Abstract
Background and purpose:
Indacaterol is a novel β2-adrenoceptor agonist in development for the treatment of chronic obstructive pulmonary disease. The aim of this study was to investigate the comparative pharmacology of indacaterol in recombinant cells expressing the common polymorphic variants of the human β2-adrenoceptor and in human primary airway smooth muscle (ASM) cells.
Experimental approach:
Chinese hamster ovarian-K1 cell lines expressing high and low levels of the common human β2-adrenoceptor variants were generated [Gly16-Glu27-Val34-Thr164(GEVT), RQVT, GQVT] and also the rare GQVI variant. Human primary ASM cells were isolated from explants of trachealis muscle. Adenosine-3′,5′-cyclic-monophosphate production was used as an outcome measure.
Key results:
In both the low- and high-expression recombinant GEVT ‘wild type’ cell lines indacaterol is a high-efficacy agonist. Salmeterol and formoterol were identified as low- and high-efficacy agonists, respectively, and showed similar potencies to indacaterol irrespective of the β2-adrenoceptor genotype. The I164 variant cell line was associated with a reduced capacity to generate adenosine-3′,5′-cyclic-monophosphate in response to β2-adrenoceptor agonist. In the human primary ASM cells indacaterol gave a maximal response intermediate between that of salmeterol and formoterol.
Conclusions and implications:
These data demonstrate that indacaterol is a high-efficacy agonist in recombinant cell systems but acts with lower efficacy in human primary ASM cells. No marked genotype-dependent effects were observed for common variants; however, changes in I164 receptor activity were identified, which were dependent on the level of expression of β2-adrenoceptors.
Keywords: asthma, chronic obstructive pulmonary disease, β2-adrenoceptor agonist, indacaterol, pharmacogenetics, polymorphism, recombinant, haplotype, cAMP
Introduction
β2-adrenoceptor agonists are the mainstay bronchodilators used in the treatment of asthma and chronic obstructive pulmonary disease. These drugs fall into two main classes, namely short acting β2-adrenoceptor agonists (SABAs) and long acting β2-adrenoceptor agonists (LABAs) (Hall and Tattersfield, 1992). The available drugs in clinical usage differ in their basic pharmacology, both in their onset and duration of action, and also in the degree of agonism, with some drugs (e.g. salmeterol) being partial agonists in most cell systems, and other drugs (e.g. formoterol) being full agonists. Recently novel LABAs have been developed for potential clinical usage; one such drug is indacaterol, which in phase 3 trials has been shown to exhibit a once-daily bronchodilator action (Battram et al., 2006; Beeh et al., 2007; Beier et al., 2007; Naline et al., 2007).
The actions of β2-adrenoceptor agonists at the human β2-adrenoceptor are potentially affected by common polymorphisms within the ADRB2 gene. A number of synonymous and non-synonymous single nucleotide polymorphisms (SNPs) have been described at this locus (Reihsaus et al., 1993). Of these, the two most frequent non-synonymous SNPs result in the presence of either an arginine (R) or a glycine (G) at codon 16 and a glutamine (Q) or a glutamate (E) at codon 27. A third polymorphism potentially results in a methionine (M) substitution for a valine (V) at position 34, but this is extremely rare and hence we have not studied it further. In addition, there is a rare but potentially important non-synonymous SNP that results in either a threonine (T) or an isoleucine (I) at codon 164. The I164 form of the receptor has been shown to be associated with reduced acute responses to most catechol ligands in fibroblast cell lines expressing the β2-adrenoceptor (Green et al., 2001). In these Chinese hamster fibroblasts and in human primary cultured airway smooth muscle (ASM) lines, codon 16 or 27 polymorphisms have not been shown to be the acute actions of isoprenaline. However, differences in down-regulation profiles have been identified, at least in transformed cell lines, with reduced receptor down-regulation with the E27 form of the receptor and enhanced down-regulation with the G16 form of the receptor (Green et al., 1994; 1995;). Codons 16 and 27 reside in the N-terminal extracellular end of the receptor, and the structure–function mechanism underlying altered receptor down-regulation is unclear at this time. Interestingly, the I164 variant resides in the fourth transmembrane domain adjacent to S165, which is predicted to interact with the β-OH group of adrenoceptor ligands. This may at least in part explain the reduced acute responses of β2-adrenoceptor agonists in the I164 variant. The reduced duration of action of the LABA salmeterol in cells expressing the I164 variant has also been rationalized by the concept of a reduced exosite binding (Green et al., 2001). It is important to note that in addition to polymorphic variation, β2-adrenoceptor expression and function is also influenced by transcriptional, translational and post-translational mechanisms.
There have been extensive clinical studies that have addressed the possible contribution of polymorphisms to clinically relevant end points. While it is unlikely these polymorphisms increase the risk of developing asthma (Hall et al., 2006), there are data to suggest they may influence disease progression (Hall et al., 2006) and also response to treatment when SABAs are administered regularly in steroid naïve patients (Israel et al., 2000; 2004;). There are conflicting data on the influence of genotype on responses to LABAs, with some studies showing significant reduction in response in individuals homozygous for the R16 form of the receptor (Wechsler et al., 2006), and other studies suggesting maintained bronchodilator responses, at least in subjects taking inhaled steroids (Bleecker et al., 2006). Whether or not the response to indacaterol is altered by genetic variants in the human β2-adrenoceptor has not to date been studied. Similarly a direct comparison of the ability of indacaterol to stimulate cAMP (adenosine-3′,5′-cyclic-monophosphate) production with other clinically relevant compounds on different genetic backgrounds has not been investigated.
The aims of the current study were: (i) to create a series of cell lines expressing the common haplotypes of the human β2-adrenoceptor matched for receptor expression; (ii) to investigate the comparative pharmacology of indacaterol in cell lines expressing the ‘wild type’ form of the β2-adrenoceptor; and (iii) to examine the possibility that genetic variation in the β2-adrenoceptor alters the response to indacaterol or other LABAs.
Methods
Plasmid construction
PCR was used to amplify the Human ADRB2 coding region from genomic DNA template using primers designed to engineer a 5'EcoRI site and a 3'XhoI site (See Table 1). PCR conditions included 100–500 ng genomic DNA template, 0.5 U platinum taq high fidelity DNA polymerase, 0.3 mmol·L−1 dNTPs, 1 mmol·L−1 magnesium sulphate, 0.2 µmol·L−1 of each primer in a reaction volume of 50 µL. Cycling conditions were 94°C for 2 min, then 30 cycles of 94°C for 1 min, 60°C for 1 min, 68°C for 2 min. The PCR product was agarose gel-purified, digested with EcoRI and XhoI and cloned into pcDNA3.1 using standard molecular biology techniques. The ADRB2 cassette of the pcDNA3-ADRB2 plasmid was sequenced using BigDye (v3.1) terminator sequencing in conjunction with the ABI-310 DNA sequencer and various sequencing primers (Table 1). Multiple genomic DNA samples were used as template, and plasmids were screened until a Gly16-Glu27-Val34-Thr164 (GEVT) haplotype was obtained. The pcDNA3-ADRB2-GEVT plasmid represents the common haplotype observed in the Caucasian population (Drysdale et al., 2000) and was used as the starting template for site-directed mutagenesis. The pcDNA3-ADRB2-GQVT, RQVT, GQVI plasmids were generated sequentially by site-directed mutagenesis of the pcDNA3-ADRB2-GEVT backbone using the Quickchange site-directed mutagenesis kit as directed by the manufacturer in conjunction with mutagenic primers (Table 1). All pcDNA3-ADRB2 plasmids were sequence-verified, and large-scale preparations were completed using the Plasmid maxi kit as directed by the manufacturer.
Table 1.
Primers
| Adrb2 expression cassette isolation | ||
| 5′primer | 3′primer | Location |
| GACT-GAATTC-CGCGCCATGGGGCAAC | GACT-CTCGAG-TTACAGCAGTGAGTCATTGGTA | 214-1461 |
| ADRB2 expression cassette sequencing | ||
| 5′primers | 3′primers | Location |
| TAATACGACTCACTATAGGG | PCDNA3/T7 | |
| CAACAGATGGCTGGCAACTA | PCDNA3/1071 | |
| TCTGCAGACGCTCGAAC | 399-415 | |
| GACATGGAAGCGGCCCTCAG | 926-945 | |
| ATTGCCTCTTCCATCGTCTC | 820-839 | |
| Site-directed mutagenesis | ||
| 5′primer | 3′primer | Location |
| CTTGCTGGCACCCAATAGAAGCCATGCGCCGG | CCGGCGCATGGCTTCTATTGGGTGCCAGCAAG | GLY16 > ARG |
| CCACGACGTCACGCAGCAAAGGGACGAGGTGTG | CACACCTCGTCCCTTGCTGCGTGACGTCGTGG | GLU27 > GLN |
| GGATTGTGTCAGGCCTTATCTCCTTCTTGCCCATTC | GAATGGGCAGAGAGGAGATAAGGCCTGACACAATCC | THR164 > ILE |
ADRB2 numbering reference sequence NM_000024 (ATG = 220).
Underlined are the EcoRI and XhoI restriction enzyme sites.
Nucleotides representing codons of interest are shown in bold.
Stable transfection of Chinese hamster ovarian-K1 cells
Chinese hamster ovarian (CHO)-K1 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal calf serum, 2 mmol·L−1 glutamine, 100 U·mL−1 penicillin, 0.1 mg·mL−1 streptomycin at 37°C/5% CO2 and passaged by using trypsin/EDTA. For transfection, 10 mL of cells (105 cells·mL−1) were plated in a 10 cm petri dish and grown for 24 h. Six micrograms of pcDNA3-ADRB2 constructs or pcDNA3 empty vector were linearized by using PvuI followed by heat inactivation. CHO-K1 cells were transfected with linearized DNA using Fugene6 as directed by the manufacturer using a 3:1 ratio of Fugene reagent to DNA. Transfected cells were grown for another 24 h followed by passaging into 24-well plates followed by a further 24 h incubation. Complete medium was then replaced by selection medium (complete medium supplemented with 500 µg·mL−1 G418), and the cells were maintained for 10–14 days with the addition of fresh selection medium every 2–3 days. Single cell clones of the transfected CHO-K1 cell lines were isolated in 96-well plates by flow cytometry using the cell sorting function (forward/side scatter) of the Coulter ultra flow cytometer. Single cell clones were maintained in complete medium using 20% foetal calf serum to promote survival; medium was replaced every 4–5 days. Clonal cell lines were maintained in selection medium for the rest of the study.
β2-adrenoceptor binding studies
Two confluent T162 flasks of cells were used for the preparation of membranes. Medium was aspirated, and cells were washed with ice-cold buffer A (10 mmol·L−1 HEPES, 0.9% NaCl, 0.2% EDTA, pH 7.4) and then incubated with buffer A at 37°C for 10 min. Cells were isolated by using a cell scraper, and a cell pellet was generated by centrifugation at 500×g for 5 min. The cell pellet was resuspended in 2 mL of buffer B (10 mmol·L−1 HEPES, 10 mmol·L−1 EDTA, pH 7.4) and two cell pellets combined and the volume made to 10 mL. The cell suspension was homogenized using an IKA Ultra Turrax T25 polytron using four bursts of 5 s with 20 s intervals between bursts. A further 15 mL of buffer B was added, and the cell suspension was centrifuged at 39 000×g for 25 min at 4°C. The cell pellet was resuspended in 10 mL buffer C (10 mmol·L−1 HEPES, 0.1 mmol·L−1 EDTA, pH 7.4), and the homogenization procedure was repeated. The cell suspension was centrifuged at 39 000×g for 25 min at 4°C and finally resuspended in 1 mL buffer C. Protein was quantified by using the Bradford protein micro-assay as directed by the manufacturer, and preparations were normalized to 1 mg·mL−1. Samples were snap-frozen in liquid nitrogen and stored at −80°C.
[125I]-iodo(±)-cyanopindolol ([125I]-iodo-CYP) saturation binding was used to determine the β2-adrenoceptor density in the membrane preparations. Briefly, 100 µL (100 µg) of membrane preparation, 50 µL assay buffer (HBSS with Ca2+ and Mg2+/0.1% BSA, pH 7.4), 50 µL containing [125I]-iodo-CYP at a range of pmol·L−1 concentrations and a further 50 µL of assay buffer for total counts or 50 µL assay buffer containing 10 µmol·L−1 alprenolol for non-specific binding determination was added to each well of a 96-well round bottom plate. Plates were incubated at room temperature for 2 h. Plates were harvested by using a Packard Filtration harvester onto GF/B filter plates and dried for 2 h at 35°C. Plates were backsealed, and 40 µL Microscintillant 20 was added to each well; the plate was sealed, and c.p.m. per well was determined by using a Packard Topcount. Kd and Bmax values were generated by using GraphPad Prism v5 (GraphPad, San Diego, CA, USA). All analyses were completed at least in duplicate.
cAMP assay
Cells were seeded in selection medium into 24-well plates at 105 cells per well and grown for 22 h prior to loading of the cells with [3H]-adenine (2 µCi per well) for 2 h. Excess radiolabel was removed by washing with Hanks HEPES buffer, and then 1 mL of Hanks HEPES buffer was added to each well. Plates were incubated at 37°C for subsequent addition of β2-adrenoceptor agonists for various time points. Reactions were terminated by the addition of 50 µL of concentrated HCl followed by freezing at −20°C. cAMP production was quantified by column chromatography, and results were corrected for adenine uptake and column efficiency using [14C]-cAMP as described previously (Freyer et al., 2004). Triplicate wells were counted for each condition, and the data are expressed as number of counts (compared with basal counts) or %isoprenaline response (100%). The response to 1 µmol·L−1 isoprenaline was routinely greater than threefold over basal counts in low-expression cell lines except the low-expression GQVI cell line that had a reduced response to all agonists tested (<1.5-fold over basal). Typically, the response to 1 µmol·L−1 isoprenaline was 10-fold greater than the basal counts in high-expression cell lines. Mean data from at least three experiments completed in triplicate are presented (±s.e.mean).
Human primary airway smooth muscle cultures
Human ASM cells were isolated from explants of trachealis muscle obtained from individuals with no history of respiratory disease as described previously (Daykin et al., 1993; Sayers et al., 2006). Briefly, a section of trachealis muscle was isolated by dissection just above the carina and washed in DMEM supplemented with penicillin (200 U·mL−1), streptomycin (200 µg·L−1) and amphotericin B (0.5 µg·L−1). Explants (0.2 × 0.2 cm) were placed in 6-well plates and allowed to adhere. Cells were allowed to grow with fresh complete medium (10% foetal calf serum and 2 mmol·L−1 glutamine) added regularly, after 7–10 days growth cells approached confluency. As cells reached confluency, explants were removed and the cells were harvested by using 5 mg·mL−1 trypsin, 2 mg·mL−1 EDTA. Primary ASM cells exhibited >95% cell staining for α-actin. ASM cells were maintained in complete medium and passaged using trypsin/EDTA. Ethical approval for the use of primary cells was obtained from the local ethics committee. Six ASM donors were used in the current analyses with genotypes: (G/R)(E/Q)VT, G(E/Q)VT, G(E/Q)VT, RQVT, GEVT and RQVT. cAMP production in response to β2-adrenoceptor agonists was measured in human ASM cells in an analogous manner to that described for the recombinant cell lines, except after seeding cells were grown for 2–3 days until ∼70–80% confluent prior to [3H]-adenine loading.
Statistical analyses
Differences between the results for the various treatment groups were compared by analysis of variance (anova) in conjunction with Bonferonni's post hoc test or Student's t-test, as appropriate. Figures represent mean values (±s.e.mean). Statistical analyses and curve fitting were completed by using GraphPad Prism v5 (GraphPad, San Diego, CA, USA); a P-value< 0.05 was considered significant.
Materials
All chemicals were analytical grade or higher. Plasticware was from Costar (High Wycombe, UK). All chemicals and reagents were purchased from Sigma-Aldrich (Poole, UK) unless otherwise stated. Platinum taq high fidelity DNA polymerase, pcDNA3.1 and DMEM were obtained from Invitrogen (Paisley, UK); EcoRI, XhoI and PvuI, Promega (Southampton, UK); ABI-310 DNA sequencer, Applied Biosystems (Warrington, UK); Quickchange site-directed mutagenesis kit, Stratagene (Amsterdam, The Netherlands); plasmid maxi kit, Qiagen (Crawley, UK); Fugene6, Roche Diagnostics (Newhaven, UK); Bradford protein micro-assay, Bio-Rad (Hemel Hempstead, UK); [125I]-iodo-CYP, Perkin Elmer (Life and Analytical Sciences, Boston, MA, USA).
Results
Creation of cell lines expressing human β2-adrenoceptor variants
Initial work was performed to create both low- (<1000 pmol·mg−1) and high-(>2000 pmol·mg−1) expressing CHO-K1 β2-adrenoceptor cell lines stably expressing the common combinations of ADRB2 polymorphisms. Because of linkage disequilibrium, the combination of arginine 16 glutamine 27 (R16Q27) and glycine 16 glutamate 27 (G16E27) are much commoner than would be expected from the respective allele frequencies of the relevant SNPs. Isoleucine (I) 164 occurs most frequently on the background G16Q27, and hence in order to compare responses of different agonists at the commoner haplotypes at this locus the following cell lines were made: G16E27V34T164 (GEVT), R16Q27V34T164 (RQVT), G16Q27V34T164 (GQVT) and G16Q27V34I164 (GQVI). These haplotypes have previously been identified in the Caucasian population with frequencies of GEVT (48.3%), GQVT (13.2%), RQVT (33.0%) and GQVI (1.0%) (Drysdale et al., 2000). In all, 18 clonal cell lines were studied for levels of β2-adrenoceptor expression using [125I]-iodo-CYP binding. Cell lines with <100 fmol·mg−1β2-adrenoceptor expression did not generate a measurable and robust cAMP response to 10 µmol·L−1 isoprenaline and were not studied further (data not shown). For each common haplotype, cell lines were chosen for further study where expression was matched as closely as was feasible. No [125I]-iodo-CYP binding was observed for the pcDNA3(-) transfected cell line, confirming that the expression of endogenous β2-adrenoceptors could not be detected in this cell line and validating the use of CHO-K1 cells as a host in order to investigate human β2-adrenoceptor function. The levels of expression for the different lines chosen for further study are shown in Table 2.
Table 2.
β2-adrenoceptor expression levels in recombinant Chinese hamster ovarian-K1 β2-adrenoceptor cell lines
| Protein (fmol·mg−1) | |
|---|---|
| High level expression | |
| GEVT | 3948 |
| GQVT | 2720 |
| RQVT | 2745 |
| GQVI | 2181 |
| Low level expression | |
| GEVT | 668.1 |
| GQVT | 204.9 |
| RQVT | 1074 |
| GQVI | 302.4 |
| pcDNA(-) | 0 |
Data from [125I]-iodo(±)-cyanopindolol saturation binding assay.
Time course of cAMP formation in ‘wild type’ (GEVT) cells to a range of β2-adrenoceptor agonists
Figure 1 shows the time course of cAMP formation in response to a range of β2-adrenoceptor agonists in low-expressing β2-adrenoceptor GEVT CHO-K1 cells. It can be seen that cAMP formation is essentially linear over the first 60 min stimulation with agonist. Agonists were used at concentrations predicted from previous work to induce near-maximal responses, that is, 1 µmol·L−1. There was a significant difference between the drug treatment groups (two-way anova, P = 0.006). Based on these single dose experiments, it appears that salmeterol (as previously described) is a relatively low-efficacy agonist, whereas indacaterol, like formoterol shows higher efficacy. Similar experiments were performed for all the cell lines expressing different variants of the β2-adrenoceptor. In all lines cAMP formation was linear over this time period, and similar findings were observed, that is, that indacaterol and formoterol are higher-efficacy agonists compared with salmeterol. Experiments with CHO-K1 cells transfected with pcDNA3 alone (i.e. without β2-adrenoceptor expression inserts) showed no increase in cAMP following exposure to any of the agonists studied complementing the [125I]-iodo-CYP binding data (n = 3, data not shown).
Figure 1.
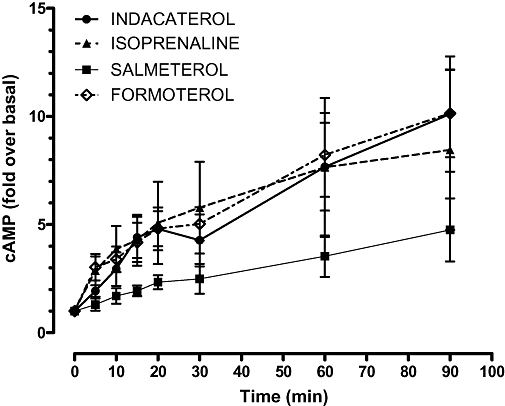
Time course of cAMP (adenosine-3′,5′-cyclic-monophosphate) production in response to 1 µmol·L−1β2-adrenocepter agonist in the low-expression GEVT ‘wild type’ cell line. Data are expressed as mean fold increase in cAMP compared with basal level; vertical lines represent s.e.mean (n = 3).
Dose–response relationships of β2-adrenoceptor agonists
Figure 2 shows the dose–response relationships for all the β2-adrenoceptor agonists studied in the four high-expression cell lines, expressing the three common haplotypes at the ADRB2 locus and also for the rare GQVI form of the receptor. Figure 3 shows the data from the same set of experiments in the low-expression cell lines. These results are in general similar, although there is somewhat greater variability in responses. EC50 and maximal response values derived from these experiments are shown in Tables 3 and 4. However, due to the low response in some cell lines we were unable to calculate accurate values for all the agonists for each cell line combination. This was particularly relevant for the low-expression GQVI cell line that gave a limited cAMP response to agonist (e.g. 1 µmol·L−1 isoprenaline response was ∼1.5-fold over basal) making accurate pEC50 and Emax calculations problematic. This low signal to background also gave the impression that salmeterol is a high-efficiency agonist on this haplotype background.
Figure 2.
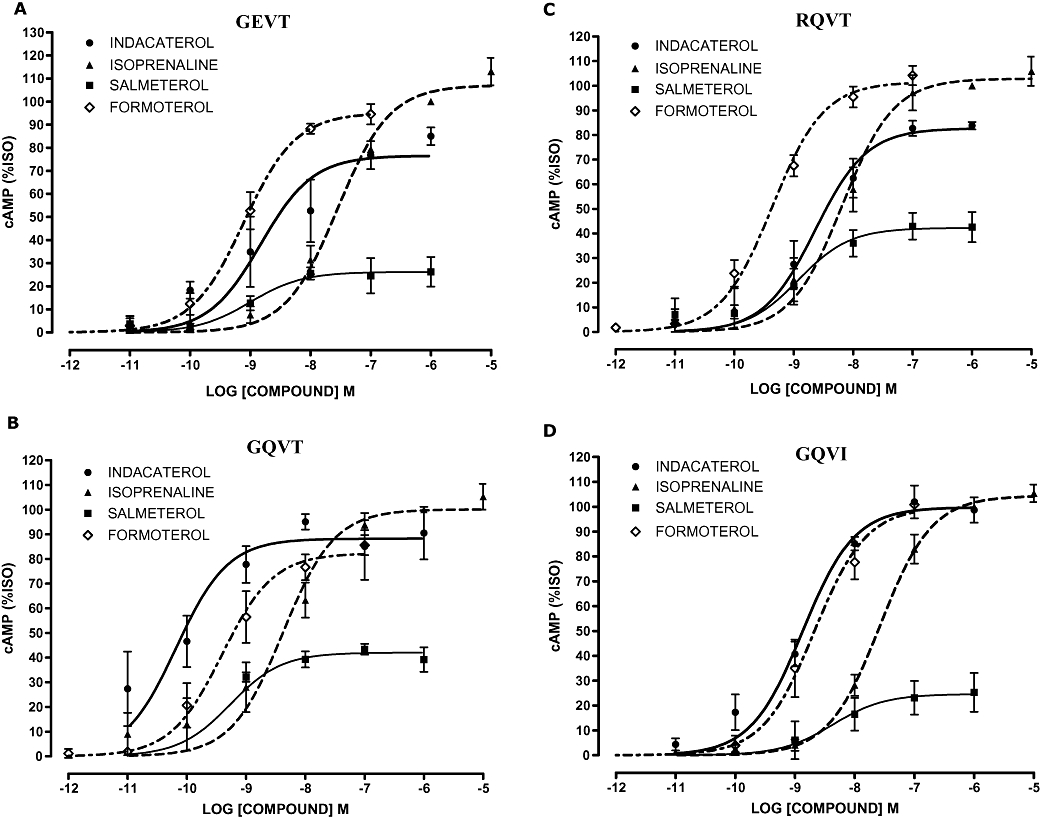
Concentration–response curves for cAMP (adenosine-3′,5′-cyclic-monophosphate) production in response to β2-adrenoceptor agonists (40 min) in the high-expression β2-adrenocepter cell lines. Variants cell lines (A) GEVT, (B) GQVT, (C) RQVT and (D) GQVI. Data were normalized to response to 1 µmol·L−1 isoprenaline (100%) in each experiment and are presented as mean percentage isoprenaline (%ISO) response; error bars represent s.e.mean (n = 3).
Figure 3.
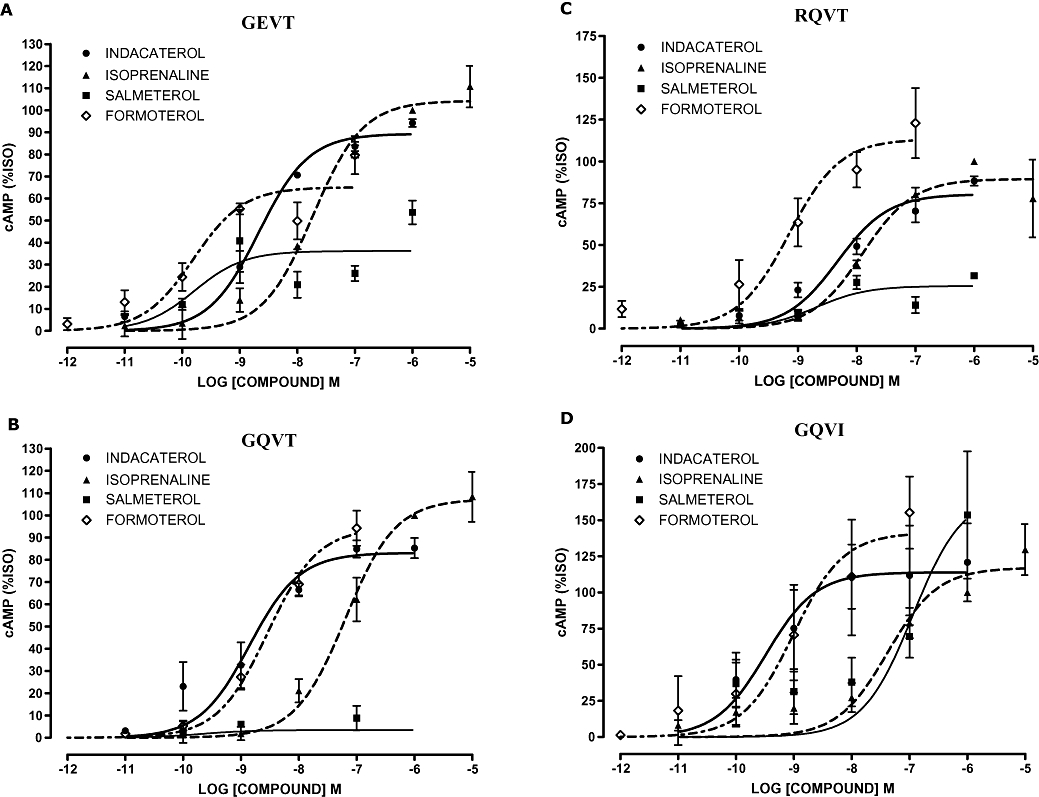
Concentration–response curves for cAMP (adenosine-3′,5′-cyclic-monophosphate) production in response to β2-adrenoceptor agonists (40 min) in the low-expression β2-adrenocepter cell lines. Variants cell lines (A) GEVT, (B) GQVT, (C) RQVT and (D) GQVI. Data were normalized to response to 1 µmol·L−1 isoprenaline (100%) in each experiment and are presented as mean percentage isoprenaline (%ISO) response; error bars represent s.e.mean (n = 3).
Table 3.
pEC50 values
| Indacaterol | Isoprenaline | Salmeterol | Formoterol | |
|---|---|---|---|---|
| High level expression | ||||
| GEVT | 8.83 ± 0.23 | 7.55 ± 0.08 | 9.00 ± 0.28 | 9.11 ± 0.08 |
| GQVT* | 10.18 ± 0.21 | 8.38 ± −0.14 | 9.27 ± 0.23$ | 9.41 ± 0.13$ |
| RQVT* | 8.64 ± 0.12 | 8.18 ± 0.12 | 8.92 ± 0.20 | 9.36 ± 0.07$ |
| GQVI | 8.87 ± 0.10 | 7.58 ± 0.05 | 8.37 ± 0.43 | 8.67 ± 0.11 |
| Low level expression | ||||
| GEVT* | 8.67 ± 0.24 | 7.73 ± 0.10 | 9.79 ± 0.49$ | 9.81 ± 0.24$ |
| GQVT | 8.85 ± 0.16 | 7.19 ± 0.11 | NC | 8.54 ± 0.10 |
| RQVT | 8.33 ± 0.13 | 7.90 ± 0.18 | 8.69 ± 0.61 | 9.15 ± 0.21 |
| GQVI | NC | NC | NC | NC |
| Human airway smooth muscle | 8.53 ± 0.16 | 6.96 ± 0.05 | 10.00 ± 0.38 | 8.65 ± 0.17 |
Chinese hamster ovarian-K1 β2-adrenoceptor data were generated by using 40 min time point (n = 3), human airway smooth muscle by using 10 min time point (n = 12 − 15). Data represent mean ± s.e.mean. NC = not calculated, experiments were completed; however, due to the low response accurate EC50 values could not be determined. EC50 values for the clinically relevant β2-adrenoceptor agonists (indacaterol, salmeterol, formoterol) were compared for each cell line by using the sum of squares F-test (GraphPad Prism v5)
P < 0.004. Statistically significant differences between indacaterol EC50 values and clinically relevant β2-adrenoceptor agonists were identified
(P < 0.05).
Table 4.
Emax values
| Indacaterol | Isoprenaline | Salmeterol | Formoterol | |
|---|---|---|---|---|
| High level expression | ||||
| GEVT* | 77 ± 6 | 100 | 26 ± 3$ | 95 ± 3$ |
| GQVT* | 88 ± 6 | 100 | 42 ± 3$ | 82 ± 4 |
| RQVT* | 83 ± 4 | 100 | 42 ± 3$ | 102 ± 3$ |
| GQVI* | 100 ± 4 | 100 | 25 ± 4$ | 100 ± 5 |
| Low level expression | ||||
| GEVT* | 89 ± 3 | 100 | 36 ± 6$ | 65 ± 6$ |
| GQVT | 83 ± 5 | 100 | NC | 94 ± 4 |
| RQVT* | 80 ± 4 | 100 | 26 ± 6$ | 113 ± 10$ |
| GQVI | NC | NC | NC | NC |
| Human airway smooth muscle * | 48 ± 2 | 100 | 10 ± 1$ | 79 ± 4$ |
Chinese hamster ovarian-K1 β2-adrenoceptor data were generated using 40 min time point (n = 3), human airway smooth muscle using 10 min time point (n = 12 − 15). Data represent mean ± s.e.mean. NC = not calculated, experiments were completed; however, due to the low response accurate Emax values could not be determined. Emax values for clinically relevant β2-adrenoceptor agonists (indacaterol, salmeterol, formoterol) were compared for each cell line by using the sum of squares F-test (GraphPad Prism v5
P < 0.001). Statistically significant differences between indacaterol Emax values and other clinically relevant β2-adrenoceptor agonists were identified
(P < 0.05).
In the low-expression cell lines there were significant differences between the EC50 values generated for the three clinically relevant β2-adrenoceptor agonists (pEC50 indacaterol 8.67 ± 0.24, salmeterol 9.79 ± 0.49 and formoterol 9.81 ± 0.24, P < 0.001) using the GEVT cell line (Table 3). The significance of the anova was driven by the reduced potency of indacaterol versus salmeterol (P = 0.007) and formoterol (P = 0.0001). Comparison of the EC50 values for indacaterol, salmeterol and formoterol in the high-expression cell lines identified significant differences for the GQVT (pEC50 10.18 ± 0.21 vs. 9.27 ± 0.23 vs. 9.41 ± 0.13 respectively) and RQVT (pEC50 8.64 ± 0.12 vs. 8.92 ± 0.20 vs. 9.36 ± 0.07 respectively) cell lines (P = 0.004 and P = 0.001 respectively). The significance of the anova was driven by the decreased potency of indacaterol versus formoterol for the RQVT cell line (P = 0.0001) and modest differences between indacaterol and salmeterol (P = 0.020) and formoterol (P = 0.013) for the GQVT cell line (Table 3). Interestingly, there was a trend in both high- and low-expression cell lines for the β2-adrenoceptor haplotype to influence agonist potency: for GEVT/RQVT, formoterol > indacaterol and for GQVT, indacaterol > formoterol.
The maximal response at 40 min post β2-adrenoceptor agonist confirmed our previous data suggesting that indacaterol and formoterol are higher-efficacy agonists compared with salmeterol (Table 4). Significant differences between the maximal responses were observed, but these were mainly driven by the low-level salmeterol responses. Indacaterol and formoterol overall had similar maximal responses (Table 4).
A comparison of indacaterol responses between cell lines expressing different β2-adrenoceptor haplotypes identified a significant difference in EC50 values in the high-expression cell lines (P < 0.0001) potentially reflecting an increased potency of indacaterol on the GQVT background. However, this was not apparent in the more physiologically relevant low-expression cell lines. Neither the low- nor the high-level β2-adrenoceptor expression cell lines were matched for receptor expression levels, confounding comparisons of agonist responses on different β2-adrenoceptor haplotypes.
The β2-adrenoceptor I164 variant was associated with lower cAMP responses to β2-adrenoceptor agonists
The responses to all agonists in the low-expression GQVI β2-adrenoceptor cell line were markedly less than in the other cell lines, and too low for accurate EC50 values to be determined for these agonists (Figure 4A). These reduced responses are unlikely to be due to β2-adrenoceptor expression differences in the GQVT and GQVI lines as these are well matched for surface receptor expression (204.9 and 302.4 fmol·mg−1 protein, respectively, Table 2). In addition, the ability of forskolin to generate a cAMP response in these two cell lines was similar (Figure 4B). Interestingly, this effect was not apparent in the high-expression GQVI cell line that had a robust cAMP response to β2-adrenoceptor agonist stimulation (16.79 ± 5.93-fold over basal, 1 µmol·L−1 isoprenaline, 30 min, n = 3, data not shown) suggesting that the genotypic effect is also dependent on the expression level of the β2-adrenoceptor. The ability of forskolin to generate cAMP was not significantly different between the four low- and four high-expression cell lines (n = 3, data not shown).
Figure 4.
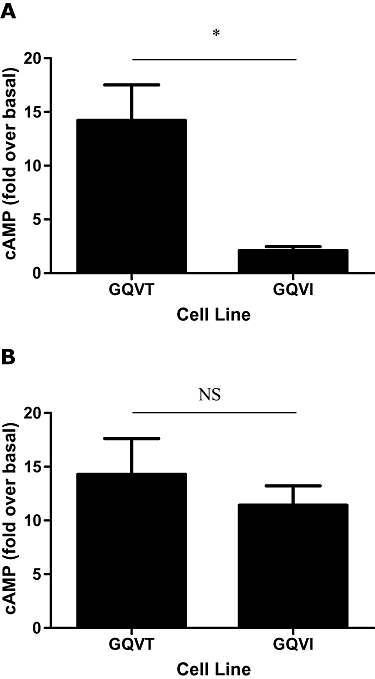
The low-expression Ile164 β2-adrenocepter cell line has a reduced capacity to respond to β2-adrenocepter agonist stimulation. (A) cAMP (adenosine-3′,5′-cyclic-monophosphate) responses to 1 µmol·L−1 isoprenaline (30 min) in the low-expression GQVT and GQVI cell cells lines. (B) cAMP responses to 10 µmol·L−1 forskolin (30 min) in the low-expression GQVT and GQVI cell lines. Data are expressed as mean fold increase in cAMP compared with basal level; error bars represent s.e.mean (n = 3). *P = 0.01 (t-test). NS, not significant.
Dose–response relationships of β2-adrenoceptor agonists in human primary ASM cells
In addition to the recombinant cell lines, we also studied a series of cell cultures of human ASM cells. Because the majority of cell lines studied were heterozygous for the polymorphisms of interest, the data presented were pooled from all the cell cultures established from different individuals (Figure 5). There were no significant differences between the EC50 values for the three clinically relevant β2-adrenoceptor agonists (indacaterol, salmeterol and formoterol) in the human primary ASM analyses (Table 3). There were significant differences between the three agonists with respect to maximal responses (Figure 5, P < 0.0001). As previously demonstrated in the CHO-K1 cell model, salmeterol was identified as a low-efficacy agonist and formoterol as a high-efficacy agonist. Interestingly, in the primary ASM cells indacaterol appears to have a reduced efficacy compared with the CHO-K1 data with a maximal response value intermediate between those to salmeterol and formoterol (Table 4; Figure 5). These data are in contrast to the data generated in the recombinant system, which suggested that indacaterol was a high-efficacy agonist when compared with isoprenaline. This finding was consistent irrespective of the genotype of the human ASM cells examined [e.g. indacaterol (%isoprenaline), GEVT 56 ± 2 (n = 4), RQVT 52 ± 2 (n = 7) data not shown].
Figure 5.
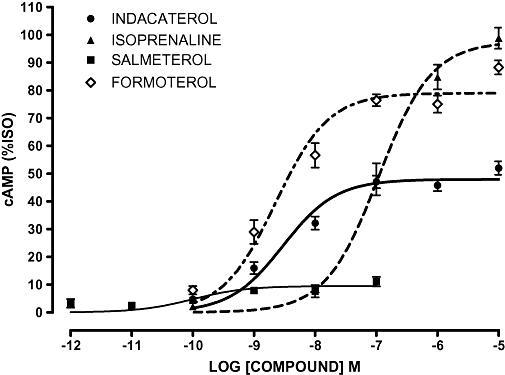
Concentration–response curves for cAMP (adenosine-3′,5′-cyclic-monophosphate) production in response to β2-adrenoceptor agonists (10 min) in human primary airway smooth muscle cells. Data were normalized to response to 1 µmol·L−1 isoprenaline (100%) in each experiment and are presented as mean percentage isoprenaline (%ISO); error bars represent s.e.mean (n = 12 − 15).
Discussion and conclusions
The data presented here make two important points. Firstly, this is the first comprehensive study describing the pharmacology of indacaterol at the β2-adrenoceptor in both recombinant cell lines expressing the human β2-adrenoceptor and primary cultures derived from human airway myocytes. Secondly, we have been able to study the effects of a range of β2-adrenoceptor agonists on cAMP responses in recombinant cell lines expressing the common β2-adrenoceptor haplotypes present in the Caucasian population.
Indacaterol acts in both low- and high-expressing CHO-K1 cell lines as a high-efficacy agonist. In this respect it is similar to formoterol, although indacaterol is a slightly less potent agonist in cells expressing the most common Caucasian people β2-adrenoceptor haplotype (GEVT). The onset of action in all the cell systems studied is rapid, again being similar to formoterol but producing a significantly greater response than salmeterol in the first 40 min, in keeping with previous studies suggesting that salmeterol is a lower-efficacy agonist. These data are in excellent agreement with previous observations using a recombinant expression system and the guinea-pig isolated trachea (Battram et al., 2006).
The data obtained from the pooled analysis in human primary ASM cells suggest that as predicted, formoterol appears to be a high-efficacy agonist and salmeterol a low-efficacy agonist. Interestingly, in contrast to the recombinant cell line data indacaterol appeared to have a lower efficacy in the ASM with a maximal response intermediate to that observed for salmeterol and formoterol. These findings in part agree with a recent study examining β2-adrenoceptor relaxation responses in human isolated bronchi (Naline et al., 2007). The order of maximal response was: formoterol [94 ± 1 (%)] > indacaterol (77 ± 5) ≥ salmeterol (74 ± 4) at resting tone (Naline et al., 2007). However, it has been noted that the characteristics of these agonists to induce relaxation can be influenced by the tone of the tissue preparation (e.g. salmeterol has low or high intrinsic activity in this system depending on bronchial tone) (Naline et al., 2007). It is important to note that relaxation of ASM (trachealis strips) is not proportional to cAMP levels suggesting cAMP-independent mechanisms are also involved (Kume et al., 1994). cAMP-independent mechanisms may at least in part explain efficacy differences observed in our cell-based/cAMP assay compared with tissue-based approaches using bronchial relaxation (Naline et al., 2007).
In the different cell systems the molecular mechanisms underlying these differences in maximal response remain to be resolved; however, a feature of the CHO-K1 system is the relatively high receptor densities in comparison with that found endogenously, for example, primary bronchial smooth muscle cells in vitro have a β2-adrenoceptor density of approximately 70 fmol·mg−1 (Green et al., 1995). In addition, we cannot exclude the possibility that the CHO-K1 signalling machinery influences our key endpoint, that is, cAMP production, and does not adequately represent that observed in human primary cells expressing endogenous β2-adrenoceptors. Overall, these data highlight the need to include primary cell and tissue-based analyses in pharmacological characterization of new compounds, but also highlight the utility of recombinant approaches to unmask interesting structure–function mechanisms.
In the next series of experiments we used both high- and low-expressing cell lines with the common haplotypes of the β2-adrenoceptor to address the possibility that agonist responses may vary by haplotype. In the Caucasian population the respective minor allele frequencies we have observed in a large population study (Hall et al., 2006) are as follows: R16 36.3% and E27 44.6%. Because of linkage disequilibrium, not all haplotypes occur at the frequency predicted by the allele frequencies per se: in particular the R16E27 combination was not seen in the 1958 birth cohort study (Hall et al., 2006), and the G16Q27 combination is also rare. I164 usually occurs on the G16Q27 background, and hence to study the effects of β2-adrenoceptor agonists on the most frequent haplotypes seen in the general population we made cell lines, both with high and low expression of the β2-adrenoceptor, with all these combinations. In all 18 cell lines were established: these were then screened to try and match expression levels as closely as possible to allow comparisons to be made. In general, cAMP responses to agonists were not detected in cell lines with expression levels <100 fmol·mg−1 protein.
In variant cell lines, the overall potency profiles of the agonists studied were similar; however, there was a trend in both the high- and low-expression cells for the receptor haplotype to influence the potency of indacaterol versus formoterol, that is, for GEVT/RQVT formoterol > indacaterol and for GQVT indacaterol > formoterol. The molecular basis of this observation based on the presence or absence of specific amino acid residues is not clear.
However, it is important to note that the genotype effects we observed were present/absent depending on the relative receptor expression, for example, the reduction in β2-adrenoceptor agonist responses for the I164 cell line was only observed in the lower-expression cell line. These data suggest that the functional effect of receptor polymorphism may only be unmasked under the appropriate expression level, which has broader implications as β2-adrenoceptor expression is cell-specific, for example, expression on human peripheral blood mononuclear cells is <700–750 receptors per cell compared with 30 000–40 000 on ASM cells (Hayes et al., 1996).
Overall, however, the data suggest that acute responses to these drugs are relatively unlikely to differ between individuals of varying genotype except in those who are homozygous for I164. Such individuals are extremely rare (Hall et al., 2006) because of the low frequency of the I164 allele. We also studied a series of primary cultures derived from human ASM cells from individuals of known genotype: as would be predicted, the number of cultures obtained that were homozygous at all loci was small, limiting our ability to determine whether there are any small differences in response by genotype in this more physiologically relevant system.
Previous functional studies on ADRB2 polymorphisms have addressed the potential to alter down-regulation profiles. In transformed cell lines the G16 genotype appears to show greater down-regulation than the R16 form of the receptor, and the E27 form appears to protect against down-regulation, an effect that is overridden by genotype at codon 16 (Green et al., 1994; 1995;). Ideally we would have liked to undertake similar studies in the CHO-K1 cell system to address down-regulation profiles with the agonists used in the current study. However, our initial studies demonstrated that while it is feasible to wash out isoprenaline from cultures and observe full recovery of cAMP responses with time [as we have previously described (Hall et al., 1993)], we were unable to wash out some of the other agonists studied. In particular, β2-adrenoceptor-mediated cAMP responses could not be recovered following prolonged exposure to indacaterol or salmeterol, suggesting these drugs remain bound to the receptor or its immediate vicinity. The refractory nature of salmeterol's stable activation of β2-adrenoceptor to wash out has been described previously (Clark et al., 1996).
We also confirmed that the I164 form of the receptor couples less efficiently with adenylate cyclase in low-expressing CHO-K1 cells, in keeping with previous work in fibroblasts with other agonists (Green et al., 2001). In the experiments comparing the response to isoprenaline in GQVT and GQVI cells, the reduced cAMP response to isoprenaline is unlikely to be related to differences in receptor expression as these two lines are well matched for expression. In addition, the abilities of both cell lines to generate cAMP responses to forskolin were similar, suggesting that reduced expression of adenylate cyclase in the GQVI cells is unlikely to provide an explanation. Our results show this effect extends to indacaterol as well as the other LABAs studied. These effects were not seen in the high-expressing GQVI cell system, suggesting that high levels of β2-adrenoceptor expression can overcome the effect of the I164 polymorphism.
Overall, these data demonstrate that indacaterol is a high-efficacy agonist in CHO-K1 cells transfected with the human β2-adrenoceptor. The data from the primary ASM analyses suggest it induces a response intermediate between those observed for salmeterol and formoterol. In keeping with data obtained with a range of other clinically relevant β2-adrenoceptor agonists there are no marked genotype-dependent effects on short-term agonist-induced receptor coupling for the common variants of the ADRB2 gene. However, for the rare I164 variant we confirmed the reduced activity associated with this variant and identified an additional level of regulation, based on receptor expression, that determines genotype effect.
Acknowledgments
This work was supported by a grant from Novartis.
Glossary
Abbreviations:
- ASM
airway smooth muscle
- cAMP
adenosine-3′,5′-cyclic-monophosphate
- CHO
Chinese hamster ovarian
- CYP
cyanopindolol
- LABA
long acting β2-adrenoceptor agonist
- SABA
short acting β2-adrenoceptor agonist
Conflict of interest
The authors state no conflict of interest.
References
- Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Farr D, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quino lin-2-one (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Derom E, Kanniess F, Cameron R, Higgins M, van As A. Indacaterol, a novel inhaled beta2-agonist, provides sustained 24-h bronchodilation in asthma. Eur Respir J. 2007;29:871–878. doi: 10.1183/09031936.00060006. [DOI] [PubMed] [Google Scholar]
- Beier J, Chanez P, Martinot JB, Schreurs AJ, Tkacova R, Bao W, et al. Safety, tolerability and efficacy of indacaterol, a novel once-daily beta(2)-agonist, in patients with COPD: a 28-day randomised, placebo controlled clinical trial. Pulm Pharmacol Ther. 2007;20:740–749. doi: 10.1016/j.pupt.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Bleecker ER, Yancey SW, Baitinger LA, Edwards LD, Klotsman M, Anderson WH, et al. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006;118:809–816. doi: 10.1016/j.jaci.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Clark RB, Allal C, Friedman J, Johnson M, Barber R. Stable activation and desensitization of beta 2-adrenergic receptor stimulation of adenylyl cyclase by salmeterol: evidence for quasi-irreversible binding to an exosite. Mol Pharmacol. 1996;49:182–189. [PubMed] [Google Scholar]
- Daykin K, Widdop S, Hall IP. Control of histamine induced inositol phospholipid hydrolysis in cultured human tracheal smooth muscle cells. Eur J Pharmacol. 1993;246:135–140. doi: 10.1016/0922-4106(93)90090-v. [DOI] [PubMed] [Google Scholar]
- Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, et al. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci USA. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer AM, Billington CK, Penn RB, Hall IP. Extracellular matrix modulates beta2-adrenergic receptor signaling in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2004;31:440–445. doi: 10.1165/rcmb.2003-0241OC. [DOI] [PubMed] [Google Scholar]
- Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- Green SA, Rathz DA, Schuster AJ, Liggett SB. The Ile164 beta(2)-adrenoceptor polymorphism alters salmeterol exosite binding and conventional agonist coupling to G(s) Eur J Pharmacol. 2001;421:141–147. doi: 10.1016/s0014-2999(01)01049-4. [DOI] [PubMed] [Google Scholar]
- Hall IP, Tattersfield AE. Beta-agonists. In: Clark TH, Godfrey S, Lee TH, editors. Asthma. 3rd. London: Chapman and Hall; 1992. pp. 341–365. [Google Scholar]
- Hall IP, Daykin K, Widdop S. Beta 2-adrenoceptor desensitization in cultured human airway smooth muscle. Clin Sci (Lond) 1993;84:151–157. doi: 10.1042/cs0840151. [DOI] [PubMed] [Google Scholar]
- Hall IP, Blakey JD, Al Balushi KA, Wheatley A, Sayers I, Pembrey ME, et al. Beta2-adrenoceptor polymorphisms and asthma from childhood to middle age in the British 1958 birth cohort: a genetic association study. Lancet. 2006;368:771–779. doi: 10.1016/S0140-6736(06)69287-8. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Qing F, Rhodes CG, Rahman SU, Ind PW, Sriskandan S, et al. In vivo quantification of human pulmonary beta-adrenoceptors: effect of beta-agonist therapy. Am J Respir Crit Care Med. 1996;154:1277–1283. doi: 10.1164/ajrccm.154.5.8912736. [DOI] [PubMed] [Google Scholar]
- Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M. Effect of indacaterol, a novel long-acting beta2-agonist, on isolated human bronchi. Eur Respir J. 2007;29:575–581. doi: 10.1183/09031936.00032806. [DOI] [PubMed] [Google Scholar]
- Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–339. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- Sayers I, Swan C, Hall IP. The effect of beta2-adrenoceptor agonists on phospholipase C (beta1) signalling in human airway smooth muscle cells. Eur J Pharmacol. 2006;531:9–12. doi: 10.1016/j.ejphar.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Wechsler ME, Lehman E, Lazarus SC, Lemanske RF, Jr, Boushey HA, Deykin A, et al. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006;173:519–526. doi: 10.1164/rccm.200509-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


