-mediated component also contributed. In an earlier paper, Lewis et al. (2007) found that capsazepine did not reduce the cough exacerbation evoked by both capsaicin and citric acid following exposure to cigarette smoke. These results show some difference with our present data, and remain unexplained, because the sole known target of capsaicin action is the TRPV1 channel, which is usually inhibited by capsazepine.
In conclusion, our study is the first to identify the TRPA1 channel as a novel initiator of the cough reflex in guinea pigs. Apart from the anecdotal evidence of the pungency of cinnamon, mustard or wasabi in man, it has been recently reported that TRPA1 is expressed in, and activates, human sensory neurons (Anand et al., 2008). Thus, it is possible that, by analogy with the TRPV1 stimulants, also TRPA1 agonists may produce cough in man. The implication of our present findings is twofold. Firstly, TRPA1 may be regarded as a novel target for cough studies in experimental animals and in man. Secondly, as recently suggested (Taylor-Clark et al., 2009b), TRPA1 antagonists may be assessed as novel anti-tussive medicines.
Acknowledgments
This study was supported by grants from MIUR (FIRB RBIP06YM29) and ARCA (Padua).
Glossary
Abbreviations:
- CAM
(+)camphor
- GM
gentamicin
- HC
HC-030031
- RR
ruthenium red
- TRP
transient receptor potential
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid type 1
Conflicts of interest
None of the authors have a real or perceived conflict of interest that relates to this paper.
References
- Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther. 2009;22:65–70. doi: 10.1016/j.pupt.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, et al. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Anderson GP. TRPV1 and cough. Thorax. 2004;59:730–731. doi: 10.1136/thx.2003.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–11252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, et al. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Aziz SM, Chapman RW. Ruthenium red decreases capsaicin and citric acid-induced cough in guinea pigs. Neurosci Lett. 1991;126:131–133. doi: 10.1016/0304-3940(91)90536-3. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Collier JG, Fuller RW. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol. 1984;81:113–117. doi: 10.1111/j.1476-5381.1984.tb10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55:643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A, et al. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37:617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, et al. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol. 2006;101:506–511. doi: 10.1152/japplphysiol.01558.2005. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Bertrand C, Baker J, Yamawaki I, Piedimonte G, Nadel JA. Ruthenium red, but not capsazepine reduces plasma extravasation by cigarette smoke in rat airways. Br J Pharmacol. 1993;108:646–650. doi: 10.1111/j.1476-5381.1993.tb12855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, Mckemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea-pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol. 1993;6:171–175. doi: 10.1006/pulp.1993.1023. [DOI] [PubMed] [Google Scholar]
- Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, et al. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol. 1989;66:2032–2038. doi: 10.1152/jappl.1989.66.5.2032. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Ambrose C, Banner K, Battram C, Butler K, Giddings J, et al. Animal models of cough: literature review and presentation of a novel cigarette smoke-enhanced cough model in the guinea-pig. Pulm Pharmacol Ther. 2007;20:325–333. doi: 10.1016/j.pupt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Lou YP, Karlsson JA, Franco-Cereceda A, Lundberg JM. Selectivity of ruthenium red in inhibiting bronchoconstriction and CGRP release induced by afferent C-fibre activation in the guinea-pig lung. Acta Physiol Scand. 1991;142:191–199. doi: 10.1111/j.1748-1716.1991.tb09147.x. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Martling CR, Saria A, Folkers K, Rosell S. Cigarette smoke-induced airway oedema due to activation of capsaicin-sensitive vagal afferents and substance P release. Neuroscience. 1983;10:1361–1368. doi: 10.1016/0306-4522(83)90117-3. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, et al. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Gatti R, Trevisani M, Geppetti P. Cough sensors. II. Transient receptor potential membrane receptors on cough sensors. Handb Exp Pharmacol. 2009;187:49–61. doi: 10.1007/978-3-540-79842-2_3. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Aizawa H, Inoue H, Koto H, Takata S, Shigyo M, et al. Eosinophilic airway inflammation induced by repeated exposure to cigarette smoke. Eur Respir J. 1998;12:387–394. doi: 10.1183/09031936.98.12020387. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Kwong KK, Taylor-Clark TE, Kollarik M, MacGlashan DW, Braun A, et al. TRPA1 expression and function in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009a;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Nassenstein C, McAlexander MA, Undem BJ. TRPA1: a potential target for anti-tussive therapy. Pulm Pharmacol Ther. 2009b;22:71–74. doi: 10.1016/j.pupt.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, et al. Antitussive activity of iodo-resiniferatoxin in guinea-pigs. Thorax. 2004;59:769–772. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Morice AH. Cough threshold in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:62–64. doi: 10.1136/thx.54.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitises the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


