Abstract
Background and purpose:
We have investigated the ability of the β3-adrenoceptor antagonist 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride (SR59230A) to affect the hyperthermia produced by methylenedioxymethamphetamine (MDMA) in conscious mice and whether α1-adrenoceptor antagonist actions are involved.
Experimental approach:
Mice were implanted with temperature probes under anaesthesia, and allowed 2 week recovery. MDMA (20 mg·kg−1) was administered subcutaneously 30 min after vehicle or test antagonist and effects on body temperature monitored by telemetry.
Key results:
Following vehicle, MDMA produced a slowly developing hyperthermia, reaching a maximum increase of 1.8°C at 130 min post injection. A low concentration of SR59230A (0.5 mg·kg−1) produced a small but significant attenuation of the slowly developing hyperthermia to MDMA. A high concentration of SR59230A (5 mg·kg−1) revealed a significant and marked early hypothermic reaction to MDMA, an effect that was mimicked by the α1-adrenoceptor antagonist prazosin. Functional and ligand binding studies revealed actions of SR59230A at α1-adrenoceptors.
Conclusions and implications:
1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride in high concentrations modulates the hyperthermic actions of MDMA in mice in two ways: by blocking an early α1-adrenoceptor-mediated component to reveal a hypothermia, and by a small attenuation of the later hyperthermic component which may possibly be β3-adrenoceptor-mediated (this seen with the low concentration of SR59230A). Hence, the major actions of SR59230A in modulating the actions of MDMA on temperature involve α1-adrenoceptor antagonism.
Keywords: MDMA, hyperthermia, hypothermia, α1-adrenoceptor, β3-adrenoceptor
Introduction
Methylenedioxymethamphetamine (MDMA) is a widely used recreational drug of abuse. Toxic effects of MDMA include a life-threatening hyperthermia that can occur particularly when the drug is used in a ‘rave’ environment, often with high ambient temperatures and excessive physical exertion. It has been shown in animal studies that MDMA disrupts thermoregulation, often causing hypothermia at low ambient temperatures and hyperthermia at high ambient temperatures (Malberg and Seiden, 1998; Green et al., 2005). MDMA has actions at monoaminergic pathways both as a direct receptor agonist and indirectly by affecting release of neurotransmitters. As 5-hydroxytryptaminergic, noradrenergic and dopaminergic neurotransmitter systems have all been implicated in the mediation of hypothermia and hyperthermia, acute increases in these neurotransmitters induced by MDMA (White et al., 1996), or agonist actions of MDMA at receptors for these neurotransmitters (Lavelle et al., 1999; McDaid and Docherty, 2001), may therefore influence the thermoregulatory system, and both central and peripheral mechanisms may be involved.
We have investigated the noradrenergic component of the hyperthermia to MDMA in a number of studies. We have demonstrated that α2- and α1-adrenoceptors (nomenclature follows Alexander et al., 2008) are involved in MDMA-induced hyperthermia and in the absence of α2A-adrenoceptors or in the presence of α2- or α1-adrenoceptor antagonists, the monophasic hyperthermic response produced by MDMA in mice became a biphasic response: hypothermia followed by hyperthermia (Bexis and Docherty, 2005; 2008;). We have further shown that more than one subtype of α1-adrenoceptor is involved in this component of the hyperthermic response to MDMA in mice, probably both α1A- and α1D-adrenoceptors (Bexis and Docherty, 2008). MDMA has been demonstrated to have an affinity for α2-, α1- and β-adrenoceptors in the brain (Battaglia et al., 1988).
In addition to α-adrenoceptors, β3-adrenoceptors have also been implicated in thermogenesis (Sprague et al., 2003; Sprague et al., 2004), although β3-adrenoceptor ligands have been associated with α1-adrenoceptor antagonism (Brahmadevara et al., 2004). The evidence for the involvement of β3-adrenoceptors in the temperature actions of MDMA is incomplete. Cyanopindolol, used as a putative β3-adrenoceptor antagonist, had no effect on rectal temperature on its own but prazosin combined with cyanopindolol caused a greater reduction in the MDMA hyperthermia than prazosin alone (Sprague et al., 2003). However, cyanopindolol has other actions in addition to β3-adrenoceptor blockade, and has similar potency to SR593230A for α1-adrenoceptors (Brahmadevara et al., 2004). Further studies examined the combination of prazosin (α1) and 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride (SR59230A) (β3) (Sprague et al., 2004), or the antagonist carvedilol (Sprague et al., 2005) and concluded that α1- and β3-adrenoceptors are involved in the hyperthermia to MDMA. However, carvedilol is more potent than SR59230A at α1-adrenoceptors, with a reported pKi of 7.7–7.9 (Qvigstad et al., 2005). Hence, in none of these studies was β3-adrenoceptor blockade alone examined against the hyperthermia to MDMA. Our hypothesis is that β3-adrenoceptors play at best a minor role in the adrenergic component of the hyperthermia to MDMA, with the major component being α1-adrenoceptor-mediated.
The main aim of this study was to investigate the role of β3-adrenoceptors in MDMA-mediated hyperthermia in mice using the selective β3-adrenoceptor subtype antagonist SR59230A (Nisoli et al., 1996) and to investigate whether the ability of SR59230A to modulate the MDMA-induced hyperthermia involved α1-adrenoceptors. We predicted that SR59230A at a high dose of 5.0 mg·kg−1 would act as an α1-adrenoceptor antagonist but that at a low dose of 0.5 mg·kg−1 would be devoid of these actions.
Some of these results have been published in abstract form (Bexis and Docherty, 2009)
Methods
All animal care and studies conform to the Declaration of Helsinki and have been approved by the Department of Health and by the RCSI Research Ethics Committee. Male C-57BL6J wild-type mice (22–35 g) were obtained from Harlan (UK).
Radiotelemetry
Animals were implanted with a radiotelemetric device under short-term ether anaesthesia, enabling measurement of core body temperature (TAC50-PXT; Data Sciences International, St Paul, MN, USA). The implant was placed in the abdominal cavity and the abdomen was then closed. Animals were given temgesic (buprenorphine hydrochloride 0.05 mg·kg−1, Schering-Plough, Welwyn, UK) s.c. postoperatively and allowed to recover for 14 days before experiments were performed.
Animals were housed individually and home cages (plastic with wire lid) and bedding (wood shavings) were used during temperature monitoring. As treatment of the animals and recordings were performed in the laboratory and not in the animal facility, animals were allowed to acclimatize, in their home cages, to the surroundings in the laboratory for 2 days (5–6 h per day) before administration of any drugs. On experimental days, a PhysiolTel-Receiver (model RPC-1) was placed under each animal cage enabling recording of core body temperature. Data signals were acquired from 90 min prior to and for 300 min after vehicle (1 mL·kg−1) or MDMA (20 mg·kg−1) administration. All recordings were obtained at room temperature (23 ± 0.2°C).
Animals were injected s.c. with the β3-adrenoceptor antagonist SR59230A (0.5 or 5 mg·kg−1) or SR59230A (5 mg·kg−1) plus prazosin (0.1 mg·kg−1). Antagonists or vehicle were administered 30 min prior to the injection s.c. of vehicle (1 mL·kg−1) or MDMA (20 mg·kg−1).
Radioligand binding assays
Membrane preparation
Membrane radioligand binding was performed using a method adapted from those of Cheung et al. (1982), Neylon and Summers (1985), Michel et al. (1989) and Connaughton and Docherty (1990). Rats were killed by overdose of CO2. The spleen and submandibular gland were removed and carefully cleaned of any adherent connective tissue. They were then weighed and chopped finely using small sharp scissors.
Tissues were diluted in five volumes (submandibular) or 10 volumes (spleen) of ice-cold initial wash buffer (Tris-HCl 50 mmol·L−1, EDTA 5 mmol·L−1: pH 7.4 at 4oC). The soluble components of the tissue were removed by a series of homogenization and centrifugation steps. The tissues were homogenized in an Ultra-Turrax homogenizer for 30 seconds and centrifuged using an Eppendorf Micro-Centrifuge (submandibular: 16 000× g; spleen: 40 000× g) for 12 min at 4oC. Following this, the supernatant was discarded and the pellet fraction was resuspended in five volumes (submandibular) or 10 volumes (spleen) of ice-cold initial wash buffer. The homogenization and centrifugation steps were repeated. The supernatant was again discarded and the pellet suspended in five volumes (submandibular) or 10 volumes (spleen) of ice-cold incubation buffer (Tris-HCl 50 mmol·L−1, EDTA 5 mmol·L−1: pH 7.4 at 25oC) and homogenized again. The homogenate was filtered through two layers of nylon 43 T-mesh gauze to remove connective tissue. Prepared membranes were stored at −20°C for later use.
Competitive radioligand binding
Competition binding assays were carried out in duplicate in 5 mL polypropylene test tubes. Membrane aliquots of 100 µL were incubated with 100 µL of [3H]-prazosin (2 nmol·L−1; Specific activity: 85 Cinmol−1, New England Nuclear), and 100 µL of unlabelled test ligand (concentrations from 1 nmol·L−1 to 0.1 mmol·L−1), incubation buffer (vehicle) or phentolamine (10 µmol·L−1). Assays were performed at 25oC for 30 min. Following the 30 min incubation period, bound and free radioligand were separated by vacuum filtration. The assays were terminated by the addition of 5 mL ice-cold wash buffer (Tris-HCl 50 mmol·L−1, EDTA 5 mmol·L−1: pH 7.4 at 4oC) to all tubes. This was followed by rapid filtration through Whatman GF/C glass fibre filters using a Brandell Call Harvester. Filters and tubes were then washed four times with 5 mL of ice-cold wash buffer. Each filter was placed in a standard polypropylene scintillation vial and 5 mL of organic liquid scintillation medium (Pico-Fluor 40, Packard) was added to each vial. The vials were left overnight before being counted on a LKB 1214 Rack Beta counter.
Specific binding was determined by subtracting the radioligand binding to non-specific sites from the total radioligand binding for all sites. Non-specific binding was determined in the presence of phentolamine (10 µmol·L−1).
Saturation radioligand binding
Saturation binding experiments were carried out as described above for competition studies but employing increasing concentrations of [3H]-prazosin (0.1–15 nmol·L−1). Prazosin KD was 0.43 nmol·L−1 (spleen) and 1.40 nmol·L−1 (submandibular) (n= 4–5).
The inhibition constant (Ki) for inhibition of radiolabelled ligand binding by test ligands was determined from the formula:
where IC50 is the concentration of competing test ligand that inhibits radioligand specific binding by 50%, KD is the dissociation constant for the radioligand prazosin and 3H is the concentration of tritiated prazosin.
Isometric contractions of rat aorta and spleen
Rats were killed by overdose of CO2. Aorta and whole spleen were removed, and the aorta was cleared of connective tissue and 4 mm rings obtained. Aortic rings or bisected spleen were attached to a fixed rod and an isometric tension transducer under 1g tension in organ baths at 37°C in Krebs-Henseleit solution and gassed with 95% O2/5%CO2. Baths contained Krebs-Henseleit solution of the following composition (mmol·L−1): NaCl 119, NaHCO3 25, D-glucose 11.1, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.0, EDTA 0.03 and ascorbic acid 0.28. In addition, cocaine (3 µmol·L−1) was present to block noradrenaline re-uptake. Vessels were allowed to equilibrate for 30 min under this passive tension. Bathing fluid was changed every 15 min except during responses to test agents. Data were recorded on a four-channel MacLab acquisition system.
After a 30 min equilibration period, tissues were contracted with noradrenaline (1 µmol·L−1) (aorta) or phenylephrine (10 µmol·L−1) (spleen). After a 30 min washout, a concentration-contractile response curve was carried out to noradrenaline (aorta) or phenylephrine (spleen). After a further 30 min washout, tissues were exposed to SR59230A (10 µmol·L−1) or vehicle for 60 min prior to and during a second concentration response curve to the test agonist. Shifts in agonist potency (pD2, –log EC50) produced by SR59230A were corrected for the effects of vehicle. Potency of SR59230A was expressed as a pKB from the effects of a single concentration of the antagonist, using the equation:
pKB =[B]+ log (DR − 1), where [B] is antagonist concentration (–log M), in this case 5, and DR − 1, the agonist dose ratio minus 1.
Statistics
Values shown are mean ± SEM from four to five (in vitro) or seven to nine (in vivo) experiments. Time course responses were compared between random groups by repeated measures (two-way) anova followed by the Bonferroni test, or one-way anova followed by Dunnett's test (to determine the significance of differences from baseline levels) or Neuman-Kuels test (to determine the significance of differences between groups). Minimum (hypothermia) and maximum (hyperthermia) temperatures reached were compared by one-way anova. Planned comparisons were between vehicle and SR59230A (0.5 mg·kg−1) and SR59230A (5 mg·kg−1), as we predicted that the low dose would be devoid of α1-adrenoceptor antagonism. Statistical and graphical analysis, including ligand binding analysis, was carried out using GraphPad Prism for Macintosh computers.
Drugs
(+/−)-3,4-methylenedioxymethamphetamine hydrochloride (MDMA) (Research Biochemicals, Natik, MA, USA and NIDA, Bethesda, MD, USA); prazosin hydrochloride (Sigma, Dublin, Ireland); SR59230A (Tocris, Bristol, UK). Drugs were dissolved in distilled water.
Results
Resting core body temperature
In this study, we looked at low and high concentrations of SR59230A (0.5 & 5 mg·kg−1 respectively) and the combination of the high concentration of SR59230A with prazosin (0.1 mg·kg−1). For comparison, the effects of prazosin (0.1 mg·kg−1) alone are also shown (taken from Bexis and Docherty, 2008).
The resting body temperatures for all groups prior to drug treatment were not significantly different (Table 1). Although baseline temperature did not significantly differ between groups prior to vehicle/antagonist and 30 min later prior to vehicle/MDMA, baseline temperatures ranged between 35.5oC and 36.2oC, whereas maximum hyperthermia was only about 1.8oC and minimum hypothermia was only about 2.0oC (see Figures 1 and 2), so that effects of drugs on response to MDMA are best seen in terms of change in temperature from baseline (Figures 1 and 2).
Table 1.
Baseline core body temperature in mice prior to treatment with vehicle/antagonist drugs and, 30 min later, vehicle/MDMA
| Treatment group | Baseline core temperature (prior to treatment) (oC) | n |
|---|---|---|
| veh + veh | 35.46 ± 0.24 | 7 |
| veh + MDMA | 35.51 ± 0.19 | 7 |
| SR (0.5) + veh | 36.04 ± 0.10 | 7 |
| SR (0.5) + MDMA | 36.08 ± 0.11 | 7 |
| SR (5) + veh | 35.77 ± 0.14 | 7 |
| SR (5) + MDMA | 36.09 ± 0.26 | 7 |
| Praz (0.1)/SR (5) + veh | 36.24 ± 0.17 | 9 |
| Praz (0.1)/SR (5) + MDMA | 35.91 ± 0.19 | 9 |
MDMA, MDMA 20 mg·kg−1; Praz, prazosin 0.1 mg·kg−1; SR (0.5), SR59230A 0.5 mg·kg−1; SR (5), SR59230A 5 mg·kg−1; veh, vehicle. Note that the baseline temperatures were obtained prior to any drug treatment. Values are the means ± SEM from n experiments.
MDMA, methylenedioxymethamphetamine; SR59230A, 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride.
Figure 1.
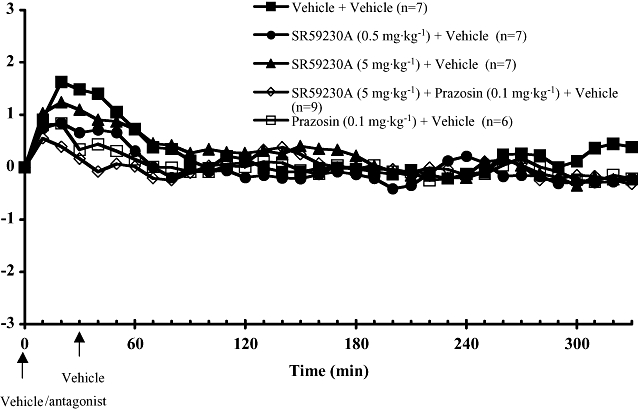
Core body temperature recordings in conscious wild-type mice treated with vehicle or antagonists 30 min prior to giving vehicle or MDMA, at room temperature. All drugs or vehicles were injected subcutaneously. Responses are expressed as change in body temperature. Vertical bars indicate the SEM from seven to nine mice. MDMA, methylenedioxymethamphetamine; SR59230A, 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride.
Figure 2.
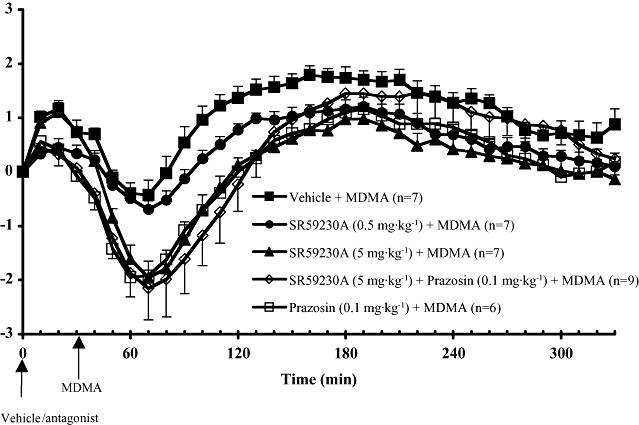
Core body temperature recordings in conscious wild-type mice administered with vehicle, SR59230A (0.5 or 5 mg·kg−1) or prazosin (0.1 mg·kg−1) plus SR59230A (5 mg·kg−1), 30 min prior to giving MDMA (20 mg·kg−1), at room temperature. Also shown are the effects of prazosin (0.1 mg·kg−1) (taken from Bexis and Docherty, 2008). All drugs were injected subcutaneously. Responses are expressed as change in body temperature. Vertical bars indicate the SEM from seven to nine mice. MDMA, methylenedioxymethamphetamine; SR59230A, 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride.
Effect of MDMA on core body temperature
Body temperature transiently increased after the administration of antagonist or antagonist vehicle (Figures 1 and 2). In all studies in which vehicle replaced MDMA, there were no significant differences between groups in the response to vehicle (Figure 1).
Following vehicle injection, the administration of MDMA (20 mg·kg−1) resulted in a slowly developing hyperthermia (Figure 2). Significant hyperthermia was observed at 70 min post MDMA with the maximal temperature rise of 1.8oC at 130 min post MDMA, after which temperature gradually declined (Figure 2). Please note that MDMA was injected at 30 min in Figure 2, so that times differ accordingly on the graphs.
Effects of the β3-adrenoceptor antagonist SR59230A on MDMA-induced hyperthermia
Following pretreatment of mice with the low concentration of SR59230A (0.5 mg·kg−1), MDMA produced qualitatively similar effects to its effects following vehicle: a slight decrease in core temperature followed by a delayed hyperthermic response (Figure 2) (no significant interaction, two-way anova). However, there was a significant difference in the column factor (temperature) (F1,33= 148.4, P < 0.0001), so that the temperature change was significantly less in the SR59230A (0.5 mg·kg−1) group (see Figure 2).
Pretreatment of mice with the high concentration of SR59230A (5 mg·kg−1) altered the monophasic hyperthermic response induced by MDMA to a biphasic response: a hypothermic response followed by a hyperthermic response (Figure 2). This change in shape of the temperature response to MDMA resulted in a significant interaction in anova (F33,1= 2.445. P < 0.0001). The onset of hypothermia in animals pretreated with the high concentration of SR59230A (5 mg·kg−1) occurred 20 min after the injection of MDMA and reached a minimum core temperature at 40 min after drug administration. The maximum core temperature was reached at 150 min after the injection of MDMA, followed by a gradual decrease in core temperature towards baseline levels (Figure 2). However, there was a significant difference in the column factor (temperature) (F33,1= 315.8, P < 0.0001), so that the temperature change was significantly lower in the SR59230A (5 mg·kg−1) group. In a Bonferroni post-test, the temperature response was significantly lower at 60–170 min and at 250–260 min (P < 0.05).
Comparison with prazosin as antagonist
In comparison with MDMA post vehicle, MDMA produced a significant hypothermia following the high concentration of SR59230A (5 mg·kg−1) or SR59230A (5 mg·kg−1) combined with prazosin (0.1 mg·kg−1) (P < 0.05, two-way anova) (Figure 2). Prazosin (0.1 mg·kg−1) alone also revealed a significant hypothermia to MDMA (Figure 2). For the low concentration of SR59230A (0.5 mg·kg−1), no significant hypothermia was seen (Figure 2).
Hypothermic minimum
When assessed by the minimum temperature reached following MDMA, SR59230A (5 mg·kg−1), alone or in combination with prazosin, but not SR59230A (0.5 mg·kg−1) revealed a significant hypothermia to MDMA, compared with the response obtained with MDMA post vehicle (P < 0.005, one-way anova).
Hyperthermic maximum
Methylenedioxymethamphetamine produced a significant hyperthermia (P < 0.05, one-way anova) in all groups, irrespective of the antagonist treatment employed, compared with baseline (Figure 2).
The maximum increases in core temperature produced by MDMA following low or high concentrations of SR59230A (0.5 or 5 mg·kg−1) were significantly reduced, compared with the response obtained with MDMA post vehicle (P < 0.05, one-way anova). However, the response to MDMA was not significantly reduced following SR59230A in combination with prazosin (Figure 2).
It is clear from Figure 2 that the degree of hypothermia can affect the later attainment of hyperthermia, so it is not surprising that the high concentration of SR59230A (5 mg·kg−1) reduced the maximum temperature reached, as this could be explained by the large initial hypothermia delaying the later hyperthermia. However, the low concentration of SR59230A (0.5 mg·kg−1) also significantly reduced the hyperthermia but, like vehicle, it did not reveal a hypothermia to MDMA (Figure 2). Hence, the low concentration of SR59230A causes a small but significant attenuation of the hyperthermia that cannot be explained by an early hypothermia (Figure 2).
Radioligand binding studies
In competition studies using membranes obtained from rat submandibular gland (α1A-adrenoceptor ligand binding sites) and spleen (α1B-adrenoceptor ligand binding sites), SR59230A displaced [3H]-prazosin binding in a competitive manner (Figure 3). From these studies, affinities of SR59230A were calculated as Ki values as shown in Table 2.
Figure 3.
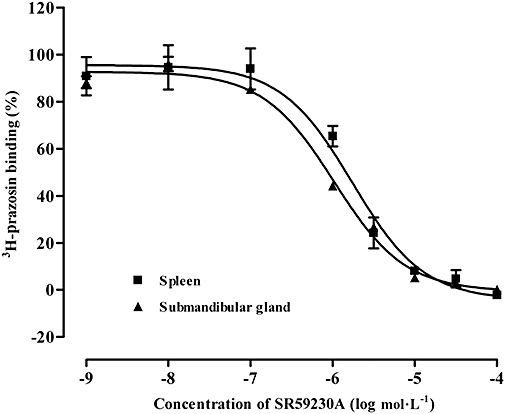
Binding curves showing the displacement by SR59230A of [3H]-prazosin binding to α1A-adrenoceptor ligand binding sites of rat submandibular gland and α1B-adrenoceptor ligand binding sites of rat spleen. Vertical bars represent SEM from four experiments. SR59230A, 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride.
Table 2.
Actions of SR59230A at α1-adrenoceptors in ligand binding (pKi) and functional (pKB) studies of rat submandibular gland (α1A), spleen (α1B) and aorta (α1D)
| Submandibularα1ApKi | Spleenα1BpKi | Spleenα1BpKB | Aortaα1DpKB |
|---|---|---|---|
| 6.35 ± 0.04 | 6.50 ± 0.07 | 6.12 ± 0.06 | 6.78 ± 0.11 |
| (n= 4) | (n= 4) | (n= 4) | (n= 5) |
Contractile studies
Phenylephrine contracted the rat spleen with a potency (pEC50, –log M) of 5.99 ± 0.16 (n= 4), and noradrenaline contracted the rat aorta with a potency of 7.78 ± 0.19 (n= 5), in the second concentration–response curve following vehicle (Figure 4). Phenylephrine was used as agonist in spleen to avoid α2-adrenoceptor-mediated contractions. In both spleen and aorta, SR59230A (10 µmol·L−1) produced significant, approximately parallel, shifts in the agonist concentration–response curve (Figure 2). Potency of SR59230A was calculated as a pKB value in spleen and aorta (Table 2).
Figure 4.
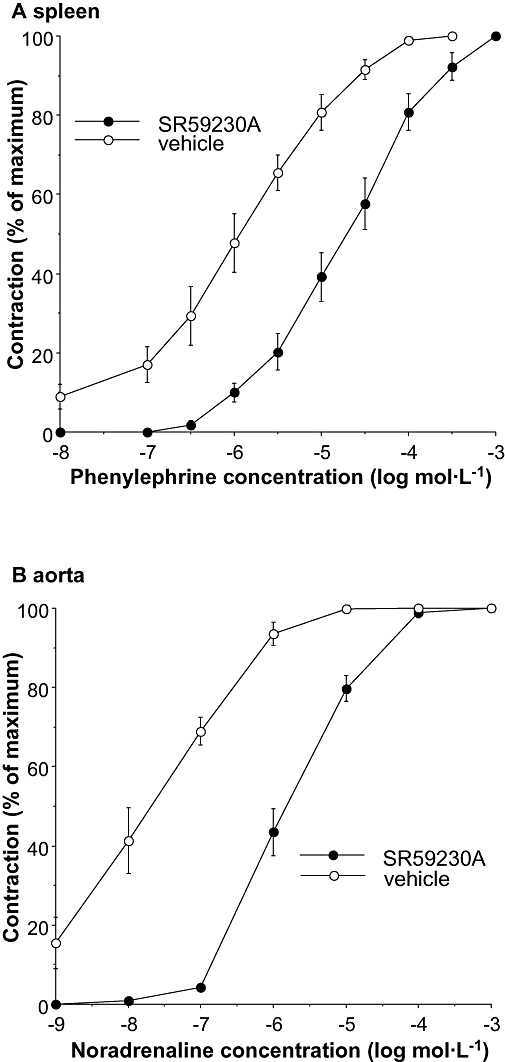
Effects of SR59230A (10 µmol·L−1) on contractions to phenylephrine in rat spleen (A) and contractions to noradrenaline in rat aorta (B). Responses shown are % of maximum for the second concentration–response curves following vehicle or SR 59230A. Vertical bars represent SEM from four (spleen) or five (aorta) experiments. In each individual experiment, shifts in agonist potency produced by SR59230A were corrected for shifts produced by vehicle, and pKB values were calculated (see Table 2). SR59230A, 1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride.
Discussion
It is well-established that hyperthermia is one of the most detrimental acute toxic effects of MDMA ingestion in humans. Consistent with clinical data, animal studies have also demonstrated that MDMA disrupts thermoregulation; however, the mechanism/s by which MDMA may disrupt thermoregulation still remain elusive, hindering therapeutic intervention.
In a recent study, we demonstrated that specific blockade of α1-adrenoceptors with prazosin (0.1 mg·kg−1) altered the monophasic hyperthermic response produced by MDMA in wild-type mice to a biphasic response with an initial hypothermic response followed by a hyperthermic response (Bexis and Docherty, 2008). Our results are consistent with other studies demonstrating the involvement of α1-adrenoceptors in the increase in core temperature seen after treatment with MDMA (Sprague et al., 2003). However, our study demonstrated that α1-adrenoceptor actions are only one component of the hyperthermic response induced by MDMA. Other studies have suggested that in addition to α1-adrenoceptors, β3-adrenoceptors may also be involved in MDMA-induced hyperthermia, at least in rats (Sprague et al., 2004; 2005;).
In the present study, pretreatment of wild-type mice prior to the administration of MDMA with a high concentration of SR59230A (5 mg·kg−1), a concentration that has been demonstrated to prevent brown adipose tissue thermogenesis (Manara et al., 1996), altered the monophasic hyperthermic response produced by MDMA. In the presence of SR59230A (5 mg·kg−1), the monophasic response produced by MDMA became biphasic, with an initial hypothermic response followed by a hyperthermic response. Both the minimum and maximum body temperatures were significantly different from those obtained for MDMA in the absence of the antagonist (post vehicle). Admittedly, the initial hypothermia may explain the reduced maximum hyperthermia. Furthermore, the hypothermic responses were similar in magnitude to the responses seen when mice were pretreated with prazosin (0.1 mg·kg−1) (Bexis and Docherty, 2008). Perhaps surprisingly, although the combination of high concentration of SR59230A and prazosin revealed a hypothermia to MDMA, there was no significant reduction in the maximum hyperthermia reached. Prazosin, alone, also fails to reduce the maximum hyperthermia to MDMA (see Bexis and Docherty, 2008). It is unclear why SR59230A did not reduce the maximum hyperthermia to MDMA in the presence of prazosin.
Although SR59230A has been commonly described as highly selective for β3-adrenoceptors (pA2 of 8.76, 1.7 nmol·L−1), there is increasing evidence, both from functional and radioligand binding studies, to suggest that SR59230A also has antagonistic actions at α1-adrenoceptors (pKB of 7.3, pKi of 6.61: Leblais et al., 2004) (pKi of 6.25: Brahmadevara et al., 2004). The present study shows that SR59230A antagonized noradrenaline/phenylephrine-induced contractions in the rat aorta (α1D-adrenoceptors) and spleen (α1B-adrenoceptors) (pKB= 6.78, 0.17 µmol, pKB= 6.12, 0.76 µmol respectively) and displaced 3H-prazosin binding in the submandibular gland (α1A-adrenoceptors) and spleen (α1B-adrencoceptors) (pKi of 6.35, 0.45 µmol·L−1 and pKi of 6.5, 0.32 µmol·L−1 respectively). Our data further support the competitive antagonist properties of SR59230A at α1-adrenoceptors. Based on the pKi and pKB values obtained in the above studies, the concentration of SR59230A (5 mg·kg−1: 13.8 µmol kg−1) used in this study would be high enough to produce marked block of α1-adrenoceptors. It can therefore be suggested that the biphasic response induced by MDMA in the presence of SR59230A (5 mg·kg−1) could be due to antagonism by SR59230A of α1-adrenoceptors, at least in part.
The fact that co-administration of prazosin (0.1 mg·kg−1) and SR59230A (5 mg·kg−1) produced a biphasic response similar to that seen when the antagonists were administered individually suggests that the two antagonists may be acting mainly through the same receptor type. This further supports the argument that SR59230A (5 mg·kg−1) may alter the hyperthermic response obtained with MDMA by antagonist actions at α1-adrenoceptors.
Although SR59230A is more than 100 times less potent than prazosin at α1-adrenoceptor subtypes, it was used in our telemetry studies at doses five or 50 times higher than the prazosin dose. Hence, α1-adrenoceptor actions are unlikely to contribute to the effects of a lower concentration of SR59230A (0.5 mg·kg−1) but may contribute to the effects of the high concentration of SR59230A (5 mg·kg−1). Even in terms of β-adrenoceptors, it has been reported that SR59230A is not selective for human β3-adrenoceptors (Vrydag and Michel, 2007).
The present study was then extended to investigate the effects of the low concentration of SR59230A (0.5 mg·kg−1 : 1.3 µmol·kg−1) on MDMA-induced hyperthermia, a dose of SR59230A that would only have threshold or minimal effects at α1-adrenoceptors (see Table 2, where affinity of SR59230A is in the range 0.16–0.76 µmol·L−1) but would still block β3-adrenoceptors (affinity of 1.7 nmol·L−1, see above). We have recently reported that SR59230A (1 µmol·L−1) abolishes relaxations to a β3-adrenoceptor agonist in mouse mesenteric arteries (Al Zubair et al., 2008). In the presence of the low concentration of SR59230A (0.5 mg·kg−1), the temperature response produced by MDMA remained monophasic but the maximum temperature reached in the delayed hyperthermia was slightly but significantly reduced when compared with the hyperthermic response induced by MDMA after pretreatment with vehicle. As no other antagonist treatment failed to reveal an initial hypothermia to MDMA, only the low concentration of SR59230A (0.5 mg·kg−1) can be directly compared with vehicle in terms of the maximum hyperthermia reached to MDMA. The other drug treatments could reduce the maximum hyperthermia reached simply by causing the initial hypothermia. Hence, it can be suggested that the low concentration of SR59230A (0.5 mg·kg−1) produced a small attenuation of the MDMA-induced hyperthermic response possibly by inhibiting β3-adrenoceptors on brown adipose tissue causing a decrease in heat production, although this effect is fairly minor. However, at the higher concentration, SR59230A (5 mg·kg−1), in addition to antagonizing β3-adrenoceptors, blocked α1-adrenoceptors, both centrally and peripherally, which lead to the attenuation of sympathetic control of brown adipose tissue and cutaneous blood vessels, causing both a decrease in heat production and an increase in heat loss leading to a hypothermic response in the presence of MDMA.
Indeed, peripheral effects of MDMA at α1-adrenoceptors can explain the findings of this study: cutaneous vasoconstriction by MDMA prevents an early hypothermic response to the drug. Such an effect may also explain the dependency of the temperature effects of MDMA on ambient temperature. At low ambient temperatures, cutaneous vasoconstriction is already marked so that MDMA produces little further vasoconstriction and the, presumed central, hypothermic actions of MDMA predominate. At high ambient temperatures, cutaneous dilatation has occurred, allowing a marked vasoconstrictor component to the actions of MDMA, and hyperthermia predominates. Hence, peripheral vasoconstrictor actions of MDMA modulate central hypo- and hyperthermic components. In addition, there may be a component of heat generation from brown fat.
In the present study, animals were housed individually. Previous studies have reported differences between group-housed and singly housed mice in the lethality of MDMA (Miller and O'Callaghan, 1995; Fantegrossi et al., 2003). Differences between studies may partly be explained in terms of differing housing conditions in addition to species and other differences. Indeed, in our previous studies of the rat, we found that MDMA produces biphasic hypo- and hyperthermia in the absence of α1-adrenoceptor antagonists (Bexis and Docherty, 2006), in contrast to the situation in the mouse where the initial hypothermic response is only revealed by the α1-adrenoceptor blockade.
In conclusion, the results of the present study suggest that the reduced hyperthermic actions of MDMA in mice by the β3-adrenoceptor antagonist SR59230A may largely involve α1-adrenoceptor antagonism. This suggests that α1-adrenoceptors are of more major importance than β3-adrenoceptors in the actions of MDMA on temperature, at least in the mouse, and that putative β3-adrenoceptor antagonists should be investigated taking into consideration possible α1-adrenoceptor antagonist actions.
Acknowledgments
This study was supported by the Health Research Board (Ireland). MDMA was generously supplied under the NIDA Drug Supply Program.
Glossary
Abbreviations:
- MDMA
methylenedioxymethamphetamine
- SR59230A
1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4,-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Zubair K, Bexis S, Docherty JR. Relaxations to beta-adrenoceptor subtype selective agonists in wild-type and NOS-3-KO mouse mesenteric arteries. Eur J Pharmacol. 2008;587:216–223. doi: 10.1016/j.ejphar.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of α2A-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2005;146:1–6. doi: 10.1038/sj.bjp.0706320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α-adrenoceptors. Br J Pharmacol. 2006;147:926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of α1-adrenoceptor subtypes in the effects of methylenedioxy methamphetamine (MDMA) on body temperature in the mouse. Br J Pharmacol. 2008;153:591–597. doi: 10.1038/sj.bjp.0707590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Modulation of the temperature effects of MDMA by the β3-adrenoceptors antagonist SR59230A in the mouse. Proceedings of BPS (in press.
- Brahmadevara N, Shaw AM, MacDonald A. 1-adrenoceptor antagonist properties of CGP 12177A and other β-adrenoceptor ligands: evidence against β beta3- or atypical β beta-adrenoceptors in rat aorta. Br J Pharmacol. 2004;142:781–787. doi: 10.1038/sj.bjp.0705840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YD, Barnett DB, Nahorski SR. 3H]Rauwolscine and [3H]yohimbine binding to rat cerebral and human platelet membranes: possible heterogeneity of alpha 2-adrenoceptors. Eur J Pharmacol. 1982;84:79–85. doi: 10.1016/0014-2999(82)90159-5. [DOI] [PubMed] [Google Scholar]
- Connaughton S, Docherty JR. Functional evidence for heterogeneity of peripheral prejunctional alpha 2-adrenoceptors. Br J Pharmacol. 1990;101:285–290. doi: 10.1111/j.1476-5381.1990.tb12702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, et al. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (‘ecstasy’) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology. 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle A, Honner V, Docherty JR. Investigation of the prejunctional α2-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Br. J. Pharmacol. 1999;128:975–980. doi: 10.1038/sj.bjp.0702875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblais V, Pourageaud F, Ivorra MD, Guibert C, Marthan R, Muller B. Role of alpha-adrenergic receptors in the effect of the beta-adrenergic receptor ligands, CGP 12177, bupranolol, and SR 59230A, on the contraction of rat intrapulmonary artery. J Pharmacol Exp Ther. 2004;309:137–145. doi: 10.1124/jpet.103.061192. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, et al. Functional identification of rat atypical beta-adrenoceptors by the first beta 3-selective antagonists, aryloxypropanolaminotetralins. Br J Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, Docherty JR. Vascular actions of MDMA involve α1 and α2-adrenoceptors in the anaesthetized rat. Br J Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Loury DN, Whiting RL. Identification of a single alpha 1-adrenoceptor corresponding to the alpha 1A-subtype in rat submaxillary gland. Br J Pharmacol. 1989;98:883–889. doi: 10.1111/j.1476-5381.1989.tb14617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol. 1995;11:177–192. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Neylon CB, Summers RJ. 3H]-rauwolscine binding to alpha 2-adrenoceptors in the mammalian kidney: apparent receptor heterogeneity between species. Br J Pharmacol. 1985;85:349–359. doi: 10.1111/j.1476-5381.1985.tb08868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective beta 3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol. 1996;49:7–14. [PubMed] [Google Scholar]
- Qvigstad E, Sjaastad I, Bøkenes J, Schiander I, Solberg L, Sejersted OM, et al. Carvedilol blockade of alpha1- and beta-adrenoceptor induced inotropic responses in rats with congestive heart failure. Eur J Pharmacol. 2005;516:51–59. doi: 10.1016/j.ejphar.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphatamine (Ecstasy) J Pharmacol Exp Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Brutcher RE, Mills EM, Caden D, Rusyniak DE. Attenuation of 3,4-methylenedioxymethamphatamine (MDMA, Ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoceptor antagonists. Br J Pharmacol. 2004;142:667–670. doi: 10.1038/sj.bjp.0705823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Moza P, Caden D, Rusyniak DE, Homes C, Goldstein DS, et al. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphatamine (MDMA, Ecstasy) in an animal model. Crit Care Med. 2005;33:1311–1316. doi: 10.1097/01.ccm.0000165969.29002.70. [DOI] [PubMed] [Google Scholar]
- Vrydag W, Michel MC. Tools to study beta3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:385–398. doi: 10.1007/s00210-006-0127-5. [DOI] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, ‘ecstasy’) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]


