Abstract
Background and purpose:
Cyclooxygenase inhibitors function to reduce levels of prostaglandin E2 (PGE2) and are broadly efficacious in models of bladder overactivity. We therefore investigated a regulation of urinary bladder function in conscious rats by modulation of the EP3 receptor for PGE2.
Experimental approach:
The activity of the EP3 receptor agonist GR63799X, and EP3 receptor antagonists, CM9 and DG041, at recombinant EP3 receptors was evaluated in vitro. In vivo, intraduodenal dosing during conscious, continuous-filling cystometry of spontaneously hypertensive rats was utilized to determine the urodynamic effect of EP3 receptor modulation.
Key results:
GR63799X dose-dependently (0.001–1 mg·kg−1) reduced bladder capacity, as indicated by a reduction in both the micturition interval and volume of urine per void. In contrast, CM9 (10 and 30 mg·kg−1) and DG041 (30 mg·kg−1) enhanced bladder capacity, as indicated by significantly longer micturition intervals and larger void volumes. CM9 and DG041 inhibited the responses to GR63799X supporting the in vivo activity of these pharmacological agents at the EP3 receptor. In addition to its effect on bladder capacity, GR63799X increased endogenous urine production. Intra-arterial infusion of saline mimicked the enhancement of urine flow observed with GR63799X, and the response was inhibited by CM9.
Conclusions and implications:
These data support the EP3 receptor as a modulator of urinary bladder activity in the conscious rat, and in addition, indicate a role for EP3 receptor activity in regulating urine flow.
Keywords: EP3 receptor, cystometry, urinary bladder, urine flow, overactive bladder, SHR
Introduction
Cyclooxygenase inhibition has been shown to regulate urinary bladder function by enhancing the functional bladder capacity in a number of species under various conditions (Takagi-Matsumoto et al., 2004; Angelico et al., 2006; Wibberley et al., 2006). Although cyclooxygenase inhibition decreases the production of many prostaglandins (PGs), these effects on bladder function are at least partially due to decreases in the levels of PGE2 (Hu et al., 2003). PGE2 is implicated as a contributor to urinary bladder overactivity as evidenced by the ability of PGE2 infused into the bladder to diminish bladder capacity (Maggi et al., 1988a; Schüssler, 1990; Ishizuka et al., 1995; McCafferty et al., 2008), and by increases in PGE2 production in bladder overactivity models (Park et al., 1999; Hu et al., 2003) as well as in overactive bladder patients (Kim et al., 2005; 2006;).
PGE2 is an agonist at EP, G protein-coupled, prostanoid receptors (EP1–EP4; nomenclature follows Alexander et al., 2008) that mediate the physiological effects of PGE2. Previously it has been suggested that the effects of PGE2 on bladder function are mediated through EP1 receptors; based on studies in knockout (KO) mice (Schröder et al., 2004) and with EP1 receptor antagonists (Maggi et al., 1988b; Lee et al., 2007). However, more recently it has been shown that EP3 receptor KO mice have a diminished response to bladder infusion of PGE2 and demonstrate an enhanced bladder capacity under basal conditions (McCafferty et al., 2008). This suggests an important contribution for EP3 receptors in the modulation of bladder function under physiological conditions, as well as under conditions of enhanced PGE2 production evoking pathological bladder overactivity.
EP3 receptors are expressed on dorsal root ganglion neurons (Oida et al., 1995; Kozaki et al., 2007; Wang et al., 2007; Su et al., 2008a) and have been shown to functionally sensitize nociceptor responses (Kumazawa et al., 1993; 1996;). Consistent with a neuronal role for EP3 receptors in controlling urinary bladder function, bladder distension-induced afferent firing is dampened by EP3 receptor antagonism (Su et al., 2008a). Therefore, we have utilized spontaneously hypertensive rats (SHR), which present with afferent-based bladder overactivity (Persson et al., 1998; Spitsbergen et al., 1998; Patra et al., 2007), to further evaluate EP3 receptors as a target in the development of treatments for overactive bladder.
We have extended our previous in vivo findings in EP3 receptor KO mice (McCafferty et al., 2008) using pharmacological agonism and antagonism of the EP3 receptor in conscious rats. We found that pharmacological activation and inhibition of the EP3 receptor resulted in a reduction and enhancement of functional bladder capacity respectively. In addition, our cystometric studies also provide evidence supporting a role for EP3 activity in urine production.
Methods
Cell-based assays of EP3 receptor ligands
U2OS cells were thawed and diluted into DMEM/F12 (HAM'S) 1:1 with L-glutamine, 15 mM HEPES, phenol red, 10% fetal bovine serum and 1% penicillin-streptomycin solution. BacMam viruses encoding human (0.25%) or rat (0.15%) EP3c (Namba et al., 1993) and Gqi5 were added to transduce U2OS cells plated into 384-well plates at 15 000 cells per well. Plates were then left at room temperature for 1 h followed by incubation at 37°C (5% CO2) for 24 h. The following day Fluo4 (2 µM) and Brilliant Black (500 µM final concentration) calcium indicator dyes were loaded into the cells by incubation for 1 h at 37°C (5% CO2) in Hank's buffered saline containing calcium, magnesium and probenecid (2.5 mM). Compounds were diluted into Hank's buffered saline containing calcium, magnesium and CHAPS (0.01%). A 20-point PGE2 response curve was generated with half log concentrations of PGE2, up to 2 µM. For each experiment an EC50 value was generated from a PGE2 curve and was used to determine the EC80 challenge for the antagonist assay [calculated via EC80= 4(EC50/Hillslope)]. Antagonists were pretreated, and fluorescence measurements were made over 65 s after agonist addition by using a FLIPR system (Fluorescence imaging plate reader, Molecular Devices). IC50 values were utilized to calculate pKi values for each compound (Cheng and Prusoff, 1973).
Conscious, continuous-filling cystometry
All animal care and experiments were approved by the institutional animal care and use committee (IACUC) of GlaxoSmithKline. Female SHR were purchased from Charles River Laboratories with urinary bladder and intraduodenal (i.d.) catheters surgically implanted by Charles River Surgical Services for saline infusion and compound dosing respectively. In some rats an additional femoral artery catheter was also implanted. After a 5 day minimum post-surgical recovery period, rats were placed in restraints (Braintree Scientific) located above a balance utilized to measure voided urine volumes. Urinary bladder catheters were extended with PE-50 tubing and connected to a pressure transducer and a saline infusion pump (Harvard Instruments) via a three-way connector. Bladder pressure was viewed and recorded via Chart software through a PowerLab data acquisition system (ADI Instruments). Bladders were continuously infused with room temperature saline at a rate of 100 µL·min−1 for the duration of studies. A 2 h infusion was used as an equilibration period allowing urodynamics to stabilize and the rats to become familiar with the restraints. After equilibration, an hour of control/basal urodynamics was obtained prior to i.d. compound dosing via the duodenal catheter. Urodynamics were continually assessed for a 2 h period post dosing. In antagonist pretreatment studies, pretreatment doses were administered 2 h prior to dosing with GR63799X. Dosing solutions of compounds were prepared in 10% Gelucire, sonicated and dosed intraduodenally (i.d.) as a suspension. In some rats saline was infused (10 µL·min−1) into the femoral artery via the chronically implanted arterial catheter in order to enhance endogenous urine production.
Data analysis and statistics
Averages of cystometric parameters were obtained for 30 min time periods post dosing. These values were normalized to the control period and expressed as a percentage of control. Data were normalized to reduce numerical variability between groups and facilitate unpaired comparisons. Baseline/control micturition interval and void volume group values were also reported. In the cases where single time point data are shown the 90–120 min values were utilized for comparison, as this time point typically demonstrated the most stable and robust response over the treatment period. Interval was measured as the time between micturition events and void volume as the average volume of urine per micturition event. Peak micturition pressures were measured as the maximum pressure reached during urine voiding. Residual volumes were assessed as the difference between the volume infused (interval*100 µL·min−1) and volume voided for a given cystometrogram. Residual volumes were then divided by the micturition interval providing a measure of endogenous urine flow.
Data were expressed as averages with associated standard errors, and statistics were performed by using GraphPad Prism version 4.02. Student's unpaired t-tests or a one-way analysis of variance (anova) followed by a Bonferroni post hoc analysis was utilized. Time course data were analysed by two-way repeated measures anova followed by a Bonferroni post hoc analysis to compare equivalent time points where applicable.
Materials
The EP3 receptor agonist GR63799X and EP3 receptor antagonists (CM9 and DG041) were synthesized in house at GlaxoSmithKline Pharmaceuticals (King of Prussia, PA, USA).
Results
In vitro potency of EP3 receptor ligands
The EP3 receptor agonist GR63799X (Bunce et al., 1991; Figure 1A) demonstrated an ability to stimulate Ca2+ entry into U2OS cells transduced with full-length human EP3 receptors demonstrating a pEC50 of 7.2 ± 0.2 (n= 6; Figure 1D). EP3 receptor antagonists CM9 (Juteau et al., 2001; Figure 1B) and DG041 (Zegar et al., 2007; Figure 1C) were confirmed as active at EP3 receptors. CM9 inhibited PGE2-induced Ca2+ entry in cells with either human or rat EP3 receptors (Figure 1E; Table 1). DG041 also dose-dependently inhibited Ca2+ influx initiated by activation of human and rat EP3 receptors (Figure 1E; Table 1). For both CM9 and DG041, the potency at the rat receptor appeared to be somewhat reduced when compared with the equivalent human EP3 receptor.
Figure 1.
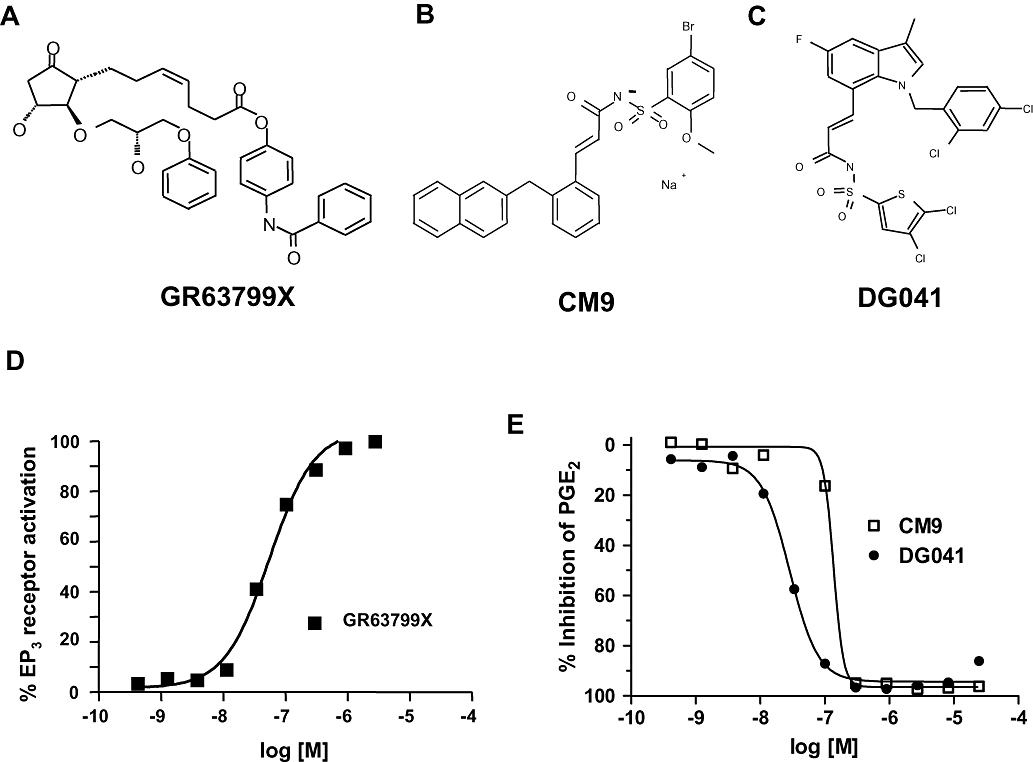
Effects of EP3 receptor modulators. (A) GR63799X, EP3 receptor agonist. (B) CM9, EP3 receptor antagonist. (C) DG041, EP3 receptor antagonist. (D) Representative dose–response to GR63799X on cells expressing human EP3 receptors. Data are expressed as a percentage of the maximum PGE2 response. (E) Representative dose–response curves to CM9 and DG041 demonstrating a complete inhibition of the PGE2-evoked response at the rat EP3 receptor.
Table 1.
Potencies of EP3 receptor antagonists in the fluorescence imaging plate reader assay
| Human EP3 | Rat EP3 | ||
|---|---|---|---|
| CM9 | pIC50 | 7.03 ± 0.04 (n= 101) | 6.58 ± 0.04 (n= 73) |
| fpKi | 7.77 ± 0.04 | 7.12 ± 0.04 | |
| DG041 | pIC50 | 7.66 ± 0.04 (n= 101) | 7.04 ± 0.03(n= 70) |
| fpKi | 8.41 ± 0.04 | 7.57 ± 0.03 | |
pIC50 values were measured in response to an EC80 challenge of the EP3 receptor agonist PGE2.
Urodynamic response to EP3 receptor activation
In conscious, continuous-filling cystometry in the SHR, GR63799X (0.1 mg·kg−1) given i.d. increased the urination frequency as compared with control (Figure 2A). This was indicated by a time-dependent decrease in the micturition interval (Figure 2B) and was also reflected by a decrease in void volume, after treatment with GR63799X (Figure 2C). Vehicle was without effect, and the GR63799X response was significantly different than vehicle, for both micturition interval and void volume. Control micturition intervals for the vehicle- (n= 10) and GR63799X (n= 4)-treated groups were 12 ± 2 and 13 ± 1 min, and control void volumes were 1.2 ± 0.2 and 1.2 ± 0.1 mL respectively. The response to GR63799X was dose-dependent with an IC50 of 0.01 mg·kg−1 i.d., as measured by the micturition interval (Figure 3). Control interval values for the GR63799X treatment groups were the following: 0.001 mg·kg−1, 11 ± 2 min (n= 8); 0.003 mg·kg−1, 12 ± 1 min (n= 7); 0.01 mg·kg−1, 12 ± 1 min (n= 7); 0.1 mg·kg−1 13 ± 1 min (n= 4); 0.3 mg·kg−1, 9 ± 1 min (n= 3); and 1 mg·kg−1 12 ± 2 min (n= 4). GR63799X had no significant effect on peak micturition pressure at the doses evaluated (percentage control: vehicle 104 ± 4%, 0.001 mg·kg−1 83 ± 7%, 0.003 mg·kg−1 108 ± 7%, 0.01 mg·kg−1 104 ± 9%, 0.1 mg·kg−1 96 ± 8%, 0.3 mg·kg−1 97 ± 6% and 1 mg·kg−1 106 ± 7%).
Figure 2.
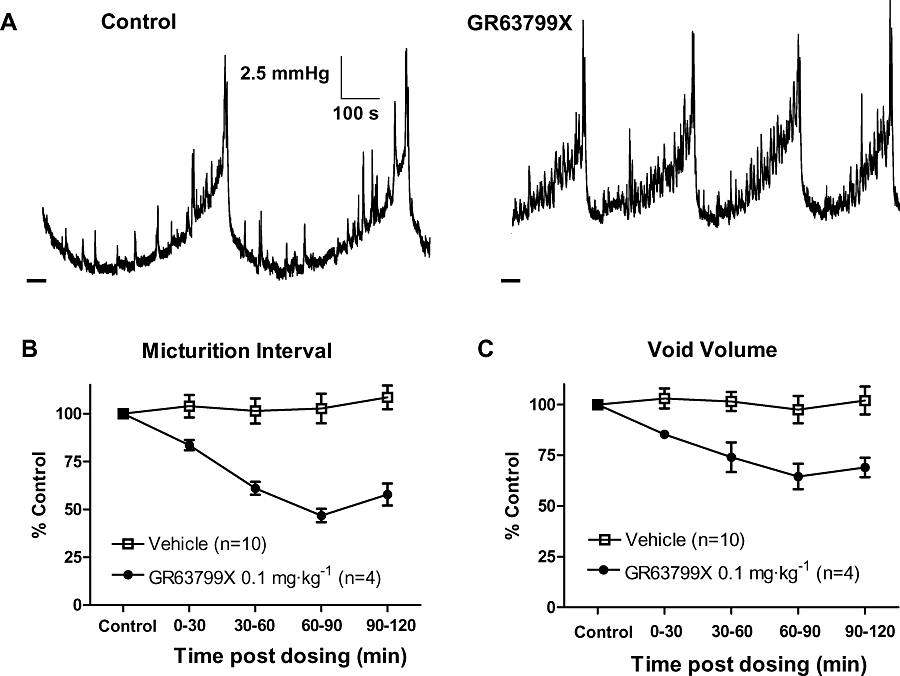
Effect of GR63799X on rat urodynamics. (A) Bladder pressure recordings before and after administration of GR63799X (0.1 mg·kg−1, intraduodenal). Dashed line at the lower left of traces is 0 mmHg. (B and C) Time course of the response to GR63799X and vehicle, demonstrating a reduction in micturition interval (P= 0.002 vs. vehicle, two-way repeated measures anova) and average void volume (P= 0.01 vs. vehicle) respectively.
Figure 3.
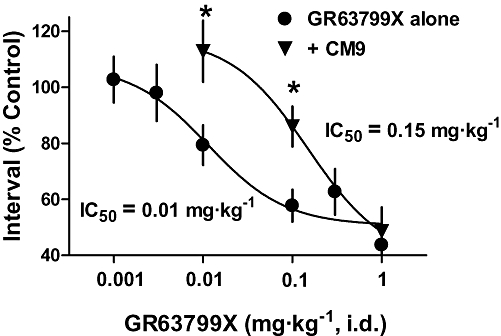
EP3 receptor antagonist CM9 antagonized the reduction in micturition interval, induced by GR63799X. Dose–response curves of the effect of GR63799X on micturition interval, in the presence (+CM9) and absence (GR63799X alone) of the EP3 receptor antagonist CM9 [30 mg·kg−1, intraduodenal (i.d.)]. Each data point represents a separate group of 3–8 rats (*P < 0.05 vs. GR63799X alone same GR63799X dose, unpaired t-test).
Urodynamic response to EP3 receptor antagonism
EP3 receptor antagonism with the chemically distinct antagonists CM9 (10 mg·kg−1, i.d.) and DG041 (30 mg·kg−1, i.d.) enhanced the bladder capacity, as indicated by significant increases in both void volume and micturition interval when compared with vehicle treatment (Figure 4B and C respectively). In this study control void volumes (mL) and intervals (min) were the following: vehicle 0.9 ± 0.1, 9 ± 1 (n= 18); CM9 0.7 ± 0.1, 7 ± 1 (n= 8); and DG041 0.66 ± 0.08, 6.1 ± 0.7 (n= 8) respectively. A representative cystometric pressure recording under control conditions and after CM9 treatment at 10 mg·kg−1 is shown in Figure 4A. As was observed with EP3 receptor activation (Figure 2), peak micturition pressure was not affected by EP3 receptor antagonism with either CM9 or DG041 (Figure 4A; percentage control: vehicle 99 ± 4%, DG041 83 ± 7% and CM9 113 ± 10%).
Figure 4.
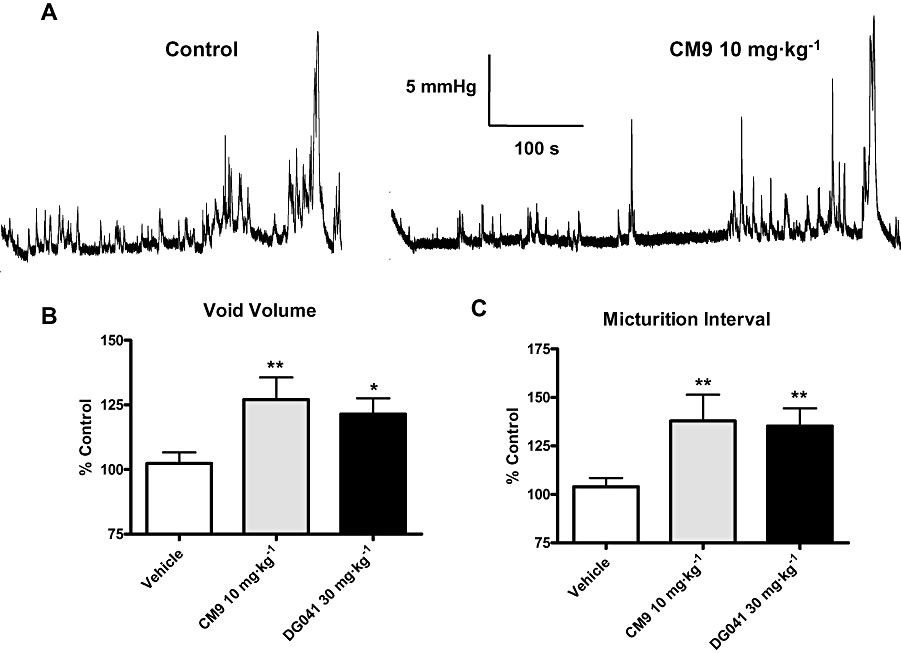
EP3 receptor antagonists CM9 and DG041 increased bladder capacity. (A) Representative recording of the effect of 10 mg·kg−1 CM9 on cystometric urodynamics. CM9 and DG041 increased void volume (B) and micturition interval (C), compared with vehicle treatment. Data are expressed as a percentage of control for each group. Vehicle (n= 18), CM9 (10 mg·kg−1, n= 8) and DG041 (30 mg·kg−1, n= 8). (*P < 0.05, **P < 0.01, vs. vehicle, unpaired t-test).
EP3 receptor antagonists inhibit the response to GR63799X
The ability of GR63799X to reduce the micturition interval and void volume was antagonized by pretreatment with CM9 (30 mg·kg−1, i.d.). Assaying micturition interval, CM9 pretreatment evoked a clear rightward shift in the GR63799X dose–response curve, shifting the IC50 value for GR63799X from 0.01 mg·kg−1 in the absence of CM9 to 0.15 mg·kg−1 in the presence of CM9 (Figure 3). The reductions in void volume and micturition interval induced by GR63799X (0.1 mg·kg−1, i.d.) were also antagonized by pretreatment with DG041 (3 and 30 mg·kg−1, i.d.) and by CM9 at 3 mg·kg−1, i.d. (Figure 5). Control void volume (mL) and interval (min) values prior to GR63799X (0.1 mg·kg−1) challenge (after respective pretreatments) were the following: vehicle 1.1 ± 0.1, 12 ± 1; DG041 (3 mg·kg−1) 0.72 ± 0.07, 6.8 ± 0.6; DG041 (30 mg·kg−1) 1.2 ± 0.2, 10 ± 2; and CM9 (3 mg·kg−1) 0.74 ± 0.09, 6 ± 1. Consistent with the effects of DG041 and CM9 on the GR63799X response being EP3 receptor-mediated, and not via an alternative competing mechanism, vehicle challenge (instead of GR63799X) of CM9 (30 mg·kg−1, i.d.)-pretreated rats was without effect (Figure 5; baseline values CM9 alone 30 mg·kg−1 0.9 ± 0.1 mL, 8.8 ± 0.9 min). As was observed with GR63799X (Figure 2), or antagonist treatment alone (Figure 4), in the GR63799X/antagonist combination studies no significant alteration in peak micturition pressure was detected with GR63799X (0.1 mg·kg−1) challenge after the various pretreatments (peak pressure as percentage control: vehicle 104 ± 6% (n= 10), DG041 (3 mg·kg−1) 104 ± 76% (n= 4), DG041 (30 mg·kg−1) 109 ± 12% (n= 4), CM9 (3 mg·kg−1) 95 ± 9% (n= 4) and CM9 (30 mg·kg−1) alone 97 ± 4% (n= 4)).
Figure 5.
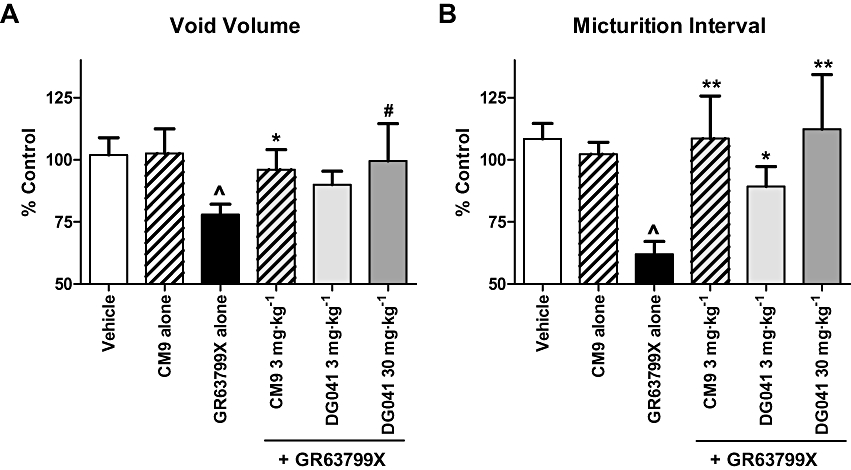
EP3 receptor antagonists CM9 and DG041 antagonized the cystometric responses to EP3 receptor activation with GR63799X. GR63799X [0.1 mg·kg−1, intraduodenal (i.d.)] evokes a reduction in void volume (A) and micturition interval (B) that was antagonized by pretreatment with CM9 (3 mg·kg−1, i.d.) or DG041 (3 and 30 mg·kg−1, i.d.). CM9 pretreatment followed by vehicle instead of GR63799X challenge was without effect (CM9 alone, 30 mg·kg−1). Vehicle and GR63799X alone n= 10 per group, and CM9 alone (30 mg·kg−1), CM9 (3 mg·kg−1), DG041 (3 mg·kg−1) and DG041 (30 mg·kg−1) n= 4 per group. (^P= 0.008 void volume vs. vehicle, ^P < 0.0001 micturition interval vs. vehicle, unpaired t-test; *P ≤ 0.05, **P < 0.01, #P= 0.07 vs. GR63799X alone, unpaired t-test).
Activation of EP3 receptors modulates urine production
As described above GR63799X evoked a significant reduction both in the micturition interval and average voided urine volume (Figure 2). However, in GR63799X-treated rats, the reduction in micturition interval was significantly greater than that observed for the void volume (Figure 6A; P= 0.01, two-way repeated measures anova). These 10 rats had an average control interval of 12 ± 1 min and void volume of 1.1 ± 0.1 mL at baseline. Comparing the volumes of urine voided to those infused into the bladder, via the bladder catheter during cystometrigrams, we noted that in the presence of GR63799X, the rats were voiding more volume than was infused into their bladders. This was reflected by the increase of urine flow in the presence of GR63799X, compared with vehicle treatment (Figure 6B). Vehicle treatment alone had no effect on urine flow. This suggests that in addition to reducing functional bladder capacity as indicated by the reduction in the average volume per void (Figure 2C), GR63799X (i.d.) also increased endogenous urine production.
Figure 6.
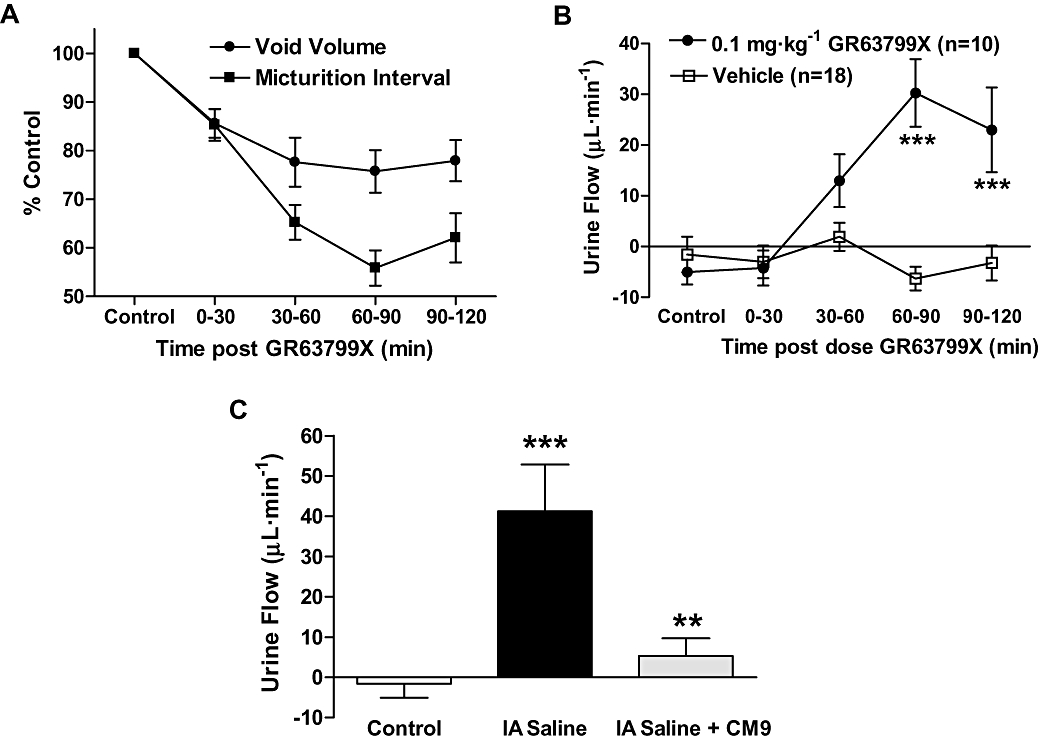
EP3 receptor modulation altered urine production as assessed during continuous-filling cystometry. (A) Comparison of the average normalized response on void volume and micturition interval in response to GR63799X treatment (n= 10). The reduction in micturition interval was significantly greater than that in void volume (P= 0.01, two-way repeated measures anova). (B) GR63799X [0.1 mg·kg−1, intraduodenal (i.d.)] increased endogenous urine flow, compared with vehicle treatment (P= 0.006, two-way repeated measures anova, ***P < 0.001 Bonferroni post hoc vs. vehicle). (C) Intra-arterial infusion of saline (IA saline) increases urine production, as indicated by the increase of urine flow. CM9 (30 mg·kg−1, i.d.) antagonized this response (P < 0.0001 one-way anova, **P < 0.01 vs. IA saline, ***P < 0.001 vs. control).
Under control conditions, in these cystometric studies, endogenous urine flow is undetectable (Figure 6B control time points and vehicle group; Figure 6C control). In order to enhance endogenous urine production during cystometry rats were intra-arterially (IA) infused with saline (10 µL·min−1), in addition to the typical continuous-filling of the bladder lumen (100 µL·min−1). This IA infusion increased the vascular blood volume and thereby significantly enhanced endogenous urine flow (Figure 6C). The increase in urine flow observed with IA infusion of saline was comparable to that observed with GR63799X treatment (compare Figures 6B and C). Administration of the EP3 receptor antagonist CM9 (30 mg·kg−1) to IA saline infused rats nearly abolished the induced urine flow (Figure 6C). This suggests that EP3 receptor antagonism could decrease endogenous urine production and was consistent with our observation that EP3 receptor activation increased urine flow.
Discussion
Here we have demonstrated that EP3 receptor modulation alters both functional urinary bladder capacity and endogenous urine flow in conscious SHR. EP3 receptor activation induced bladder overactivity by decreasing the functional bladder capacity (void volume), and EP3 receptor antagonism, with two chemically distinct antagonists, was shown to increase functional bladder capacity. In addition, in the same rats, activation of EP3 receptors evoked diuresis, and EP3 receptor antagonism induced an antidiuretic effect. Therefore, in addition to a role for EP3 receptors in regulating bladder activity it appears that the EP3 receptor has a role in regulating urine production.
We conclude that the observed effects on bladder activity and urine flow involve two mechanisms of action, and that the enhancement of urine flow was not the sole underlying cause of bladder overactivity induced by EP3 receptor activation. As a result of an enhanced urine production alone, under these study conditions it would be expected that the volume of urine per micturition (void volume) would remain unaffected and a decrease in the interval would occur, or alternatively the void volume could increase, as observed in dogs and rabbits in response to diuresis (Levin et al., 1995; McCafferty et al., 2009). Because the void volume demonstrated a significant decrease in response to EP3 receptor activation, this indicates that a kidney-independent, bladder-based, effect is likely. A bladder-based effect is further supported by a response to GR63779X delivered locally to the bladder lumen of mice (McCafferty et al., 2008). Bladder infusion of GR63799X into mice did not evoke a detectable increase in urine production; probably as a result of the local delivery of the compound to the bladder. However, dosing GR63799X i.d. provides access to the urinary bladder and to the renal and cardiovascular systems, and has revealed the additional diuretic consequence of systemic EP3 receptor activation shown here. Consistent with a dual functional contribution (bladder activity and urine flow) to the effect of GR63799X on micturition interval, GR63799X demonstrated a significantly larger reduction on the interval than on the void volume. The interval reduction due to GR63799X represents the additive effect of activating EP3 receptors on bladder function and on urine production, whereas the smaller response on the void volume presumably represents the effect on bladder function alone.
Previously we have shown that inhibiting EP3 receptor function by genetic ablation of the EP3 receptor gene enhanced conscious bladder capacity under basal conditions (McCafferty et al., 2008). Our pharmacological data presented here utilizing conscious rats demonstrated an enhancement of bladder capacity by pharmacological inhibition of the EP3 receptor with chemically distinct antagonists. These findings in conscious rats are consistent with the phenotype displayed by EP3 receptor knockout mice. EP3 receptor-mediated modulation of bladder activity has been proposed to occur via an afferent mechanism (McCafferty et al., 2008; Su et al., 2008a). Therefore, SHR were chosen for the current study as they have been reported to display afferent-based urinary bladder hyperactivity (Persson et al., 1998; Spitsbergen et al., 1998). However, no differences in the expression or function of EP3 receptors in the SHR have been observed compared with normal rats (Su et al., 2008a), suggesting that the EP3 receptor-mediated responses reported here may well be observed in other rat strains. This is supported by equivalent EP3 receptor-mediated responses observed in anaesthetized bladder models performed in several strains (Su et al., 2008a).
The responses to activation of EP3 receptors with GR63799X on void volume and micturition interval were inhibited by EP3 receptor antagonism with the two chemically distinct EP3 receptor antagonists, CM9 and DG041, suggesting that the reported effects of GR63799X are indeed mediated via the EP3 receptor. The selectivity of GR63799X for EP3 receptor is further supported by the loss of GR63799X-induced bladder overactivity in EP3 receptor KO mice (McCafferty et al., 2008), along with previously published binding studies (Bunce et al., 1991; Kiriyama et al., 1997). Using a FLIPR-based calcium influx assay we demonstrated the ability of CM9 and DG041 to antagonize the rat EP3 receptor and confirmed activity at the human receptor (Su et al., 2008b). In general DG041 was more potent than CM9, and antagonism at the human EP3 receptor was more potent than at the rat receptor for both compounds under our conditions. The selectivity of CM9 and DG041 for the EP3 receptor is supported by binding assays to other members of the EP receptor family (EP1, EP2 and EP4) and other PG receptors (IP, TP and FP; Coleman et al., 1994; Su et al., 2008b). DG041 demonstrates a low nanomolar affinity for the PGD2 (DP) receptor (Su et al., 2008b). This is not the case for the chemically distinct antagonist, CM9, which we have used to replicate the effects of DG041, and therefore DG041 acting via the DP receptor is unlikely to explain the functional DG041 responses reported here in the conscious rat. Furthermore, the ability of CM9 and DG041 to antagonize the response to GR63799X in vivo strongly suggests that these compounds are active at the EP3 receptor at these doses in the rat. The possibility that CM9 was working via an independent competing mechanism in these experiments was ruled out by the lack of response (increase in void volume and interval) in CM9-pretreated rats, challenged with vehicle in the place of GR63799X. Taken together, our in vivo and in vitro selectivity data support the functional effects observed here with CM9 and DG041 as being mediated by EP3 receptors.
The mRNA for EP3 receptors has been specifically detected in L6/S1 dorsal root ganglia that innervate the rat urinary bladder, and this expression was comparable to that detected in the intact rat bladder (Su et al., 2008a). Expression of EP3 receptors in intact bladders may represent their expression on nerve terminals located within the bladder wall. Alternatively, or in addition to a neuronal expression, EP3 receptors may be expressed in the smooth muscle, urothelium and/or other cell types present within the intact bladder. A functional role for the bladder-based EP3 receptor expression is supported by the ability of GR63799X delivered to the bladder to selectively induce bladder overactivity in EP3 receptor wild-type mice (McCafferty et al., 2008), while the functional importance of an afferent-based expression is supported by the finding that inhibition of peripheral EP3 receptors decreased bladder-induced afferent firing (Su et al., 2008a). In addition, efficacy in anaesthetized, bladder models has also been described via central delivery of EP3 receptor antagonists suggesting a modulatory role for EP3 receptors in the central control of the micturition reflex (Su et al., 2008b). The effects on conscious rat bladder urodynamics described here with i.d. administration of compounds were likely to have been mediated via modulation of peripheral EP3 receptors as DG041 does not penetrate the blood–brain barrier (Su et al., 2008a). Expression of EP3 receptors on afferent nerves is likely to play an important functional role in the regulation of urodynamics. We cannot, however, eliminate a contribution from EP3 receptors expressed in other cell types, in the modulation of bladder function.
EP1 receptors have been implicated in urinary bladder function based on studies utilizing EP1 receptor KO mice (Schröder et al., 2004) and EP1 receptor antagonists (Maggi et al., 1988b; Lee et al., 2007). In these studies PGE2-induced bladder overactivity was inhibited by EP1 receptor KO or antagonist administration. Although the EP3 receptor KO yielded a reduced sensitivity to infusion of PGE2 into the bladder, the response was not completely prevented (McCafferty et al., 2008). Therefore, under pathological conditions of bladder overactivity when PGE2 levels are increased, as occurs in preclinical models and patients (Park et al., 1999; Hu et al., 2003; Kim et al., 2005; 2006;), EP1 and EP3 receptors may both play a role in PGE2-mediated overactivity. The data provided here in conscious rats using pharmacological modulators further supports an involvement of EP3, in addition to EP1 receptors, in regulating conscious urodynamics.
PGE2 is produced by various cell types within the kidney, regulates blood flow and the permeability of various segments of the nephron, including the collecting ducts (Hao and Breyer, 2008). In this study EP3 receptor activation with GR63799X increased urine flow indicating that EP3 receptor activity favours the production of urine, while EP3 receptor antagonism with CM9 inhibited urine flow elicited by arterial saline infusion indicating that EP3 receptors play a functional role during volume overload. These results are consistent with studies in EP3 receptor KO mice suggesting that EP3 receptor activity functions to regulate urine osmolality (Fleming et al., 1998). EP3 receptors are highly expressed in the renal medulla and cortical collecting duct (Breyer et al., 1993; Sugimoto et al., 1994; Takeuchi et al., 1994a,b; Taniguchi et al., 1994) where PGE2 has been shown to inhibit water re-absorption by reducing epithelial cAMP levels (Hébert et al., 1991; Hao and Breyer, 2008). Furthermore, EP3 receptors are coupled to Gi proteins that reduce cAMP and thus promote diuresis as exemplified by its ability to inhibit the antidiuretic response of vasopressin (Grantham and Burg, 1966; Takeuchi et al., 1994a,b; Breyer et al., 1996; Nielsen et al., 1999).
In addition, the changes in urine flow, dependent on EP3 receptors, described here could result from altered renal blood flow (Edwards, 1985; Tang et al., 2000; Audoly et al., 2001), and/or changes in mean arterial pressure in response to the EP3 receptor modulators (Audoly et al., 1999; Zhang et al., 2000; van Rodijnen et al., 2007). Volume overload by saline infusion may increase EP3 receptor activity in the renal/cardiovascular system and contribute to the induced urine flow. While it is likely that EP3 receptor-mediated modulation of renal output involves an alteration in tubular permeability, haemodynamic changes or other undescribed EP3 receptor-mediated responses, may also contribute.
In conclusion, we have demonstrated in conscious rats that activation of EP3 receptors has a dual functional consequence inducing bladder overactivity and diuresis, and that antagonism of EP3 receptors has the expected, opposing response of reducing bladder activity and urine output. A development of EP3 receptor antagonists as a therapy for bladder overactivity would require a particular consideration of the renal consequences of EP3 receptor modulation.
Acknowledgments
This study was supported by GlaxoSmithKline Pharmaceuticals. The authors would like to thank Dr Christine Schnackenberg for insight on renal function.
Glossary
Abbreviations:
- EP
receptor
- EP-type
prostanoid receptor
- FLIPR
fluorescence imaging plate reader
- i.d.
intraduodenal
- KO
knockout
- PGE2
prostaglandin E2
- SHR
spontaneously hypertensive rat
Conflict of interest
No conflicts of interest. All the authors are employees of GlaxoSmithKline Pharmaceuticals.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelico P, Guarneri L, Velasco C, Cova R, Leonardi A, Clarke DE, et al. Effect of cyclooxygenase inhibitors on the micturition reflex in rats: correlation with inhibition of cyclooxygenase isozymes. BJU Int. 2006;97:837–846. doi: 10.1111/j.1464-410X.2006.06003.x. [DOI] [PubMed] [Google Scholar]
- Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, et al. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol. 1999;277:H924–H930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- Audoly LP, Ruan X, Wagner VA, Goulet JL, Tilley SL, Koller BH, et al. Role of EP(2) and EP(3) PGE(2) receptors in control of murine renal hemodynamics. Am J Physiol. 2001;280:H327–H333. doi: 10.1152/ajpheart.2001.280.1.H327. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Jacobson HR, Davis LS, Breyer RM. In situ hybridization and localization of mRNA for the rabbit prostaglandin EP3 receptor. Kidney Int. 1993;44:1372–1378. doi: 10.1038/ki.1993.391. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Jacobson HR, Breyer RM. Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol. 1996;7:8–17. doi: 10.1681/ASN.V718. [DOI] [PubMed] [Google Scholar]
- Bunce KT, Clayton NM, Coleman RA, Collington EW, Finch H, Humphray JM, et al. GR63799X – a novel prostanoid with selectivity for EP3 receptors. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:379–382. [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Edwards RM. Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Physiol. 1985;248:F779–F784. doi: 10.1152/ajprenal.1985.248.6.F779. [DOI] [PubMed] [Google Scholar]
- Fleming EF, Athirakul K, Oliverio MI, Key M, Goulet J, Koller BH, et al. Urinary concentrating function in mice lacking EP3 receptors for prostaglandin E2. Am J Physiol. 1998;275:F955–F961. doi: 10.1152/ajprenal.1998.275.6.F955. [DOI] [PubMed] [Google Scholar]
- Grantham JJ, Burg MB. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966;211:255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- Hébert RL, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest. 1991;87:1992–1998. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol. 2003;284:R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- Ishizuka O, Mattiasson A, Andersson KE. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins? J Urol. 1995;153:2034–2038. doi: 10.1016/s0022-5347(01)67397-x. [DOI] [PubMed] [Google Scholar]
- Juteau H, Gareau Y, Labelle M, Sturino CF, Sawyer N, Tremblay N, et al. Structure–activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg Med Chem. 2001;9:1977–1984. doi: 10.1016/s0968-0896(01)00110-9. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol. 2005;12:875–880. doi: 10.1111/j.1442-2042.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol. 2006;175:1773–1776. doi: 10.1016/S0022-5347(05)00992-4. [DOI] [PubMed] [Google Scholar]
- Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki Y, Kambe F, Hayashi Y, Ohmori S, Seo H, Kumazawa T, et al. Molecular cloning of prostaglandin EP3 receptors from canine sensory ganglia and their facilitatory action on bradykinin-induced mobilization of intracellular calcium. J Neurochem. 2007;100:1636–1647. doi: 10.1111/j.1471-4159.2006.04320.x. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Koda H. Involvement of EP3 subtype of prostaglandin E receptors in PGE2-induced enhancement of the bradykinin response of nociceptors. Brain Res. 1993;632:321–324. doi: 10.1016/0006-8993(93)91169-s. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Koda H, Fukusako H. EP receptor subtypes implicated in the PGE2-induced sensitization of polymodal receptors in response to bradykinin and heat. J Neurophysiol. 1996;75:2361–2368. doi: 10.1152/jn.1996.75.6.2361. [DOI] [PubMed] [Google Scholar]
- Lee T, Hedlund P, Newgreen D, Andersson KE. Urodynamic effects of a novel EP1 receptor antagonist in normal rats and rats with bladder outlet obstruction. J Urol. 2007;177:1562–1567. doi: 10.1016/j.juro.2006.11.070. [DOI] [PubMed] [Google Scholar]
- Levin RM, Wein AJ, Eika B, Tammela TL, Longhurst PA. Effects of diuresis on micturition. Neurourol Urodyn. 1995;14:169–176. doi: 10.1002/nau.1930140209. [DOI] [PubMed] [Google Scholar]
- McCafferty GP, Misajet BA, Laping NJ, Edwards RM, Thorneloe KS. Enhanced bladder capacity and reduced prostaglandin E2 mediated bladder hyperactivity in EP3 receptor knock-out mice. Am J Physiol. 2008;295:F507–F514. doi: 10.1152/ajprenal.00054.2008. [DOI] [PubMed] [Google Scholar]
- McCafferty GP, Coatney RW, Laping NJ, Thorneloe KS. Urodynamics by radio-telemetry in the conscious, freely-moving beagle dogs. J Urol. 2009;181:1444–1451. doi: 10.1016/j.juro.2008.10.137. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Conte B, Furio M, Santicioli P, Meli P, et al. Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur J Pharmacol. 1988a;145:105–112. doi: 10.1016/0014-2999(88)90221-x. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Patacchini R, Conte B, Furio M, Santicioli P, et al. The effect of SC-19220, a prostaglandin antagonist, on the micturition reflex in rats. Eur J Pharmacol. 1988b;152:273–279. doi: 10.1016/0014-2999(88)90722-4. [DOI] [PubMed] [Google Scholar]
- Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, et al. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;365:166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frøkiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol. 1999;10:647–663. doi: 10.1681/ASN.V103647. [DOI] [PubMed] [Google Scholar]
- Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, et al. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Yang T, Arend LJ, Schnermann JB, Peters CA, Freeman MR, et al. Obstruction stimulates COX-2 expression in bladder smooth muscle cells via increased mechanical stretch. Am J Physiol. 1999;276:F129–F136. doi: 10.1152/ajprenal.1999.276.1.F129. [DOI] [PubMed] [Google Scholar]
- Patra PB, Jugus MJ, Laping NJ. Sex differences in cystometry of normal and hypertensive conscious rats. Curr Urol. 2007;1:84–88. [Google Scholar]
- Persson K, Pandita RK, Spitsbergen JM, Steers WD, Tuttle JB, Andersson KE. Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. Am J Physiol. 1998;275:R1366–R1373. doi: 10.1152/ajpregu.1998.275.4.R1366. [DOI] [PubMed] [Google Scholar]
- van Rodijnen WF, Korstjens IJ, Legerstee N, Ter Wee PM, Tangelder GJ. Direct vasoconstrictor effect of prostaglandin E2 on renal interlobular arteries: role of the EP3 receptor. Am J Physiol. 2007;292:F1094–F1101. doi: 10.1152/ajprenal.00351.2005. [DOI] [PubMed] [Google Scholar]
- Schröder A, Newgreen D, Andersson KE. Detrusor responses to prostaglandin E2 and bladder outlet obstruction in wild-type and Ep1 receptor knockout mice. J Urol. 2004;172:1166–1170. doi: 10.1097/01.ju.0000134186.58854.2c. [DOI] [PubMed] [Google Scholar]
- Schüssler B. Comparison of the mode of action of prostaglandin E2 (PGE2) and sulprostone, a PGE2-derivative, on the lower urinary tract in healthy women. A urodynamic study. Urol Res. 1990;18:349–352. doi: 10.1007/BF00300786. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Clemow DB, McCarty R, Steers WD, Tuttle JB. Neurally mediated hyperactive voiding in spontaneously hypertensive rats. Brain Res. 1998;790:151–159. doi: 10.1016/s0006-8993(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Su X, Lashinger ES, Leon LA, Hoffman BE, Hieble JP, Gardner SD, et al. An excitatory role for peripheral EP3 receptors in bladder afferent function. Am J Physiol. 2008a;295:F585–F594. doi: 10.1152/ajprenal.90273.2008. [DOI] [PubMed] [Google Scholar]
- Su X, Leon LA, Wu CW, Morrow DM, Jaworski JP, Hieble JP, et al. Modulation of bladder function by prostaglandin EP3 receptors in the central nervous system. Am J Physiol. 2008b;295:F984–F994. doi: 10.1152/ajprenal.90373.2008. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Shigemoto R, Negishi M, Ichikawa A, Narumiya S. Distinct cellular localization of mRNAs for three subtypes of prostaglandin E receptor in kidney. Am J Physiol. 1994;266:F823–8. doi: 10.1152/ajprenal.1994.266.5.F823. [DOI] [PubMed] [Google Scholar]
- Takagi-Matsumoto H, Ng B, Tsukimi Y, Tajimi M. Effects of NSAIDs on bladder function in normal and cystitis rats: a comparison study of aspirin, indomethacin, and ketoprofen. J Pharmacol Sci. 2004;95:458–465. doi: 10.1254/jphs.fp0040098. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Takahashi N, Abe T, Abe K. Two isoforms of the rat kidney EP3 receptor derived by alternative RNA splicing: intrarenal expression co-localization. Biochem Biophys Res Commun. 1994a;199:834–840. doi: 10.1006/bbrc.1994.1304. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Takahashi N, Abe T, Ito O, Tsutsumi E, Taniyama Y, et al. Functional difference between two isoforms of rat kidney prostaglandin receptor EP3 subtype. Biochem Biophys Res Commun. 1994b;203:1897–1903. doi: 10.1006/bbrc.1994.2409. [DOI] [PubMed] [Google Scholar]
- Tang L, Loutzenhiser K, Loutzenhiser R. Biphasic actions of prostaglandin E(2) on the renal afferent arteriole : role of EP(3) and EP(4) receptors. Circ Res. 2000;86:663–670. doi: 10.1161/01.res.86.6.663. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Watanabe T, Nakao A, Seki G, Uwatoko S, Kurokawa K. Detection and quantitation of EP3 prostaglandin E2 receptor mRNA along mouse nephron segments by RT-PCR. Am J Physiol. 1994;266:C1453–C1458. doi: 10.1152/ajpcell.1994.266.5.C1453. [DOI] [PubMed] [Google Scholar]
- Wang C, Li GW, Huang LY. Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain. 2007;3:22–34. doi: 10.1186/1744-8069-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibberley A, McCafferty GP, Evans C, Edwards RM, Hieble JP. Dual, but not selective, COX-1 and COX-2 inhibitors, attenuate acetic acid-evoked bladder irritation in the anaesthetised female cat. Br J Pharmacol. 2006;148:154–161. doi: 10.1038/sj.bjp.0706715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegar S, Tokar C, Enache LA, Rajagopol V, Zeller W, O'Connell M, et al. Development of a scalable process for DG-041, a potent EP3 receptor antagonist via tandem heck reactions. Org Process Res Dev. 2007;11:747–753. [Google Scholar]
- Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E2 receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]


