Abstract
Neuromedin U (NMU) has been paired with the G-protein-coupled receptors (GPRs) NMU1 (formely designated as the orphan GPR66 or FM-3) and NMU2 (FM-4 or hTGR-1). Recently, a structurally related peptide, neuromedin S (NMS), which shares an amidated C-terminal heptapeptide motif, has been identified in both rat and human, and has been proposed as a second ligand for these receptors. Messenger RNA encoding NMU receptor subtypes shows differential expression: NMU1 is predominantly expressed in peripheral tissues, particularly the gastrointestinal tract, whereas NMU2 is abundant within the brain and spinal cord. NMU peptide parallels receptor distribution with highest expression in the gastrointestinal tract and specific structures within the brain, reflecting its major role in the regulation of energy balance. The NMU knockout mouse has an obese phenotype and, in agreement, the Arg165Trp amino acid variant of NMU-25 in humans, which is functionally inactive, co-segregated with childhood-onset obesity. Emerging physiological roles for NMU include vasoconstriction mediated predominantly via NMU1 with nociception and bone remodelling via NMU2. The NMU system has also been implicated in the pathogenesis of septic shock and cancers including bladder carcinoma and acute myeloid leukaemia. Intriguingly, NMS is more potent at NMU2 receptors in vivo where it has similar central actions in suppression of feeding and regulation of circadian rhythms to NMU. Taken together with its vascular actions, NMU may be a functional link between energy balance and the cardiovascular system and may provide a future target for therapies directed against the disorders that comprise metabolic syndrome.
Keywords: energy homeostasis, neuromedin S, neuromedin U, NMU1, NMU2, smooth muscle contraction
Introduction
Neuromedin U (NMU), first isolated from porcine spinal cord and named for its potent contractile effect on rat uterus (Minamino et al., 1985), exists in two major molecular forms: an extended 23 (NMU-23) or 25 (NMU-25) amino acid peptide, or truncated 8 (NMU-8) or 9 (NMU-9) amino acid C-terminal fragments. More recently NMU-17 has been isolated from the skin secretions of the Chinese red belly toad, Bombina maxima (Lee et al., 2005), and splice variants NMU-21, NMU-25 and NMU-38 from goldfish brain (Maruyama et al., 2008). NMU is therefore widely conserved throughout nature with almost absolute conservation of the amidated C-terminal pentapeptide (-Phe-Arg-Pro-Arg-Asn-NH2; Figure 1), suggesting a ‘strong evolutionary pressure’ to retain this peptide (Brighton et al., 2004a). This is further exemplified by the discovery of homologues in invertebrates, namely the pyrokinins (-FXPRXamide), Cap2b-like peptides (-FPRXamide) and ecdysis triggering hormones (-PRXamide); these are ligands for four receptors in Drosophila, which fall into the same clade as vertebrate NMU receptors (Park et al., 2002). In particular, cockroach (Periplaneta americana) pyrokinin shares four out of five of the C-terminal pentapeptide amino acid residues with NMU (Melcher et al., 2006). The focus of this review is the distribution and functions of NMU in vertebrates.
Figure 1.
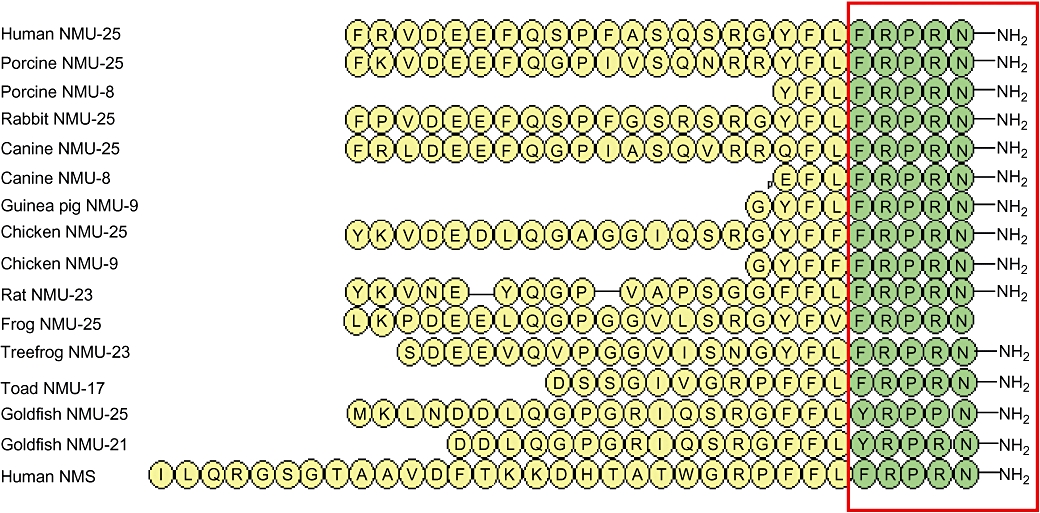
Amino acid sequences of neuromedin U (NMU) from mammalian (Minamino et al., 1985; Minamino et al., 1988; Murphy et al., 1990; Kage et al., 1991; O'Harte et al., 1991a; Austin et al., 1995), avian (O'Harte et al., 1991b; Domin et al., 1992), amphibian (Domin et al., 1989; Salmon et al., 2000; Lee et al., 2005) and piscine (Maruyama et al., 2008) species, and human neuromedin S (NMS). The red box, highlighting the C-terminal pentapeptide, shows conservation of this sequence in vertebrates, except goldfish. In mammalian species, the C-terminal heptapeptide is fully conserved. Amidation of the C-terminus of frog NMU-25 could not be confirmed by the study by Domin et al. (1989). NMU shares some structural features with pancreatic polypeptide (PP) and vasoactive intestinal polypeptide (VIP). Both NMU and PP have a C-terminal sequence of –Arg–Pro–Arg–X–CONH2, whereas VIP is also amidated at the C-terminus. However, neither PP nor VIP have shown any binding or activity at NMU receptors (Hosoya et al., 2000; Kojima et al., 2000; Szekeres et al., 2000).
Structure-activity relationship of NMU peptides
Minimal active fragment and conservation of the C-terminus
NMU in most species has two major features: amidation of the C-terminus and a conserved C-terminal pentapeptide (Figure 1). Amidation is crucial for receptor activation; NMU-8 lacking an amide group failed to activate human and murine receptors at concentrations up to 10 µmol·L−1 (Hedrick et al., 2000; Funes et al., 2002), and had no effect on either rat uterine contractility or blood pressure (Minamino et al., 1985; Sakura et al., 1991) showed that residues 19–23 (FRPRN-NH2) in rat NMU-23 retained biological activity in rat uterus but the smallest fragment with which it was possible to achieve maximum activity was residues 17–22 (FLFRPR-NH2). Individual alanine or glycine substitution of each residue in NMU-8 resulted in reduced potency of this peptide in calcium flux (Funes et al., 2002) and chicken crop smooth muscle assays (Hashimoto et al., 1991), respectively; however, it became clear that Arg7 was most important for the activity as responses were completely abolished when this residue was replaced.
Proteolytic processing of NMU
Several differences exist in the amino acid sequence of NMU between species. For instance, there is controversy surrounding whether the extended forms of NMU are intermediate precursors of the truncated forms. The dibasic cleavage site Arg16–Arg17 is present in NMU-25 of both pig (Minamino et al., 1985) and dog (O'Harte et al., 1991a), preceding the sequence for NMU-8. O'Harte et al. (1991b) suggested that NMU-25 in chicken is a labile intermediate for NMU-9, implying Arg16–Gly17 is also a cleavage site. Additionally, Murphy et al. (1990) observed a larger molecular weight NMU-like immunoreactivity (NMU-LI) than NMU-9, which has an N-terminal Gly residue, in guinea pig small intestine; although described as an extraction artefact, this could be an extended form of NMU. Indeed, Domin et al. (1986) showed larger molecular weight NMU-LI in guinea pig ileum and spinal cord. In contrast, rabbit, human and frog NMU-25 possess the Arg16–Gly17 site (Domin et al., 1989; Kage et al., 1991; Austin et al., 1995), though only the extended form has been isolated in these species, whereas Domin et al. (1986) demonstrated NMU-25-like peptides predominate in pig and human. Species differences in proteolytic enzyme expression may account for these observations. In rat NMU-23, Arg16–Gly17 is replaced by Gly14–Gly15, thought to be non-cleavable, indicating only the extended form is present, as confirmed by HPLC in rat brain and intestine (Domin et al., 1987; Honzawa et al., 1990).
N-terminus – species variation
Whereas the C-terminus is conserved between species, the N-terminus is more variable and is thought to be responsible for determining potency and duration of response to NMU. For example, rat NMU-23 is twice as potent as porcine NMU-25 (Minamino et al., 1988), but three times as potent as toad NMU-17 (Lee et al., 2005) in the rat uterus contraction assay. To date, the potencies of both porcine and toad NMU have not been assessed in tissue from their native species. Therefore, further studies are required to determine whether species differences in the peptide-receptor interaction also exist such that porcine and toad NMU have the same potency at their respective receptors. Nevertheless, porcine NMU-25 elicited a more potent contractile effect on rat uterus (Minamino et al., 1985) and chicken crop (Okimura et al., 1992) than NMU-8 and, furthermore, NMU-8 was less potent than NMU-23 at competing for [125I]-NMU-23 binding from rat uterus membrane preparations (Nandha et al., 1993), suggesting that the N-terminus also influences the affinity of NMU peptide-receptor interactions. Highlighting differences that can arise between tissue and cell-based assays, in the latter, porcine NMU-8 was equipotent to NMU-25 (Hosoya et al., 2000; Szekeres et al., 2000; Johnson et al., 2004) and bound with comparable affinity (Hosoya et al., 2000; Howard et al., 2000; Raddatz et al., 2000; Aiyar et al., 2004) in functional and binding assays using human NMU receptors.
Some similarities within the N-terminus exist between species. Sakura et al. (1991) observed large increases in potency when the C-terminal octapeptide of rat NMU-23 was gradually extended, in particular positions 6–9 and 13–15 were important for enhancing activity. Positions 13–15 correspond to the tripeptide preceding the sequence for NMU-8; in the same way, the Asn15–Arg16–Arg17 tripeptide in porcine NMU-25 was shown to augment activity (Okimura et al., 1992). Moreover, some N-terminal residues are conserved, particularly Glu5 (only absent from goldfish NMU-25), Gln8 and Pro10 (absent from chicken NMU-25).
Finally, canine NMU-8 contains a pyroglutamate residue at position 1; O'Harte et al. (1991a) demonstrated that substituting the tyrosine residue at position 1 for this entity in porcine NMU-8 resulted in a greater contractile effect on rat uterus. Similarly, substitution of Tyr1 with the D-form of this amino acid enhanced activity in chicken crop preparations (Hashimoto et al., 1991; Sakura et al., 1995) proceeded to show that this was a result of conferring resistance to aminopeptidases, reducing degradation of canine NMU-8 relative to its porcine counterpart.
NMU in humans
In humans, prepro-NMU (Figure 2) comprises 174 residues, including a 34 amino acid signal peptide, suggesting secretion of this peptide. The mature peptide, NMU-25, is located towards the C-terminus with a pair of basic residues on either side at which cleavage occurs. It has been proposed that further cleavage at sites located closer to the N-terminus results in generation of a 33 amino acid peptide, which is 95% homologous to its equivalent in rats (Austin et al., 1995) and is shown to stimulate the release of prolactin (Mori et al., 2005).
Figure 2.
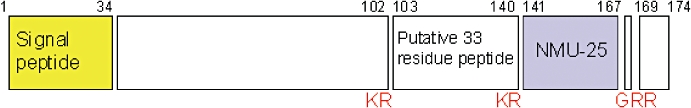
Schematic structure of prepro-neuromedin U (NMU) in humans. Numbers refer to residues and cleavage sites given in red.
Peripheral distribution of NMU peptide
The distribution of NMU has been extensively investigated in human and rat (Table 1) but yet to be reported in other species. In the latter, highest levels of NMU-LI have been observed in small intestine (Domin et al., 1987; Augood et al., 1988; Honzawa et al., 1990). In humans, all regions of the gastrointestinal tract (stomach through to rectum) had comparable levels of NMU-25 precursor mRNA expression, though NMU-LI was greatest in the jejunum (Austin et al., 1995). Immunohistochemical studies have shown localization of NMU-LI to both cell bodies and nerve fibres of the myenteric and submucous plexuses within the enteric nervous system of rat small intestine (Augood et al., 1988; Ballesta et al., 1988; Honzawa et al., 1990); interestingly, no NMU-LI was associated with the smooth muscle layers, though rapid turnover of peptide may result in levels below the limit of detection (Ballesta et al., 1988). The densest area of immunoreactive nerve fibres was in the mucosa around the crypts and extending up the villi (Augood et al., 1988; Ballesta et al., 1988; Honzawa et al., 1990).
Table 1.
Distribution of neuromedin U (NMU)-like immunoreactivity (NMU-LI) and mRNA in rat and human
| Species Technique |
Domin et al. (1987) |
Fujii et al. (2000) |
Szekeres et al. (2000) |
|---|---|---|---|
| Rat | Rat | Human | |
| RIA | qRT-PCR | qRT-PCR | |
| Whole brain | + | + | + |
| Spinal cord | ++ | + | |
| Dorsal root ganglia | +++ | ||
| Oesophagus | + | ||
| Stomach | + | + | ++ |
| Duodenum | +++ | +++ | |
| Jejunum | +++ | +++ | |
| Ileum | +++ | ++ | +++ |
| Colon | +++ | ++ | |
| Rectum | +++ | ++ | |
| Liver | − | − | − |
| Spleen | − | + | |
| Pancreas | − | + | + |
| Heart | − | + | |
| Lung | + | + | |
| Trachea | + | ||
| Kidney | − | − | + |
| Ureter | + | ||
| Bladder | + | − | |
| Testis | + | + | |
| Epididymis | + | ||
| Vas deferens | ++ | ||
| Seminal vesicle | − | − | |
| Prostate | + | + | |
| Penis | + | ||
| Ovary | + | + | |
| Fallopian tube | + | ||
| Uterus | + | + | |
| Urethra | + | ||
| Vagina | + | ||
| Pituitary | +++ | +++ | +++ |
| Adrenal | + | ||
| Thyroid | + | ||
| Thymus | + | ||
| Salivary gland | + | ||
| Skeletal muscle | − | − | |
| Adipose | ++ | ||
| Mammary gland | − | ||
| Skin | − | ||
| Bone | + | + | |
| Bone marrow | + | +++ | |
| Costal cartilage | + | − | |
| Lymphocytes | ++ | ||
| Macrophages | − |
For Domin et al. (1987), + denotes <10 pmol·g−1 NMU-LI, ++ 10–50 pmol·g−1, +++ >50 pmol·g−1. Blank boxes indicate the tissue was not reported and − indicates below levels of detection. Highest levels of NMU-LI within rat brain were detected in nucleus accumbens, septum and hypothalamus. For Fujii et al. (2000), + denotes <2 copies ×10−3·ng−1 poly(A)+RNA, ++ 2–4 and +++ >4. For Szekeres et al. (2000), + denotes <250 copies of gene's mRNA detected/ng mRNA pool, ++ 250–750 and +++ >750.
qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; RIA, radioimmunoassay.
Is NMU a locally acting or circulating peptide?
Domin et al. (1987) failed to detect NMU-LI in mucosal endocrine cells, which, together with low levels of NMU-LI in plasma (<0.5 pmol·L−1), suggested NMU is a neuropeptide rather than circulating hormone. The localization of NMU-LI is largely the same in guinea pig small intestine, yet there are some subtle differences: NMU-LI was observed in nerve fibres around submucous arterioles, whereas in rat there was no consistent relationship between nerve fibres and blood vessels (Ballesta et al., 1988), and a small population of NMU-LI positive endocrine cells in mucosal crypts were identified (Furness et al., 1989), collectively suggesting that NMU could have access to the circulation. In man, low picomolar levels of NMU-25-LI were detected in plasma, suggesting NMU is a locally acting peptide rather than a circulating hormone (Mitchell et al., 2009). Further sources of NMU include vascular endothelial cells, adipose tissue (Mitchell et al., 2009) and keratinocytes (Moriyama et al., 2005), consistent with emerging roles in vascular reactivity, energy balance and local inflammation.
Central distribution of NMU peptide
NMU-LI also has widespread distribution in the central nervous system (CNS) (Honzawa et al., 1987; Ballesta et al., 1988). NMU mRNA tends to be confined to the discrete regions of the brain; hence, levels in the whole brain are low. Relative to intestinal NMU mRNA expression, moderate levels were detected in the striatum, hypothalamus and medulla oblongata of rat (Fujii et al., 2000), and the cingulate gyrus and medial frontal gyrus of human, with low to moderate levels in hypothalamus, locus coeruleus, thalamus, medulla oblongata and substantia nigra (Szekeres et al., 2000). A high expression of NMU-LI was detected in the rat anterior pituitary gland by Domin et al. (1987), subsequently localized to corticotrophs (Ballesta et al., 1988). In the rat spinal cord, NMU-LI levels were greater in the dorsal than ventral horn, implying a sensory role for NMU (Domin et al., 1987).
NMU receptors – NMU1 and NMU2
NMU receptors have been designated NMU1 and NMU2, according to the Guide to Receptors and Channels (Alexander et al., 2008), published by the British Journal of Pharmacology. Although high affinity, saturable and specific binding sites for [125I]-NMU-23 in rat had previously been characterized (Nandha et al., 1993), the molecular identity of the receptor was not known until NMU was reported by several groups to be a cognate ligand for the previously designated ‘orphan’ class A G-protein-coupled receptors (GPCR) NMU1 (GPR66, FM-3; Fujii et al., 2000; Hedrick et al., 2000; Howard et al., 2000; Kojima et al., 2000; Raddatz et al., 2000; Szekeres et al., 2000) and NMU2 (FM-4, TGR-1; Hosoya et al., 2000; Howard et al., 2000; Raddatz et al., 2000; Shan et al., 2000), the genes for which are located on human chromosomes 2 and 5 respectively. There are two distinct isoforms of NMU2: a haplotype with four missense amino acid changes is found at a frequency of 15% (Bhattacharyya et al., 2004); the functional significance, if any, is yet to be identified.
Nandha et al. (1993) had initially hinted that the NMU receptor would be coupled to a G-protein, as reduced specific binding was observed in the presence of the non-hydrolysable analogue of GTP, GTPγS. Five years later, NMU1, then named FM-3, was cloned from a murine T-cell cDNA library and subsequently from a human P1-derived artificial chromosome library based on its homology with another GPCR, human ghrelin (previously growth hormone secretagogue) receptor (Tan et al., 1998), with which it shares 33% protein sequence identity. In turn, NMU2 was discovered because of its sequence similarity with NMU1 (Hosoya et al., 2000; Howard et al., 2000; Raddatz et al., 2000; Shan et al., 2000). NMU1 and NMU2, possibly the result of gene duplication (Shan et al., 2000), share 51% protein sequence identity in human (Howard et al., 2000). These receptors are 73 and 75% identical to rat NMU1 and NMU2, respectively (Howard et al., 2000) and 79 and 81% identical to murine NMU receptors (Funes et al., 2002). Much of the inter-receptor and inter-species variation arise from sequence differences in the N- and C-termini; in addition, the third intracellular loop in NMU2 is considerably shorter than in NMU1 (Shan et al., 2000).
Using ‘reverse pharmacology’, these receptors were artificially expressed in cell lines and screened with libraries of ligands in conjunction with a functional assay, such as calcium mobilization or arachidonic acid release, resulting in the pairing of NMU with NMU1 and NMU2 (Table 2). Overwhelmingly, data suggest that NMU is equipotent at these receptors, at least in cell-based studies. Extensive radioligand binding studies have also been performed in cells expressing either receptor, demonstrating sub-nanomolar affinity (Table 2). Whether NMU receptor subtypes have similar binding and functional characteristics in native tissue is yet to be determined.
Table 2.
Binding and functional data from cells expressing NMU1 or NMU2
| Radioligand | Cell type | Receptor | KD nmol·L−1 | Assay | Ligand | EC50 nmol·L−1 | |
|---|---|---|---|---|---|---|---|
| Howard et al. (2000) | [125I]-NMU-23 (rat) | HEK-293 | hNMU1 | 0.3 | ↑[Ca2+]i | NMU-25 (human) | 1.0 |
| NMU-23 (rat) | 0.75 | ||||||
| NMU-8 (pig) | 0.11 | ||||||
| NMU-25 (pig) | 1.36 | ||||||
| hNMU2 | ↑[Ca2+]i | NMU-25 (human) | 1.0 | ||||
| NMU-23 (rat) | 2.9 | ||||||
| NMU-8 (pig) | 0.3 | ||||||
| NMU-25 (pig) | 2.8 | ||||||
| Fujii et al. (2000) | [125I]-NMU-8 (pig) | CHO | hNMU1 | 0.066 | ↑[Ca2+]i | NMU-23 (rat) | 1.3 |
| Hosoya et al. (2000) | [125I]-NMU-8 (pig) | CHO | hNMU2 | 0.022 | ↑AA release | NMU-25 (human) | 4.0 |
| NMU-23 (rat) | |||||||
| NMU-25 (pig) | 1.4–2.0 | ||||||
| NMU-8 (pig) | |||||||
| Raddatz et al. (2000) | [125I]-NMU-23 (rat) | COS-7 | hNMU1 | 0.61 | ↑[Ca2+]i | NMU-25 (human) | 4.0 |
| NMU-23 (rat) | 2.1 | ||||||
| NMU-25 (pig) | 5.2 | ||||||
| NMU-8 (pig) | 1.1 | ||||||
| hNMU2 | 0.81 | NMU-25 (human) | 2.4 | ||||
| NMU-23 (rat) | 5.0 | ||||||
| NMU-25 (pig) | 3.0 | ||||||
| NMU-8 (pig) | 1.2 | ||||||
| 125I]-NMU-8 (pig) | hNMU1 | 1.2 | |||||
| hNMU2 | 0.83 | ||||||
| Szekeres et al. (2000) | HEK-293 | hNMU1 | ↑[Ca2+]i | NMU-25 (pig) | 0.38 | ||
| NMU-8 (pig) | 0.21 | ||||||
| NMU-23 (rat) | 0.17 | ||||||
| ↑IP | NMU-25 (pig) | 0.28 | |||||
| Shan et al. (2000) | HEK-293 | hNMU2 | ↑[Ca2+]i | NMU-25 (human) | 5 | ||
| Hedrick et al. (2000) | HEK-293 | hNMU1 | ↑[Ca2+]i | NMU-25 (human) | 12 | ||
| NMU-8 (pig) | 10 | ||||||
| Kojima et al. (2000) | CHO | hNMU1 | ↑[Ca2+]i | NMU-25 (human) | 0.25 | ||
| NMU-23 (rat) | 7.0 | ||||||
| NMU-25 (pig) | 0.8 | ||||||
| NMU-8 (pig) | 2.8 | ||||||
| Funes et al. (2002) | HEK-293 | mNMU1 | ↑[Ca2+]i | NMU-25 (human) | 20 | ||
| NMU-23 (mouse) | 8.8 | ||||||
| NMU-8 (pig) | 9.5 | ||||||
| mNMU2 | NMU-25 (human) | 2.2 | |||||
| NMU-23 (mouse) | 1.6 | ||||||
| NMU-8 (pig) | 3 | ||||||
| Brighton et al. (2004b) | [125I]-NMU-25 (human) | HEK-293 | hNMU1 | 0.14 | ↑[Ca2+]i | NMU-25 (human) | 0.72 |
| ↑IP | 0.39 | ||||||
| ↓cAMP | 0.08 | ||||||
| hNMU2 | 0.11 | ↑[Ca2+]i | 1.07 | ||||
| ↑IP | 0.43 | ||||||
| ↓cAMP | 0.09 | ||||||
| Johnson et al. (2004) | [125I]-NMU-25 (human) | T-cell clone | mNMU1 | 0.36 | ↑[Ca2+]i | NMU-25 (human) | 4.8 |
| NMU-23 (rat) | 10.1 | ||||||
| NMU-25 (pig) | 6.0 | ||||||
| NMU-8 (pig) | 4.8 | ||||||
| Aiyar et al. (2004) | [125I]-NMU-25 (human) | HEK-293 | hNMU1 | 0.08 | ↑IP | NMU-25 (human) | 1.6 |
| hNMU2 | 0.16 | 1.5 | |||||
| hNMU1 | ↑[Ca2+]i | 0.50 | |||||
| hNMU2 | 0.50 | ||||||
| Garlton et al. (2004) | HEK-293 | hNMU1 | ↑[Ca2+]i | NMU-23 (rat) | 1.25 | ||
| hNMU2 | 1.10 | ||||||
| Shukla et al. (2007) | [125I]-NMU-23 (rat) | Yeast | hNMU2 | 0.79 | |||
| BHK | 0.96 | ||||||
| Xia et al. (2008) | [125I]-NMU-23 (rat) | BHK | hNMU1 | 0.14 |
‘h’ in front of NMU1 or NMU2 denotes human and ‘m’ murine.
AA, arachidonic acid; BHK, baby hamster kidney; cAMP, cyclic adenosine monophosphate; CHO, Chinese hamster ovary; HEK, human embryonic kidney; IP, inositol phosphates; NMU, neuromedin U.
NMU receptor distribution – NMU1 peripheral and NMU2 central
To distinguish between the two NMU receptor subtypes, several studies have investigated their tissue distribution at the mRNA level (Table 3) in human (Hedrick et al., 2000; Howard et al., 2000; Raddatz et al., 2000; Shan et al., 2000; Szekeres et al., 2000; Westfall et al., 2002; Garlton et al., 2004) and rat (Fujii et al., 2000; Hosoya et al., 2000; Guan et al., 2001; Garlton et al., 2004) using a range of techniques, including quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), in situ hybridization, Northern blot analysis and dot blot analysis.
Table 3.
Tissue distribution of mRNA for NMU1 and NMU2 in human (Raddatz et al., 2000) and rat (Fujii et al., 2000; Hosoya et al., 2000)
|
Human |
Rat |
|||
|---|---|---|---|---|
| NMU1 | NMU2 | NMU1 | NMU2 | |
| Amygdala | + | + | ||
| Cerebellum | ++ | + | + | + |
| Cortex | + | ++ | + | + |
| Hippocampus | + | +++ | + | + |
| Hypothalamus | + | ++ | + | +++ |
| Medulla oblongata | + | +++ | + | + |
| Pontine ret. form. | + | +++ | ||
| Striatum | + | + | ||
| Thalamus | + | +++ | + | + |
| Spinal cord | + | +++ | + | ++ |
| DRG | + | + | ||
| Adrenal | ++ | + | − | − |
| Pituitary | + | + | − | |
| Thyroid | + | − | ||
| Thymus | + | + | ||
| Skeletal muscle | + | + | − | − |
| Adipose | + | + | ||
| Salivary gland | + | − | − | + |
| Spleen | + | − | + | − |
| Heart | + | + | − | − |
| Lung | ++ | ++ | +++ | + |
| Trachea | ++ | + | + | − |
| Liver | − | − | − | − |
| Pancreas | ++ | − | + | − |
| Kidney | ++ | + | + | − |
| Small intestine | +++ | + | +++ | + |
| Large intestine | + | + | ||
| Stomach | ++ | + | + | + |
| Mammary gland | + | + | + | − |
| Bladder | + | + | ||
| Prostate | ++ | + | ||
| Seminal vesicle | − | − | ||
| Testis | +++ | +++ | − | + |
| Ovary | − | ++ | ||
| Uterus | ++ | + | + | +++ |
| Skin | − | − | ||
| Bone | + | − | ||
| Bone marrow | − | − | ||
| Costal cartilage | + | + | ||
For the human study, + denotes <100 copies/ng cDNA, ++ 100–300, +++ >300 and − below level of detection. For the rat study, + denotes <1 copies ×10−3/ng poly(A)+RNA, ++ 1–2 and +++ >2. Blank boxes indicate tissue levels not reported.
DRG, dorsal root ganglia; pontine ret. form., pontine reticular formation.
The studies are largely in agreement: NMU1 mRNA was more abundant in peripheral tissues, whereas NMU2 mRNA was located more centrally. In addition, the distribution of receptors overlaps with that of NMU peptide (Table 1). At the protein level, Mangold et al. (2008) investigated the localization of NMU binding sites in rat brain; high densities were observed in the hypothalamus, as expected from mRNA studies, and hippocampal formation.
NMU receptor signalling
Calcium mobilization as a result of phospholipase C activation and subsequent production of inositol phosphates can occur through pertussis toxin (PTX)-insensitive Gq/11 protein and/or PTX-sensitive Gi/o protein pathways. The overwhelming majority of studies favour the former for NMU receptors (Raddatz et al., 2000; Shan et al., 2000; Szekeres et al., 2000; Funes et al., 2002); however, Aiyar et al. (2004) showed that PTX caused partial inhibition of inositol phosphate accumulation in cells expressing either NMU1 or NMU2, though this effect was more pronounced in the latter. Lack of a PTX-sensitive component of calcium mobilization does not rule out receptor coupling to Gi/o proteins as this pathway has other downstream effectors. Indeed, studies in cells have shown NMU receptor-mediated inhibition of forskolin-induced cAMP accumulation, prevented by pretreatment with PTX (Hosoya et al., 2000; Aiyar et al., 2004; Brighton et al., 2004b) suggesting coupling to Gi/o proteins, but have failed to demonstrate PTX-sensitive NMU receptor-mediated accumulation of inositol phosphates (Brighton et al., 2004b). Brighton et al. (2004b) have further demonstrated coupling of NMU1 and NMU2 to both Gq/11 and Gi proteins using [35S]-guanosine 5′-O-[gamma-thio]triphosphate binding and immuno-precipitation of Gα subunits, but were unable to directly compare levels of G protein activation. More recently, using chimeric Gs proteins, Hsu and Luo (2007) have demonstrated that NMU1 primarily signals through Gq/11 proteins and NMU2 through Gi proteins. Few studies have been performed using endogenous NMU receptors. In rat colonic smooth muscle cells, NMU receptors were shown to couple with both Gq/11 and Gi proteins (Brighton et al., 2008). The NMU receptor subtype composition for rat colon is not clear; similar levels of mRNA encoding each subtype were detected by qRT-PCR (Fujii et al., 2000; Hosoya et al., 2000), but these do not necessarily reflect protein levels.
Agonist and antagonist studies
At present, no publications have reported the development of NMU receptor subtype-selective antagonists. However, using cell-based functional assays, natural products EUK2010, EUK2011 and EUK2012 have been described as NMU2-specific agonists (Fang et al., 2006), and icariin from Herba epimedii has been identified as an NMU2-selective agonist (Zheng et al., 2005), though its activity at NMU2 was compared with the muscarinic 1 and melanocortin 4 receptors as opposed to NMU1. Meanwhile, Meng et al. (2008) have discovered two synthetic low molecular weight non-selective NMU receptor agonists. Funes et al. (2002) showed that substitution of Arg5 in NMU-8 with alanine resulted in a peptide with 15-fold greater potency at NMU2 than at NMU1. Although this alanine-substituted peptide had no antagonistic action, this information may be useful in the design of receptor subtype-specific compounds.
Functions of NMU
The focus of most research has been the role of NMU in smooth muscle contraction and regulation of feeding, reflecting the brain–gut distribution of this peptide. Like structure, function also seems to be well conserved throughout nature; pyrokinin-2, an NMU homologue encoded by the Drosophila hugin gene, has myostimulatory activity and has been implicated in the regulation of feeding (Melcher et al., 2006). However, research into the NMU system is still in its infancy and subsequently functions continue to emerge, many of which appear disparate. This should be expected as both NMU peptide and receptors have widespread dissemination in addition to their major brain–gut axis of expression. It has also been suggested that NMU may be involved in a number of pathophysiological processes, particularly in the fields of oncology and inflammation.
Smooth muscle contraction
NMU has been shown to directly contract smooth muscle of the gastrointestinal and genitourinary systems in a range of species (Table 4). However, in mouse colon (Dass et al., 2007), and mouse and rat vas deferens (Prendergast et al., 2006), NMU was only effective in potentiating electrically induced contractions, indicating an indirect neuronally-mediated action. Such actions suggest a role for NMU in the regulation of motility within these systems; indeed, NMU has been shown to elicit a prokinetic effect in mouse colon, reducing the interval between successive peristaltic waves (Dass et al., 2007). Therefore, it can be speculated that the NMU system may represent a future therapeutic target for the treatment of intestinal motility disorders. Firstly, it is necessary to determine whether the role of NMU in the animal model reflects that observed in humans. NMU has been shown to cause direct contraction of human ascending colon (Jones et al., 2006), though it is not reported whether NMU could also potentiate electrically induced contractions. In fact, a number of species differences relating to the contractile activity of NMU exist. For example, NMU contracted human and canine urinary bladder smooth muscle but was without effect in bladder preparations from mouse, rat, guinea pig, rabbit and ferret (Westfall et al., 2002), even though NMU1 and NMU2 mRNA have been detected in this tissue in rat (Table 3).
Table 4.
Summary of studies investigating direct contractile effect of NMU on smooth muscle preparations
| Species | Smooth muscle preparation | |
|---|---|---|
| Rat | Uterus | Minamino et al. (1985) |
| Fundus of stomach | Benito-Orfila et al. (1991) | |
| LOS | Prendergast et al. (2006) | |
| Ileum | ||
| Colon | Brighton et al. (2008) | |
| Human | Ileum | Maggi et al. (1990) |
| Urinary bladder | ||
| Gall bladder | Jones et al. (2006) | |
| Colon (ascending) | ||
| Dog | Urinary bladder | Westfall et al. (2002) |
| Stomach | ||
| Ileum | ||
| Colon | ||
| Mouse | LOS | Prendergast et al. (2006) |
| Gall bladder | ||
| Uterus | ||
| Fundus of stomach | ||
| Guinea pig | Uterus | Prendergast et al. (2006) |
| Turtle | Small intestine | Bockman et al. (1989) |
LOS, lower oesophageal sphincter.
Secondly, studies using NMU receptor knock-out mice have sought to determine the NMU receptor subtype responsible for smooth muscle contractile activity. Dass et al. (2007) demonstrated that responses to NMU in stomach and electrically-stimulated colon were similar in NMU2−/− and wild-type mice, implying NMU acts through NMU1 in these tissues. Prendergast et al. (2006) reported loss of responses to NMU in stomach and gall bladder of NMU1−/− mice, indicating contraction was mediated by NMU1; in contrast, responses in uterus and vas deferens were unchanged in these mice, suggesting a role for NMU2. Alternatively, NMU1 may be able to compensate for a loss of NMU2 and vice versa in some tissues; precise delineation requires receptor subtype-selective antagonists.
Central energy homeostasis
Evidence from intracerebroventricular (i.c.v.) NMU administration to rats, NMU knock-out mice and mutations in man
Intracerebroventrial administration of NMU produced a reduction in food intake and body weight in both ad libitum-fed and fasted rats (Howard et al., 2000; Kojima et al., 2000; Nakazato et al., 2000; Niimi et al., 2001; Ivanov et al., 2002; Wren et al., 2002; Hanada et al., 2003). In support, i.c.v. anti-NMU antisera resulted in increased feeding in rats (Kojima et al., 2000; Jethwa et al., 2005) whereas i.c.v. NMU also decreased food intake in Japanese quail (Shousha et al., 2005), chicks (Kamisoyama et al., 2007) and goldfish (Maruyama et al., 2008). Furthermore, NMU knock-out mice were hyperphagic, resulting in an obese phenotype (Hanada et al., 2004) and, as expected, i.c.v. NMU reduced their fat mass (Sato et al., 2007). Transgenic mice, overexpressing the NMU gene, were leaner than wild types, even when fed a high-fat diet (Kowalski et al., 2005). All of these studies implicate NMU in the regulation of food intake and are consistent with its hypothalamic expression.
Consistent with this hypothesis, in humans, the Ala19Glu polymorphism correlated with an overweight or obese phenotype in middle-aged Caucasians; this amino acid change is located in the signal peptide of pre-pro-NMU and is believed to reduce export (Hainerova et al., 2006). Rarer is the Arg165Trp mutation, discovered in a Czech family and associated with hypertriglyceridaemia and childhood-onset obesity (Hainerova et al., 2006); the mutation is situated within the C-terminal pentapeptide of mature NMU and, from structure-activity relationship data, is expected to abolish any functional responses (Funes et al., 2002). Indeed, unlike NMU-25, the Arg165Trp amino acid variant of NMU-25 was without vasoconstrictor effect in saphenous vein (Mitchell et al., 2008). As well as reducing energy intake, i.c.v. NMU has also been shown to amplify energy expenditure in rats, producing increases in locomotion, core body temperature and oxygen consumption (Howard et al., 2000; Nakazato et al., 2000; Hanada et al., 2001; Ivanov et al., 2002; Wren et al., 2002; Garlton et al., 2004).
Upon i.c.v. administration of NMU to rats, increased c-Fos-LI, a marker for cellular activation, was observed in the paraventricular (PVN), arcuate (Arc) and supraoptic (SON) nuclei of the hypothalamus, the dorsomedial hypothalamus (DMH), the lateral hypothalamic area, the amygdala and the parabrachial nucleus, nucleus tractus solitarius (NTS) and the ventrolateral medulla of the brainstem (Niimi et al., 2001; Ivanov et al., 2002; Ozaki et al., 2002). However, the prime candidate for the location at which NMU exerts the aforementioned effects is the PVN as direct microinjection of NMU into this area in rats resulted in reduced food intake and increased physical activity (Wren et al., 2002; Novak et al., 2006). NMU most likely acts through NMU2, the gene for which is expressed in the PVN of both rats and mice (Howard et al., 2000; Guan et al., 2001; Graham et al., 2003). Furthermore, the specific NMU2 agonist EUK2010, a natural compound, was shown to reduce body weight of rats and mice (Fang et al., 2006). However, NMU2 knockout mice were not obese and had a similar level of motor activity relative to wild types, suggesting NMU was operating through NMU1, even though its central expression is low, or an unidentified receptor (Zeng et al., 2006). Nevertheless, these mice were refractory to i.c.v. NMU-induced reductions in food intake (Zeng et al., 2006), demonstrating the importance of NMU2 in the central regulation of feeding. Reduced food intake and increased physical activity were also observed after intra-Arc administration of NMU to rats (Wren et al., 2002; Novak et al., 2006); only recently have NMU receptors been localized to this area of rat brain (Graham et al., 2003; Mangold et al., 2008). In mice, NMU2 mRNA was also abundant in cells located on the periphery of the ventromedial hypothalamus (Graham et al., 2003), an area associated with satiety (Hoebel, 1965).
The source of NMU involved in control of energy balance is uncertain. Howard et al. (2000) showed a reduction in NMU mRNA in the Arc of fasted rats. In accordance, Ballesta et al. (1988) and Graham et al. (2003) demonstrated the presence of NMU-LI and NMU mRNA respectively in this structure in rat. Conflictingly, neither Honzawa et al. (1987) using immunohistochemistry nor Ivanov et al. (2002) using in situ hybridization could detect NMU in cell bodies of rat Arc. Other potential sources are the NTS of the brainstem and pars tuberalis of the pituitary. Ivanov et al. (2004) located neurones expressing the gene for NMU in rat NTS and subsequently showed that peripheral administration of cholecystokinin, a satiety hormone, increased c-fos expression in approximately one-third of these neurones; furthermore, it is known that a population of neurones project from the NTS to the PVN (Doyle et al., 2004). NMU mRNA was detected in rat pars tuberalis (Ivanov et al., 2002; Graham et al., 2003), but levels increased upon fasting and decreased after central administration of leptin (Nogueiras et al., 2006), which is not in accordance with an anorexigenic action for this source of NMU. In mice, NMU mRNA was most abundantly expressed in DMH and ventromedial hypothalamus; levels in the DMH were augmented in food-deprived mice compared with their ad libitum-fed littermates (Graham et al., 2003), again at odds with the anorectic effect of NMU. In addition, a peripheral source should not be discounted, though it is yet to be determined whether NMU can cross the blood–brain barrier. It was previously believed that peptides were unable to penetrate this structure; however, it has been shown that peptides, such as vasoactive intestinal polypeptide, can cross by passive diffusion (Dogrukol-Ak et al., 2004), whereas others have saturable transporter systems, for example ghrelin (Banks et al., 2002). Nevertheless, current evidence suggests NMU is a locally acting rather than circulating peptide (Domin et al., 1987; Mitchell et al., 2008).
Interactions with corticotropin-releasing hormone (CRH) and leptin systems
Transmitter systems interact to form complicated regulatory networks. It is therefore unlikely that the NMU system operates in isolation. An understanding of such interactions allows prediction of the potential wider consequences of disrupting the NMU system should it be considered as a future therapeutic target. CRH has been shown to reduce food intake (Morley and Levine, 1982) and was released from rat hypothalamic explants upon exposure to NMU (Wren et al., 2002). After central administration of NMU, c-Fos-LI was upregulated in neurones immunoreactive for CRH and CRH mRNA was increased in rat PVN (Hanada et al., 2004; Yokota et al., 2004). In addition, reduced expression of CRH mRNA was observed in the PVN of NMU knock-out mice (Hanada et al., 2004) and the NMU-induced decrease in food intake and increases in locomotion, core body temperature and oxygen consumption were abolished in CRH knock-out mice (Hanada et al., 2001; 2003;). The reduced anorexigenic action of CRH observed with chronic central administration (Krahn et al., 1990) even mirrors the effect of chronic delivery of intra-PVN NMU to rats (Thompson et al., 2004). However, a study by Kowalski et al. (2005) casts doubt on the role of CRH in NMU-regulated feeding behaviour: mice overexpressing the NMU gene in the PVN had similar levels of hypothalamic CRH mRNA to wild types.
Several studies have investigated the relationship between NMU and the well-characterized adipostatic factor leptin (Campfield et al., 1995). Leptin stimulated the release of NMU from rat hypothalamic explants (Wren et al., 2002), and i.c.v. administration of anti-NMU antisera to rats reduced the satiety effect of i.p. leptin (Jethwa et al., 2005). These findings suggest NMU acts downstream of leptin. In contrast, NMU resulted in weight loss in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice and leptin receptor-deficient (Zucker fatty) rats equivalent to that observed in controls (Hanada et al., 2004), implying leptin does not operate downstream of NMU. However, not all studies support a role for NMU in leptin-mediated inhibition of feeding (Hanada et al., 2004): NMU knockout mice lost the same amount of weight as control mice when leptin was administered and, in rat Arc, i.c.v. leptin neither affected NMU mRNA expression nor induced c-Fos expression in NMU immunoreactive neurones. However, the major source of NMU involved in maintenance of energy homeostasis still remains unclear; such studies are required in other central structures implicated in NMU-mediated control of energy balance, e.g. NTS.
The NMU system: a novel target for anti-obesity therapy?
Findings indicate that NMU has an important role in maintaining energy balance in a number of species and, therefore, suggest that NMU may be an attractive target in the fight against obesity. However, two problems can be foreseen from recent studies. Chronic administration of this peptide was without effect on the food intake of ad libitum-fed rats (Thompson et al., 2004). This could be a result of receptor down-regulation or up-regulation of peptide degradative pathways, although effects of chronic NMU on the hypothalamo–pituitary–adrenal (HPA) axis did not differ from that when NMU is acutely administered. Most studies have shown that NMU reduces feeding in ad libitum-fed rats; in contrast, Ivanov et al. (2002) found no effect on food intake in satiated compared with fasted rats. It is suggested that the NMU system is already operating at maximum activity in the former and, therefore, exogenous NMU has no effect (Thompson et al., 2004). A further explanation reflects the observation that rats did not experience a normal growth rate during the study, masking the effects of NMU, if any, on body weight (Thompson et al., 2004).
The second problem is that ‘central sensitivity’ to NMU is reduced in obese animals: before a high-fat diet, diet-induced obese (DIO) and diet-resistant (DR) rats had similar levels of increased physical activity post-NMU administration; after 1 month, NMU produced greater physical activity in DR rats than DIO rats (Novak et al., 2007). As NMU has an anorexigenic action, such a reduced functional response may contribute to the development of obesity and/or exacerbate an existing obese phenotype. A mechanism for this phenomenon is yet to be established. Although NMU receptor expression within the PVN was not investigated, receptor down-regulation or desensitization are unlikely as no difference in NMU-LI within the PVN of DR and DIO rats was detected (Novak et al., 2007). Instead, reduced sensitivity to NMU may be secondary to a decrease in expression of downstream effectors. For example, in DIO relative to DR rats, lower levels of CRH mRNA were demonstrated within the brain (Michel et al., 2004).
Peripheral energy homeostasis
NMU may also have peripheral actions controlling energy balance. Messenger RNA for NMU1 has been observed in rat pancreas (Fujii et al., 2000) and, more recently, this receptor has been detected at the protein level in the pancreatic islets (Kaczmarek et al., 2006). Application of NMU to isolated pancreatic islets resulted in an inhibition of insulin secretion (Kaczmarek et al., 2006); a reduction of this anabolic hormone is in accordance with the anorexigenic action of centrally administered NMU.
Ion transport within the gut and gastric emptying
In addition to contracting smooth muscle in the gastrointestinal system, NMU also increased electrogenic ion transport in porcine jejunum, the physiological relevance of which is unknown (Brown and Quito, 1988), and reduced gastric acid secretion and gastric emptying upon central administration (Mondal et al., 2003) in keeping with its anorexigenic action. Control of gastric acid secretion was dependent on CRH and the sympathetic nervous system, as both anti-CRH antisera and yohimbine, an α2-adrenergic receptor antagonist, prevented the inhibitory action of NMU (Mondal et al., 2003).
Cardiovascular actions of NMU
Initial studies demonstrated that NMU elicited a rapid and sustained increase in systemic blood pressure in anaesthetized rats upon intravenous (i.v.) administration (Minamino et al., 1985). Further studies in conscious rats (Gardiner et al., 1990; Chu et al., 2002) and anaesthetized dogs (Sumi et al., 1987; Westfall et al., 2002) supported a pressor effect for i.v. NMU but found it to be smaller and transient. More detailed experiments in chronically-instrumented rats showed NMU at lower doses could reduce superior mesenteric blood flow without changes in systemic blood pressure and renal and hindquarter blood flows, suggesting local vasoconstriction (Gardiner et al., 1990). Similarly, in anaesthetized dogs, i.v. NMU decreased blood flow in the superior mesenteric artery and portal vein but had no effect on blood flow in the axillary artery (Sumi et al., 1987). These results suggest NMU is an important regulator of intestinal blood flow. However, until recently, little was known about the direct effect of NMU on blood vessels. Using radioligand binding assays, [125I]-NMU-25 binding sites were detected in human heart and coronary artery (dissociation constant, KD= 0.26 nmol·L−1). Messenger RNA encoding NMU1 predominated in these tissues and NMU1-LI was subsequently localized to the medial smooth muscle layer of both intra-myocardial and large conduit blood vessels (Mitchell et al., 2009). Consistent with this localization and the contractile activity of NMU in other hollow organs, constrictor responses to NMU-25 have been characterized in human isolated coronary and mammary artery and saphenous vein, similar in potency and maximum contractile response to angiotensin II (Mitchell et al., 2009). Human in vivo vascular studies in healthy volunteers and appropriate patient groups are required to determine whether the NMU system is worth pursuing as a target for the treatment of vascular diseases such as hypertension. It could be speculated that NMU released from adipose tissue could contribute to an elevated blood pressure in obesity. Meanwhile, the effect of i.v. NMU on heart rate is ambiguous. Although rat NMU-23 produced an increase in heart rate in the chronically instrumented rat model (Gardiner et al., 1990), although whether this is a direct or indirect effect remains uncertain, Chu et al. (2002) observed no effect on heart rate post-administration of NMU.
NMU has also been shown to exert a central influence over cardiovascular function. In both chronically-instrumented conscious and anaesthetized rats, i.c.v. administration of NMU resulted in increased systemic blood pressure and heart rate (Chu et al., 2002). Furthermore, the increase in blood pressure detected in mice when the temperature of their environment is increased is not observed in NMU knockout mice (Nakahara et al., 2004a). In contrast, microinjection of NMU into rat NTS, involved in integration of autonomic control of the cardiovascular system, produced a decrease in systemic blood pressure and heart rate (Tsubota et al., 2003). Thus the central actions of NMU on the cardiovascular system are unclear at present and warrant further investigation.
Stress response
Evidence supports roles for NMU in both the central and peripheral control of the stress response. i.c.v. Administration of NMU brought about stress-related behaviour in rats such as increased grooming (Garlton et al., 2004; Kojima et al., 2000; Hanada et al., 2001; Wren et al., 2002; Zeng et al., 2006), dampened by i.c.v. administration of anti-CRH antisera and the CRH receptor antagonist, α-helical CRH (Hanada et al., 2001). Furthermore, intra-PVN administration of NMU to rats produced increases in plasma levels of the stress-related hormones, adrenocorticotropic hormone (ACTH) and corticosterone (Thompson et al., 2004; Wren et al., 2002); expectedly, low plasma corticosterone levels were present in NMU knockout mice (Hanada et al., 2004), even when subjected to immobilization stress (Nakahara et al., 2004a). Leptin has also been described as a ‘stress-related peptide’ (Bornstein, 1997); anti-NMU antisera reduced the release of CRH from rat hypothalamic explants exposed to leptin and, when administered centrally, lowered leptin-induced increases in plasma ACTH and corticosterone (Jethwa et al., 2006). In summary, findings indicate that NMU signals through a CRH pathway in the PVN, resulting in the activation of the HPA axis, and may be a route through which leptin exerts its ‘stress-related’ actions. Recently, central, but not peripheral, administration of NMU has also been shown to increase secretion of the catecholamine adrenaline, which has a major role in the acute stress response, from the adrenal medulla in rat (Sasaki et al., 2008).
Peripheral administration of NMU to rats resulted in increased plasma levels of ACTH and corticosterone (Malendowicz et al., 1993). A direct action for NMU on the rat adrenal gland was investigated in vitro. Prerequisites for NMU-induced secretion of adrenal steroids were medullary chromaffin cells and the intra-medullary CRH/ACTH system; NMU had no effect on adrenal explants that lacked medullary chromaffin cells whereas exposure to both α-helical CRH and corticotropin-inhibiting peptide, antagonists of the CRH and ACTH receptors, respectively, reduced steroid secretion from cortical cells (Malendowicz et al., 1994a). Intriguingly, the action of NMU on the HPA axis appears to be biphasic: low doses yielded hypertrophy of the zona fasciculata with subsequently amplified plasma levels of corticosterone and increased secretion of this steroid from adrenal explants; conversely, high doses produced hypertrophy of pituitary corticotrophs and increased plasma levels of ACTH together with a reduction in adrenal weight and no effect on corticosterone secretion (Malendowicz et al., 1994b). The receptor subtype NMU1 is most likely responsible for the direct adrenal actions of NMU. Although Fujii et al. (2000) found no evidence of mRNA for NMU1 in rat adrenal gland, several studies have since detected NMU1 in cortex and medulla at both mRNA and protein levels with no indication of NMU2 expression (Rucinski et al., 2007; Trejter et al., 2008; Ziolkowska et al., 2008).
Reproductive actions of NMU
Studies investigating the reproductive actions of NMU are yet to achieve clarity. In particular, it is difficult to relate the effects of NMU on ovariectomized (OVX) rats, in which sex steroids are absent, to the physiological scenario. In both pubertal and adult rats, i.c.v. NMU produced increases in serum luteinizing hormone (LH) (Vigo et al., 2007a). In contrast, i.c.v. NMU reduced LH secretion in OVX rats and counteracted the increase in LH in pubertal rats receiving the pro-puberty hormone, kisspeptin-10 (KP-10) (Quan et al., 2003; Vigo et al., 2007a). In both OVX and KP-10-treated rats, LH levels are raised; this poses the question whether NMU has a bimodal action on LH release, suppressing secretion when LH levels are high but otherwise promoting secretion. However, in NMU knock-out mice, vaginal opening occurred earlier than in wild-type mice and, likewise, the LH/follicle-stimulating hormone (FSH) ratio increased at an earlier time point (Fukue et al., 2006). Furthermore, NMU suppressed LH release from rat anterior pituitary cells (Fukue et al., 2006). Both vaginal opening and the LH/FSH ratio are indicators for the onset of puberty and, therefore, this study suggests NMU delays puberty by suppressing gonadotropin secretion.
Factors governing the reproductive function of NMU include sex steroids and energy balance. Hypothalamic NMU mRNA levels were observed to fluctuate during post-natal maturation and the oestrous cycle, with high expression in pubertal (30 days) rats and prior to the proestrous LH surge in cycling adults, decreasing on approach to oestrus (Vigo et al., 2007a). After ovariectomy, hypothalamic expression of NMU mRNA was reduced in rat; however, this effect was not observed in animals implanted with an oestrdiol and progesterone releasing device (Vigo et al., 2007a). Changes in sex steroid levels may therefore be responsible for the increased NMU mRNA expression observed during puberty and the oestrous cycle. Pituitary NMU mRNA expression has also been shown to be greater during puberty in rat, decreasing towards maturity. In contrast to the hypothalamic expression of NMU mRNA, in cultured pituitary cells, such expression was reduced by oestradiol treatment. Therefore, the increase in oestradiol levels seen at puberty may inhibit NMU expression as maturity approaches and consequently prevent any suppressive effect of NMU on LH secretion (Shimizu et al., 2008). Peripherally, although NMU receptor density did not alter during the oestrous cycle in rat uterus, a 60% decrease in receptor density post-ovariectomy was observed, which was rectified by treatment with oestradiol (Nandha et al., 1999).
A 48 hour fasting period was shown to augment the inhibition of i.c.v. NMU on LH secretion in OVX rats (Quan et al., 2003), an effect reduced by astressin, a CRH receptor antagonist, suggesting CRH, at least in part, mediates this synergistic inhibitory effect of NMU and fasting on pulsatile LH secretion (Quan et al., 2004). It can be speculated that this effect may contribute to prevention of ovulation in animals whose body condition may not be suitable for pregnancy.
NMU and nociception
Domin et al. (1987) detected greater levels of NMU-LI in the dorsal horn of rat spinal cord than the ventral horn, suggesting a sensory function for NMU. Indeed, both electrophysiological and behavioural studies support a role for NMU in nociception. NMU increased excitability of neurones in the dorsal horn of the spinal cord in vitro and in vivo whereas animals receiving NMU intrathecally experienced thermal hyperalgesia, mechanical allodynia and greater nociceptive flexor reflexes in response to touch or pinch stimuli. In addition, a behavioural response comprising scratching, biting or licking of lower body parts was observed (Cao et al., 2003; Yu et al., 2003; Moriyama et al., 2004; Nakahara et al., 2004a). As further evidence, NMU knock-out mice had reduced nociceptive reflexes and increased expression of NMU mRNA in the spinal cord after injecting mice with formalin was detected (Nakahara et al., 2004a).
By autoradiography, [125I]-NMU-23 binding sites were localized to laminae I and II of the spinal cord in rat; more specifically, molecular studies indicated NMU2 mRNA was present in lamina I and the outer part of lamina II of the dorsal horn (Yu et al., 2003), the site of most nociceptive neurones (Light and Willcockson, 1999). NMU1 mRNA was detected in small to medium diameter neurones of the dorsal root ganglia but not spinal cord (Yu et al., 2003), even though Fujii et al. (2000) had previously reported its presence in this tissue. However, NMU2 does seem the most likely candidate through which NMU exerts its nociceptive action. NMU2 knock-out mice had reduced sensitivity to hot plate, capsaicin and formalin tests, and both NMU-induced enhanced nociceptive responses to formalin administration and increased frequency of excitatory post-synaptic currents in lamina II neurones were absent (Zeng et al., 2006; Torres et al., 2007). NMU1 knock-out mice had similar sensitivity to nociceptive stimuli as wild types (Torres et al., 2007), implying this receptor subtype is not involved.
NMU and the circadian ‘clock’
The presence of NMU, NMU1 and NMU2 mRNA within the suprachiasmatic nucleus (SCN) (Nakahara et al., 2004b), the location of the circadian ‘clock,’ and increased c-Fos-LI in the SCN when i.c.v. NMU was administered to rats (Nakahara et al., 2004b) suggest a role for this peptide in regulation of circadian rhythms. Indeed, i.c.v. NMU produced a phase shift of circadian locomotor activity in rats and resulted in increased expression of mRNA for period homologue 1, a ‘circadian regulator’ (Albrecht et al., 1997), in the SCN (Nakahara et al., 2004b). In addition, re-entrainment in NMU knock-out mice took longer when the light–dark cycle was shifted than in wild-types (Nakahara et al., 2004a). Expression of NMU itself demonstrated a circadian rhythm: mRNA content oscillated within the SCN, peaking during the light phase or subjective day in animals exposed to light–dark cycling (Graham et al., 2005) or constant darkness (Nakahara et al., 2004b), respectively, the latter indicating an endogenous rhythm that persists in the absence of environmental cues.
Taken together with anatomical considerations, namely that the SCN has projections to the PVN (Leak and Moore, 2001), these results suggest that NMU may exert circadian control over its PVN-mediated actions, in particular feeding. The finding that NMU mRNA within the SCN peaks during the day concords with nocturnal feeding of rodents, such that appetite would be suppressed in hours of light. However, this would also imply increased physical activity during the day, which is at odds with rodent nocturnal behaviour. In addition, the majority of evidence suggests that CRH is a downstream effector of NMU; however, no relationship between the circadian rhythms for NMU expression in the SCN and expression of CRH in the PVN was evident in mouse (Graham et al., 2005). Whether the same is true in rat, which has higher levels of NMU2 expression in the PVN (Graham et al., 2003), remains to be studied.
Effects of NMU on bone
Sato et al. (2007) proposed that NMU acts centrally as a negative regulator of bone formation. Firstly, NMU was shown not to directly affect osteoblasts, suggesting a central action, and secondly, NMU knock-out mice had a higher bone mass than controls, exhibiting a larger population of osteoblasts and subsequently a greater rate of bone formation. This action of NMU is believed to occur downstream of leptin, as leptin was no longer effective at inhibiting bone formation in NMU knock-out mice. In contrast, Rucinski et al. (2008) have shown that NMU can directly stimulate proliferation of cultured rat calvarial osteoblast-like cells. Both of these effects are thought to be mediated through NMU2.
Pathophysiological roles for NMU
Role of NMU in immunity and inflammation
Messenger RNA for NMU has been detected in monocytes and dendritic cells and NMU1 mRNA in T cells, macrophages and natural killer cells (Hedrick et al., 2000; Johnson et al., 2004; Moriyama et al., 2006a), suggesting an immunoregulatory role. Indeed, NMU induced secretion of interleukins (IL)-4, 5, 6, 10 and 13 from murine T cells (Johnson et al., 2004); in NMU knock-out mice, mortality rate was lower after administration of lipopolysaccharide (LPS; endotoxin), a result of decreased production of IL-6 by macrophages and subsequent reduced septic shock (Moriyama et al., 2006a). Peripherally, NMU appears to perpetuate the deleterious effects of LPS; however, NMU ameliorated LPS-induced poor performance of mice in the Y-maze test when administered centrally and reduced death of cultured hippocampal neurones when subjected to LPS. Although NMU had no effect on cytokine levels or inflammation within the brain, its up-regulation of brain-derived neurotrophic factor in the hippocampus, shown at mRNA and protein levels, is believed to offer protection against ‘neuroinflammation-induced amnesia’ (Iwai et al., 2008).
NMU1 mRNA has also been observed in mast cells and eosinophils (Moriyama et al., 2005; 2006b;). Intraplantar injection of complete Freund's adjuvant (CFA) caused degranulation of mast cells with ensuing paw oedema and up-regulation of IL-6, tumour necrosis factor-alpha, macrophage inflammatory protein-2 and leukocyte adhesion molecules, responses not seen in NMU knock-out mice (Moriyama et al., 2005). Similarly, subcutaneous administration of NMU produced plasma extravasation and paw oedema in wild-type and NMU knock-out mice but not in mast cell-deficient mice (Moriyama et al., 2005), providing convincing evidence that NMU has a role in mast cell-mediated inflammation. The source of NMU in the paw was thought to be keratinocytes and, indeed, reduced NMU-LI at this site was observed after CFA administration (Moriyama et al., 2005). NMU has also been shown to promote eosinophilia, allowing cell adhesion and chemotaxis in vitro; in addition, NMU knock-out mice have a reduced eosinophilic response in the ovalbumin-mediated asthma model (Moriyama et al., 2006b). These findings propose that the NMU system is a potential target for the treatment of diseases characterized by mast cell or eosinophil mediated inflammation, such as inflammatory bowel disease and bronchial asthma respectively.
NMU and cancer
Changes in NMU mRNA expression have been noted in several forms of cancer (Table 5). In oesophageal squamous cell carcinoma, expression was decreased or silenced in both cell lines and primary tumours (Yamashita et al., 2002). As NMU suppressed the colony-forming ability of cells derived from this cancer (Yamashita et al., 2002), reduced expression results in greater tumourogenicity. In contrast, expression was increased in bladder carcinoma (Wu et al., 2007), ovarian carcinoma (Euer et al., 2005), non-small cell lung cancers (NSCLCs) (Takahashi et al., 2006) and acute myeloid leukaemia (AML) (Shetzline et al., 2004). Although the consequences of increased NMU mRNA expression in ovarian carcinoma were not reported (Euer et al., 2005), NMU promoted proliferation of primary AML cells (Shetzline et al., 2004) and exposure of NSCLC cells to short interfering RNA against NMU reduced cell growth (Takahashi et al., 2006). In human bladder cancer cells, the NMU gene was negatively regulated by RhoGDI2, a Rho-guanine diphosphate dissociation inhibitor shown to suppress metastasis. In nude mice, injection of such cells overexpressing NMU not only increased tumour formation but also metastasis (Wu et al., 2007). In bladder carcinoma, reduced expression of RhoGDI2 heralds a lower 5-year survival rate (Theodorescu et al., 2004). Therefore, it can be hypothesized that reduced RhoGDI2 expression in this cancer results in increased NMU expression and subsequent metastasis. NMU was also shown to contribute to cancer-associated cachexia (Wu et al., 2007), potentially through its anorectic effects but also through increased secretion of the cachectic factor IL-6 (Strassmann et al., 1992; Kuroda et al., 2007) from macrophages and T cells (Johnson et al., 2004; Moriyama et al., 2006a).
Table 5.
Summary of cancers in which NMU mRNA has been detected
| Cancer type | Change in NMU mRNA expression | Action of NMU | |
|---|---|---|---|
| Oral tumours | ↓ | Alevizos et al., (2001) | |
| Oesophageal SCC | ↓ | Reduced growth of oesophageal SCC cells | Yamashita et al. (2002) |
| Ovarian carcinoma | ↑ | Euer et al., (2005) | |
| Bladder carcinoma | ↑, secondary to down-regulation of Rho-GDP dissociation inhibitor | Increased tumourogenicity, pulmonary metastasis and cancer-associated cachexia | Wu et al. (2007) |
| Non-small cell lung cancers | ↑ | Increased growth and invasion of tumour cells; NMU thought to act at a heterodimer of NTS1 and splice variant of ghrelin receptor | Takahashi et al. (2006) |
| AML | ↑ | Proliferation of AML cells | Shetzline et al., (2004) |
AML, acute myeloid leukaemia; GDP, G-protein-coupled receptors; NTS1, neurotensin receptor 1; SCC, squamous cell carcinoma.
Evidence suggests that NMU has a tumourogenic action in AML, NSCLC and bladder carcinoma, suggesting the NMU system may provide a novel therapeutic target in the battle against cancer. In addition, serum samples from patients with such cancers should be analysed to determine whether NMU could be a marker of disease and diagnostic aid (Euer et al., 2005).
Neuromedin S (NMS)
In 2005, a 36 amino acid peptide related to NMU was discovered in rat brain, named NMS owing to its high expression in the SCN (Mori et al., 2005). These two peptides share the same amidated C-terminal heptapeptide (Figure 1) and bind to the same receptors, NMU1 and NMU2, though NMU2 has a greater affinity for NMS than NMU (Mori et al., 2005). However, their genes are located on different chromosomes (NMS on 2q11.2 and NMU on 4q12) and NMS mRNA has a more limited distribution, at least in rat, with the highest levels of expression in the hypothalamus, spleen and testis (Mori et al., 2005). NMS has also recently been identified in the human heart (Mitchell et al., 2008). Akin to the discovery of NMU analogues in the skin secretions of the treefrog, Litoria caerulea (Salmon et al., 2000), and toad, Bombina maxima (Lee et al., 2005), NMS analogues, NMS-17 and NMS-33, have been isolated from the dermal venoms of bombinid toads, including B. maxima (Chen et al., 2006). Both produced functional responses in cells expressing either human NMU1 or NMU2 with similar potency to human and rat NMS.
Functionally, NMU and NMS have similar putative physiological actions; both cause contraction of smooth muscle preparations and increase systemic blood pressure in rats when administered i.v. (Mori et al., 2005). In man, NMS elicited vasoconstriction in isolated saphenous vein with comparable potency with NMU-25 but significantly reduced maximum contractile response, suggesting NMS is a partial agonist at least in this tissue (Mitchell et al., 2009). Like NMU, NMS also has roles in regulation of feeding (Ida et al., 2005; Shousha et al., 2006), circadian rhythms (Mori et al., 2005), the HPA axis (Jaszberenyi et al., 2007) and LH secretion (Vigo et al., 2007b). Most recently, i.c.v. NMS has been shown to increase plasma levels of arginine-vasopressin, subsequently reducing urine production (Sakamoto et al., 2007), and to stimulate oxytocin release in response to suckling (Sakamoto et al., 2008) in rats.
Interestingly, although NMS induced contraction of chick rectum and elevation of rat systemic blood pressure with potencies similar to NMU (Mori et al., 2005), central effects of NMS were more potent. i.c.v. Administration of NMS, compared with i.c.v. NMU, resulted in more potent phase-shifting of circadian rhythms (Mori et al., 2005), suppression of feeding (Ida et al., 2005), reduction of urine volume (Sakamoto et al., 2007) and stimulation of oxytocin release (Sakamoto et al., 2008). The mechanism behind this phenomenon is yet to be identified; it is speculated that NMU receptors in the CNS could be under the control of receptor-activity-modifying proteins (Mori et al., 2005), though these primarily interact with class B GPCRs (Hay et al., 2006).
Conclusion
The NMU system has a widespread distribution and diverse physiological actions, with key roles in contraction of smooth muscle and regulation of energy balance. Knock-out mice have been useful tools for elucidating functions for the NMU receptor subtypes; however, precise delineation requires the development of subtype-specific antagonists. Perhaps most intriguing are the obese phenotypes of NMU knock-out mice and individuals with the Arg165Trp amino acid variant of NMU-25. Taken together with its vascular actions, NMU may be a functional link between energy balance and the cardiovascular system and may provide a future target for therapies directed against the disorders that comprise metabolic syndrome.
Acknowledgments
We thank the British Heart Foundation for their support (grant numbers PS/02/001, FS/05/020/18408, PG/05/127/19872).
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- AML
acute myeloid leukaemia
- Arc
arcuate nucleus
- CNS
central nervous system
- CRH
corticotropin-releasing hormone
- DIO
diet-induced obese
- DMH
dorsomedial hypothalamus
- DR
diet-resistant
- DRG
dorsal root ganglia
- FSH
follicle-stimulating hormone
- GDP
guanine diphosphate
- GPCR
G-protein-coupled receptor
- HPA
hypothalamo–pituitary–adrenal
- IL
interleukin
- LH
luteinizing hormone
- LOS
lower oesophageal sphincter
- LPS
lipopolysaccharide
- NMS
neuromedin S
- NMU
neuromedin U
- NMU1
neuromedin U receptor 1
- NMU2
neuromedin U receptor 2
- NSCLC
non-small cell lung cancer
- NTS
nucleus tractus solitarius
- NTS1
neurotensin receptor 1
- OVX
ovariectomized
- pontine ret. form.
pontine reticular formation
- PP
pancreatic polypeptide
- PTX
pertussis toxin
- PVN
paraventricular nucleus
- SCC
squamous cell carcinoma
- SCN
suprachiasmatic nucleus
- SON
supraoptic nucleus
- VIP
vasoactive intestinal polypeptide
Conflict of interest
None declared.
References
- Aiyar N, Disa J, Foley JJ, Buckley PT, Wixted WE, Pullen M, et al. Radioligand binding and functional characterization of recombinant human NMU1 and NMU2 receptors stably expressed in clonal human embryonic kidney-293 cells. Pharmacology. 2004;72:33–41. doi: 10.1159/000078630. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Alevizos I, Mahadevappa M, Zhang X, Ohyama H, Kohno Y, Posner M, et al. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene. 2001;20:6196–6204. doi: 10.1038/sj.onc.1204685. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augood SJ, Keast JR, Emson PC. Distribution and characterization of neuromedin-U-like immunoreactivity in rat brain and intestine and in guinea-pig intestine. Regul Pept. 1988;20:281–292. doi: 10.1016/0167-0115(88)90063-8. [DOI] [PubMed] [Google Scholar]
- Austin C, Lo G, Nandha KA, Meleagros L, Bloom SR. Cloning and Characterization of the cDNA encoding the human neuromedin U (NMU) precursor – NMU expression in the human gastrointestinal tract. J Mol Endocrinol. 1995;14:157–169. doi: 10.1677/jme.0.0140157. [DOI] [PubMed] [Google Scholar]
- Ballesta J, Carlei F, Bishop AE, Steel JH, Gibson SJ, Fahey M, et al. Occurrence and developmental pattern of neuromedin U-immunoreactive nerves in the gastrointestinal-tract and brain of the rat. Neuroscience. 1988;25:797–816. doi: 10.1016/0306-4522(88)90037-1. [DOI] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Benito-Orfila MA, Domin J, Nandha KA, Bloom SR. The motor effect of neuromedin-U on rat stomach in vitro. Eur J Pharmacol. 1991;193:329–333. doi: 10.1016/0014-2999(91)90147-i. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Luan J, Farooqi IS, Keogh J, Montague C, Brennand J, et al. Studies of the neuromedin U-2 receptor gene in human obesity: evidence for the existence of two ancestral forms of the receptor. J Endocrinol. 2004;183:115–120. doi: 10.1677/joe.1.05830. [DOI] [PubMed] [Google Scholar]
- Bockman CS, Abel PW, Hicks JW, Conlon JM. Evidence that neuromedin-U may regulate gut motility in reptiles but not in mammals. Eur J Pharmacol. 1989;171:255–257. doi: 10.1016/0014-2999(89)90117-9. [DOI] [PubMed] [Google Scholar]
- Bornstein SR. Is leptin a stress related peptide? Nat Med. 1997;3:937–937. doi: 10.1038/nm0997-937. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Szekeres PG, Willars GB. Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacol Rev. 2004a;56:231–248. doi: 10.1124/pr.56.2.3. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Szekeres PG, Wise A, Willars GB. Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to G alpha(q/11) and G alpha(i) and an irreversible ligand-receptor interaction. Mol Pharmacol. 2004b;66:1544–1556. doi: 10.1124/mol.104.002337. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Wise A, Dass NB, Willars GB. Paradoxical behavior of neuromedin U in isolated smooth muscle cells and intact tissue. J Pharmacol Exp Ther. 2008;325:154–164. doi: 10.1124/jpet.107.132803. [DOI] [PubMed] [Google Scholar]
- Brown DR, Quito FL. Neuromedin-U octapeptide alters ion-transport in porcine jejunum. Eur J Pharmacol. 1988;155:159–162. doi: 10.1016/0014-2999(88)90415-3. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse Ob protein – evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cao CQ, Yu XH, Dray A, Filosa A, Perkins MN. A pro-nociceptive role of neuromedin U in adult mice. Pain. 2003;104:609–616. doi: 10.1016/S0304-3959(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Chen TB, Zhou M, Walker B, Harriot P, Mori K, Miyazato M, et al. Structural and functional analogs of the novel mammalian neuropeptide, neuromedin S (NmS), in the dermal venoms of Eurasian bombinid toads. Biochem Biophys Res Commun. 2006;345:377–384. doi: 10.1016/j.bbrc.2006.04.103. [DOI] [PubMed] [Google Scholar]
- Chu CP, Jin QH, Kunitake T, Kato K, Nabekura T, Nakazato M, et al. Cardiovascular actions of central neuromedin U in conscious rats. Regul Pept. 2002;105:29–34. doi: 10.1016/s0167-0115(01)00381-0. [DOI] [PubMed] [Google Scholar]
- Dass NB, Bassil AK, North-Laidler VJ, Morrow R, Aziz E, Tuladhar BR, et al. Neuromedin U can exert colon-specific, enteric nerve-mediated prokinetic activity, via a pathway involving NMU receptor activation. Br J Pharmacol. 2007;150:502–508. doi: 10.1038/sj.bjp.0707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrukol-Ak D, Tore F, Tuncel N. Passage of VIP/PACAP/secretin family across the blood-brain barrier: therapeutic effects. Curr Pharm Des. 2004;10:1325–1340. doi: 10.2174/1381612043384934. [DOI] [PubMed] [Google Scholar]
- Domin J, Ghatei MA, Chohan P, Bloom SR. Characterization of neuromedin-U like immunoreactivity in rat, guinea-pig and human tissue extracts using a specific radioimmunoassay. Biochem Biophys Res Commun. 1986;140:1127–1134. doi: 10.1016/0006-291x(86)90752-7. [DOI] [PubMed] [Google Scholar]
- Domin J, Ghatei MA, Chohan P, Bloom SR. Neuromedin-U – a study of its distribution in the rat. Peptides. 1987;8:779–784. doi: 10.1016/0196-9781(87)90058-1. [DOI] [PubMed] [Google Scholar]
- Domin J, Yiangou Y, Spokes RA, Aitken A, Parmar KB, Chrysanthou BJ, et al. The distribution, purification and pharmacological action of an amphibian neuromedin-U. J Biol Chem. 1989;264:20881–20885. [PubMed] [Google Scholar]
- Domin J, Benito-Orfila MA, Nandha KA, Aitken A, Bloom SR. The purification and sequence-analysis of an avian neuromedin-U. Regul Pept. 1992;41:1–8. doi: 10.1016/0167-0115(92)90508-r. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods. 2004;137:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Euer NI, Kaul S, Deissler H, Mobus VJ, Zeillinger R, Weidle UH. Identification of L1CAM, Jagged2 and neuromedin U as ovarian cancer-associated antigens. Oncol Rep. 2005;13:375–387. [PubMed] [Google Scholar]
- Fang LY, Zhang MC, Li CX, Dong SZ, Hu YH. Chemical genetic analysis reveals the effects of NMU2R on the expression of peptide hormones. Neurosci Lett. 2006;404:148–153. doi: 10.1016/j.neulet.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Habata Y, Hinuma S, et al. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. J Biol Chem. 2000;275:21068–21074. doi: 10.1074/jbc.M001546200. [DOI] [PubMed] [Google Scholar]
- Fukue Y, Sato T, Teranishi H, Hanada R, Takahashi T, Nakashima Y, et al. Regulation of gonadotropin secretion and puberty onset by neuromedin U. FEBS Lett. 2006;580:3485–3488. doi: 10.1016/j.febslet.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Yang SJ, Shan LX, Bayne M, Monsma FJ, et al. Cloning and characterization of murine neuromedin U receptors. Peptides. 2002;23:1607–1615. doi: 10.1016/s0196-9781(02)00097-9. [DOI] [PubMed] [Google Scholar]
- Furness JB, Pompolo S, Murphy R, Giraud A. Projections of neurons with neuromedin U-like immunoreactivity in the small-intestine of the guinea-pig. Cell Tissue Res. 1989;257:415–422. doi: 10.1007/BF00261844. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Bennett T, Domin J, Bloom SR. Regional hemodynamic effects of neuromedin-U in conscious rats. Am J Physiol. 1990;258:R32–R38. doi: 10.1152/ajpregu.1990.258.1.R32. [DOI] [PubMed] [Google Scholar]
- Garlton J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, et al. Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology (Berl) 2004;177:1–14. doi: 10.1007/s00213-004-1918-3. [DOI] [PubMed] [Google Scholar]
- Graham ES, Turnbull Y, Fotheringham P, Nilaweera K, Mercer JG, Morgan PJ, et al. Neuromedin U and neuromedin U receptor-2 expression in the mouse and rat hypothalamus: effects of nutritional status. J Neurochem. 2003;87:1165–1173. doi: 10.1046/j.1471-4159.2003.02079.x. [DOI] [PubMed] [Google Scholar]
- Graham ES, Littlewood P, Turnbull Y, Mercer JG, Morgan PJ, Barrett P. Neuromedin-U is regulated by the circadian clock in the SCN of the mouse. Eur J Neurosci. 2005;21:814–819. doi: 10.1111/j.1460-9568.2005.03923.x. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Jiang Q, Van Der Ploeg LH, Liu Q. Distribution of neuromedin U receptor subtype 2 mRNA in the rat brain. Brain Res Gene Expr Patterns. 2001;1:1–4. doi: 10.1016/s1567-133x(00)00002-8. [DOI] [PubMed] [Google Scholar]
- Hainerova I, Torekov SS, Ek J, Finkova M, Borch-Johnsen K, Jorgensen T, et al. Association between neuromedin U gene variants and overweight and obesity. J Clin Endocrinol Metab. 2006;91:5057–5063. doi: 10.1210/jc.2006-1442. [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Murakami N, Sakihara S, Yoshimatsu H, Toshinai K, et al. A role for neuromedin U in stress response. Biochem Biophys Res Commun. 2001;289:225–228. doi: 10.1006/bbrc.2001.5945. [DOI] [PubMed] [Google Scholar]
- Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, et al. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med. 2004;10:1067–1073. doi: 10.1038/nm1106. [DOI] [PubMed] [Google Scholar]
- Hanada T, Date Y, Shimbara T, Sakihara S, Murakami N, Hayashi Y, et al. Central actions of neuromedin U via corticotropin-releasing hormone. Biochem Biophys Res Commun. 2003;311:954–958. doi: 10.1016/j.bbrc.2003.10.098. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Masui H, Uchida Y, Sakura N, Okimura K. Agonistic and antagonistic activities of neuromedin-U-8 analogs substituted with glycine or D-amino-acid on contractile activity of chicken crop smooth-muscle preparations. Chem Pharm Bull (Tokyo) 1991;39:2319–2322. doi: 10.1248/cpb.39.2319. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPS. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hedrick JA, Morse K, Shan LX, Qiao XD, Pang L, Wang S, et al. Identification of a human gastrointestinal tract and immune system receptor for the peptide neuromedin U. Mol Pharmacol. 2000;58:870–875. doi: 10.1124/mol.58.4.870. [DOI] [PubMed] [Google Scholar]
- Hoebel BG. Hypothalamic lesions by electrocauterization: disinhibition of feeding and self-stimulation. Science. 1965;149:452–453. doi: 10.1126/science.149.3682.452. [DOI] [PubMed] [Google Scholar]
- Honzawa M, Sudoh T, Minamino N, Tohyama M, Matsuo H. Topographic localization of neuromedin U-like structures in the rat-brain – an immunohistochemical study. Neuroscience. 1987;23:1103–1122. doi: 10.1016/0306-4522(87)90185-0. [DOI] [PubMed] [Google Scholar]
- Honzawa M, Sudoh T, Minamino N, Kangawa K, Matsuo H. Neuromedin-U-like immunoreactivity in rat intestine – regional distribution and immunohistochemical study. Neuropeptides. 1990;15:1–9. doi: 10.1016/0143-4179(90)90153-p. [DOI] [PubMed] [Google Scholar]
- Hosoya M, Moriya T, Kawamata Y, Ohkubo S, Fujii R, Matsui H, et al. Identification and functional characterization of a novel subtype of neuromedin U receptor. J Biol Chem. 2000;275:29528–29532. doi: 10.1074/jbc.M004261200. [DOI] [PubMed] [Google Scholar]
- Howard AD, Wang RP, Pong SS, Mellin TN, Strack A, Guan XM, et al. Identification of reeptors for neuromedin U and its role in feeding. Nature. 2000;406:70–74. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Luo CW. Molecular dissection of G protein preference using G(s)alpha chimeras reveals novel ligand signaling of GPCRs. Am J Physiol Endocrinol Metab. 2007;293:E1021–E1029. doi: 10.1152/ajpendo.00003.2007. [DOI] [PubMed] [Google Scholar]
- Ida T, Mori K, Miyazato M, Egi Y, Abe S, Nakahara K, et al. Neuromedin S is a novel anorexigenic hormone. Endocrinology. 2005;146:4217–4223. doi: 10.1210/en.2005-0107. [DOI] [PubMed] [Google Scholar]
- Ivanov TR, Lawrence CB, Stanley PJ, Luckman SM. Evaluation of neuromedin U actions in energy homeostasis and pituitary function. Endocrinology. 2002;143:3813–3821. doi: 10.1210/en.2002-220121. [DOI] [PubMed] [Google Scholar]
- Ivanov TR, Le Rouzic P, Stanley PJ, Ling WY, Parello R, Luckman SM. Neuromedin U neurones in the rat nucleus of the tractus solitarius are catecholaminergic and respond to peripheral cholecystokinin. J Neuroendocrinol. 2004;16:612–619. doi: 10.1111/j.1365-2826.2004.01210.x. [DOI] [PubMed] [Google Scholar]
- Iwai T, Iinuma Y, Kodani R, Oka JI. Neuromedin U inhibits inflammation-mediated memory impairment and neuronal cell-death in rodents. Neurosci Res. 2008;61:113–119. doi: 10.1016/j.neures.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Jaszberenyi M, Bagasi Z, Thurzo B, Foldesi I, Telegdy G. Endocrine and behavioral effects of neuromedin S. Horm Behav. 2007;52:631–639. doi: 10.1016/j.yhbeh.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Jethwa PH, Small CJ, Smith KL, Seth A, Darch SJ, Abbott CR, et al. Neuromedin U has a physiological role in the regulation of food intake and partially mediates the effects of leptin. Am J Physiol Endocrinol Metab. 2005;289:E301–E305. doi: 10.1152/ajpendo.00404.2004. [DOI] [PubMed] [Google Scholar]
- Jethwa PH, Smith KL, Small CJ, Abbott CR, Darch SJ, Murphy KG, et al. Neuromedin U partially mediates leptin-induced hypothalamo-pituitary adrenal (HPA) stimulation and has a physiological role in the regulation of the HPA axis in the rat. Endocrinology. 2006;147:2886–2892. doi: 10.1210/en.2005-0983. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Appelbaum ER, Carptenter DC, Cox RF, Disa J, Foley JJ, et al. Neuromedin U elicits cytokine release in murine Th2-type T cell clone D10.G4.1. J Immunol. 2004;173:7230–7238. doi: 10.4049/jimmunol.173.12.7230. [DOI] [PubMed] [Google Scholar]
- Jones NA, Morton MF, Prendergast CE, Powell GL, Shankley NP, Hollingsworth SJ. Neuromedin U stimulates contraction of human long saphenous vein and gastrointestinal smooth muscle in vitro. Regul Pept. 2006;136:109–116. doi: 10.1016/j.regpep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kaczmarek P, Malendowicz LK, Pruszynska-Oszmalek E, Wojciechowicz T, Szczepankiewicz D, Szkudelski T, et al. Neuromedin U receptor 1 expression in the rat endocrine pancreas and evidence suggesting neuromedin U suppressive effect on insulin secretion from isolated rat pancreatic islets. Int J Mol Med. 2006;18:951–955. [PubMed] [Google Scholar]
- Kage R, O'Harte F, Thim L, Conlon JM. Rabbit neuromedin U-25 – lack of conservation of a posttranslational processing site. Regul Pept. 1991;33:191–198. doi: 10.1016/0167-0115(91)90213-z. [DOI] [PubMed] [Google Scholar]
- Kamisoyama H, Honda K, Saneyasu T, Sugahara K, Hasegawa S. Central administration of neuromedin U suppresses food intake in chicks. Neurosci Lett. 2007;420:1–5. doi: 10.1016/j.neulet.2007.03.062. [DOI] [PubMed] [Google Scholar]
- Kojima M, Haruno R, Nakazato M, Date Y, Murakami N, Hanada R, et al. Purification and identification of neuromedin U as an endogenous ligand for an orphan receptor GPR66 (FM3) Biochem Biophys Res Commun. 2000;276:435–438. doi: 10.1006/bbrc.2000.3502. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Spar BD, Markowitz L, Maguire M, Golovko A, Yang S, et al. Transgenic overexpression of neuromedin U promotes leanness and hypophagia in mice. J Endocrinol. 2005;185:151–164. doi: 10.1677/joe.1.05948. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraints decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, et al. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007;69:113–117. doi: 10.1016/j.urology.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lee WH, Liu SB, Shen JH, Jin Y, Lai R, Zhang Y. Identification and molecular cloning of a novel neuromedin U analog from the skin secretions of toad Bombina maxima. Regul Pept. 2005;129:43–47. doi: 10.1016/j.regpep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Light AR, Willcockson HH. Spinal laminae I-II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. J Neurophysiol. 1999;82:3316–3326. doi: 10.1152/jn.1999.82.6.3316. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Patacchini R, Giuliani S, Turini D, Barbanti G, Rovero P, et al. Motor response of the human isolated small-intestine and urinary bladder to porcine neuromedin U-8. Br J Pharmacol. 1990;99:186–188. doi: 10.1111/j.1476-5381.1990.tb14675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz LK, Andreis PG, Markowska A, Nowak M, Warchol JB, Neri G, et al. Effects of neuromedin U-8 on the secretory activity of the rat adrenal cortex – evidence for an indirect action requiring the presence of the zona medullaris. Res Exp Med (Berl) 1994a;194:69–79. doi: 10.1007/BF02576368. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Nussdorfer GG, Markowska A, Tortorella C, Nowak M, Warchol JB. Effects of neuromedin-U (NMU)-8 on the rat hypothalamo-pituitary-adrenal axis – evidence of a direct effect of NMU-8 on the adrenal gland. Neuropeptides. 1994b;26:47–53. doi: 10.1016/0143-4179(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Malendowicz LM, Nussdorfer GG, Nowak KW, Mazzocchi G. Effects of neuromedin U-8 on the rat pituitary-adrenocortical axis. In Vivo (Athens) 1993;7:419–422. [PubMed] [Google Scholar]
- Mangold C, Ksiazek I, Yun SW, Berger E, Binkert C. Distribution of neuromedin U binding sites in the rat CNS revealed by in vitro receptor autoradiography. Neuropeptides. 2008;42:377–386. doi: 10.1016/j.npep.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Konno N, Ishiguro K, Wakasugi T, Uchiyama M, Shioda S, et al. Isolation and characterisation of four cDNAs encoding neuromedin U (NMU) from the brain and gut of goldfish, and the inhibitory effect of a deduced NMU on food intake and locomotor activity. J Neuroendocrinol. 2008;20:71–78. doi: 10.1111/j.1365-2826.2007.01615.x. [DOI] [PubMed] [Google Scholar]
- Melcher C, Bader R, Walther S, Simakov O, Pankratz MJ. Neuromedin U and its putative Drosophila homolog hugin. PLoS Biol. 2006;4:316–316. doi: 10.1371/journal.pbio.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T, Su HR, Binkert C, Fischli W, Zhou L, Shen JK, et al. Identification of non-peptidic neuromedin U receptor modulators by a robust homogeneous screening assay. Acta Pharmacol Sin. 2008;29:517–527. doi: 10.1111/j.1745-7254.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- Michel C, Dunn-Meynell A, Levin BE. Reduced brain CRH and GR mRNA expression precedes obesity in juvenile rats bred for diet-induced obesity. Behav Brain Res. 2004;154:511–517. doi: 10.1016/j.bbr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H. Neuromedin-U-8 and neuromedin-U-25 – novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem Biophys Res Commun. 1985;130:1078–1085. doi: 10.1016/0006-291x(85)91726-7. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Honzawa M, Matsuo H. Isolation and structural determination of rat neuromedin-U. Biochem Biophys Res Commun. 1988;156:355–360. doi: 10.1016/s0006-291x(88)80848-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Maguire JJ, Kuc RE, Davenport AP. Expression and vasoconstrictor function of anorexigenic peptides neuromedin U-25 & S in the human cardiovascular system. Cardiovasc Res. 2009;81:353–361. doi: 10.1093/cvr/cvn302. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Date Y, Murakami N, Toshinai K, Shimbara T, Kangawa K, et al. Neuromedin U acts in the central nervous system to inhibit gastric acid secretion via CRH system. Am J Physiol Gastrointest Liver Physiol. 2003;284:G963–G969. doi: 10.1152/ajpgi.00218.2002. [DOI] [PubMed] [Google Scholar]
- Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, et al. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. EMBO J. 2005;24:325–335. doi: 10.1038/sj.emboj.7600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Furue H, Katafuchi T, Teranishi H, Sato T, Kano T, et al. Presynaptic modulation by neuromedin U of sensory synaptic transmission in rat spinal dorsal horn neurones. J Physiol – London. 2004;559:707–713. doi: 10.1113/jphysiol.2004.070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Sato T, Inoue H, Fukuyama S, Teranishi H, Kangawa K, et al. The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J Exp Med. 2005;202:217–224. doi: 10.1084/jem.20050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Matsukawa A, Kudoh S, Takahashi T, Sato T, Kano T, et al. The neuropeptide neuromedin U promotes IL-6 production from macrophages and endotoxin shock. Biochem Biophys Res Commun. 2006a;341:1149–1154. doi: 10.1016/j.bbrc.2006.01.075. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Fukuyama S, Inoue H, Matsumoto T, Sato T, Tanaka K, et al. The neuropeptide neuromedin U activates eosinophils and is involved in allergen-induced eosinophilia. Am J Physiol Lung Cell Mol Physiol. 2006b;290:L971–L977. doi: 10.1152/ajplung.00345.2005. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Corticotropin releasing-factor, grooming and ingestive behavior. Life Sci. 1982;31:1459–1464. doi: 10.1016/0024-3205(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Murphy R, Turner CA, Furness JB, Parker L, Giraud A. Isolation and microsequence analysis of a novel form of neuromedin-U from guinea-pig small-intestine. Peptides. 1990;11:613–617. doi: 10.1016/0196-9781(90)90066-e. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Kojima M, Hanada R, Egi Y, Ida T, Miyazato M, et al. Neuromedin U is involved in nociceptive reflexes and adaptation to environmental stimuli in mice. Biochem Biophys Res Commun. 2004a;323:615–620. doi: 10.1016/j.bbrc.2004.08.136. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hanada R, Murakami N, Teranishi H, Ohgusu H, Fukushima N, et al. The gut-brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem Biophys Res Commun. 2004b;318:156–161. doi: 10.1016/j.bbrc.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Hanada R, Murakami N, Date Y, Mondal MS, Kojima M, et al. Central effects of neuromedin U in the regulation of energy homeostasis. Biochem Biophys Res Commun. 2000;277:191–194. doi: 10.1006/bbrc.2000.3669. [DOI] [PubMed] [Google Scholar]
- Nandha KA, Benitoorfila MA, Smith DM, Bloom SR. Characterization of the rat uterine neuromedin-U receptor. Endocrinology. 1993;133:482–486. doi: 10.1210/endo.133.2.8393763. [DOI] [PubMed] [Google Scholar]
- Nandha KA, Benito-Orfila MA, Jamal H, Akinsanya KO, Bloom SR, Smith DM. Effect of steroids and the estrous cycle on uterine neuromedin U receptor expression. Peptides. 1999;20:1203–1209. doi: 10.1016/s0196-9781(99)00124-2. [DOI] [PubMed] [Google Scholar]
- Niimi M, Murao K, Taminato T. Central administration of neuromedin U activates neurons in ventrobasal hypothalamus and brainstem. Endocrine. 2001;16:201–206. doi: 10.1385/ENDO:16:3:201. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tovar S, Mitchell SE, Barrett P, Rayner DV, Dieguez C, et al. Negative energy balance and leptin regulate neuromedin-U expression in the rat pars tuberalis. J Endocrinol. 2006;190:545–553. doi: 10.1677/joe.1.06577. [DOI] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA. Neuromedin U in the paraventricular and arcuate hypothalamic nuclei increases non-exercise activity thermogenesis. J Neuroendocrinol. 2006;18:594–601. doi: 10.1111/j.1365-2826.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA. Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity. Brain Res. 2007;1169:57–68. doi: 10.1016/j.brainres.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Harte F, Bockman CS, Abel PW, Conlon JM. Isolation, structural characterization and pharmacological activity of dog neuromedin-U. Peptides. 1991a;12:11–15. doi: 10.1016/0196-9781(91)90159-m. [DOI] [PubMed] [Google Scholar]
- O'Harte F, Bockman CS, Zeng WY, Abel PW, Harvey S, Conlon JM. Primary structure and pharmacological activity of a nonapeptide related to neuromedin-U isolated from chicken intestine. Peptides. 1991b;12:809–812. doi: 10.1016/0196-9781(91)90138-f. [DOI] [PubMed] [Google Scholar]
- Okimura K, Sakura N, Ohta S, Kurosawa K, Hashimoto T. Contractile activity of porcine neuromedin U-25 and various neuromedin U-related peptide-fragments on isolated chicken crop smooth muscle. Chem Pharm Bull (Tokyo) 1992;40:1500–1503. doi: 10.1248/cpb.40.1500. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Onaka T, Nakazato M, Saito J, Kanemoto K, Matsumoto T, et al. Centrally administered neuromedin U activates neurosecretion and induction of c-fos messenger ribonucleic acid in the paraventricular and supraoptic nuclei of rat. Endocrinology. 2002;143:4320–4329. doi: 10.1210/en.2002-220201. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA. 2002;99:11423–11428. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast CE, Morton MF, Figueroa KW, Wu XD, Shankley NP. Species-dependent smooth muscle contraction to Neuromedin U and determination of the receptor subtypes mediating contraction using NMU1 receptor knockout mice. Br J Pharmacol. 2006;147:886–896. doi: 10.1038/sj.bjp.0706677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H, Funabashi T, Furuta M, Kimura F. Effects of neuromedin U on the pulsatile LH secretion in ovariectomized rats in association with feeding conditions. Biochem Biophys Res Commun. 2003;311:721–727. doi: 10.1016/j.bbrc.2003.10.052. [DOI] [PubMed] [Google Scholar]
- Quan H, Funabashi T, Kimura F. Intracerebroventricular injection of corticotropin-releasing hormone receptor antagonist blocks the suppression of pulsatile luteinizing hormone secretion induced by neuromedin U in ovariectomized rats after 48 hours of fasting. Neurosci Lett. 2004;369:33–38. doi: 10.1016/j.neulet.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Raddatz R, Wilson AE, Artymyshyn R, Bonini JA, Borowsky B, Boteju LW, et al. Identification and characterization of two neuromedin U receptors differentially expressed in peripheral tissues and the central nervous system. J Biol Chem. 2000;275:32452–32459. doi: 10.1074/jbc.M004613200. [DOI] [PubMed] [Google Scholar]
- Rucinski M, Ziolkowska A, Neri G, Trejter M, Zemleduch T, Tyczewska M, et al. Expression of neuromedins S and U and their receptors in the hypothalamus and endocrine glands of the rat. Int J Mol Med. 2007;20:255–259. [PubMed] [Google Scholar]
- Rucinski M, Ziolkowska A, Tyczewska M, Szyszka M, Malendowicz LK. Neuromedin U directly stimulates growth of cultured rat calvarial osteoblast-like cells acting via the NMU receptor 2 isoform. International. J Mol Med. 2008;22:363–368. [PubMed] [Google Scholar]
- Sakamoto T, Mori K, Nakahara K, Miyazato M, Kangawa K, Sameshima H, et al. Neuromedin S exerts an antidiuretic action in rats. Biochem Biophys Res Commun. 2007;361:457–461. doi: 10.1016/j.bbrc.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Mori K, Miyazato M, Kangawa K, Sameshima H, Nakahara K, et al. Involvement of neuromedin S in the oxytocin release response to suckling stimulus. Biochem Biophys Res Commun. 2008;375:49–53. doi: 10.1016/j.bbrc.2008.07.124. [DOI] [PubMed] [Google Scholar]
- Sakura N, Ohta S, Uchida Y, Kurosawa K, Okimura K, Hashimoto T. Structure-activity-relationships of rat neuromedin-U for smooth muscle contraction. Chem Pharm Bull (Tokyo) 1991;39:2016–2020. doi: 10.1248/cpb.39.2016. [DOI] [PubMed] [Google Scholar]
- Sakura N, Kurosawa K, Hashimoto T. Structure-activity-relationships of neuromedin-U.1. Contractile activity of dog neuromedin-U-related peptides on isolated chicken crop smooth muscle. Chem Pharm Bull (Tokyo) 1995;43:1148–1153. doi: 10.1248/cpb.43.1148. [DOI] [PubMed] [Google Scholar]
- Salmon AL, Johnsen AH, Bienert M, McMurray G, Nandha KA, Bloom SR, et al. Isolation, structural characterization, and bioactivity of a novel neuromedin U analog from the defensive skin secretion of the Australasian tree frog, Litoria caerulea. J Biol Chem. 2000;275:4549–4554. doi: 10.1074/jbc.275.7.4549. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shimizu T, Wakiguchi H, Yokotani K. Centrally administered neuromedin U elevates plasma adrenaline by brain prostanoid TP receptor-mediated mechanisms in rats. Eur J Pharmacol. 2008;592:81–86. doi: 10.1016/j.ejphar.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, et al. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13:1234–1240. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- Shan LX, Qiao XD, Crona JH, Behan J, Wang S, Laz T, et al. Identification of a novel neuromedin U receptor subtype expressed in the central nervous system. J Biol Chem. 2000;275:39482–39486. doi: 10.1074/jbc.C000522200. [DOI] [PubMed] [Google Scholar]
- Shetzline SE, Rallapalli R, Dowd KJ, Zou SM, Nakata Y, Swider CR, et al. Neuromedin U: a Myb-regulated autocrine growth factor for human myeloid leukemias. Blood. 2004;104:1833–1840. doi: 10.1182/blood-2003-10-3577. [DOI] [PubMed] [Google Scholar]
- Shimizu F, Matsuzaki T, Iwasa T, Minakuchi M, Kuwahara A, Yasui T, et al. Estradiol suppresses NMU mRNA expression during sexual maturation in the female rat pituitary. Int J Dev Neurosci. 2008;26:381–384. doi: 10.1016/j.ijdevneu.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Shousha S, Nakahara K, Miyazato M, Kangawa K, Murakami N. Endogenous neuromedin U has anorectic effects in the Japanese quail. Gen Comp Endocrinol. 2005;140:156–163. doi: 10.1016/j.ygcen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Shousha S, Nakahara K, Sato M, Mori K, Miyazato M, Kangawa K, et al. Effect of neuromedin S on feeding regulation in the Japanese quail. Neurosci Lett. 2006;391:87–90. doi: 10.1016/j.neulet.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Haase W, Reinhart C, Michel H. Heterologous expression and comparative characterization of the human neuromedin U subtype II receptor using the methylotrophic yeast Pichia pastoris and mammalian cells. Int J Biochem Cell Biol. 2007;39:931–942. doi: 10.1016/j.biocel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin-6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi S, Inoue K, Kogire M, Doi R, Takaori K, Suzuki T, et al. Effect of synthetic neuromedin U-8 and U-25, novel peptides identified in porcine spinal-cord, on splanchnic circulation in dogs. Life Sci. 1987;41:1585–1590. doi: 10.1016/0024-3205(87)90725-9. [DOI] [PubMed] [Google Scholar]
- Szekeres PG, Muir AI, Spinage LD, Miller JE, Butler SI, Smith A, et al. Neuromedin U is a potent agonist at the orphan G protein-coupled receptor FM3. J Biol Chem. 2000;275:20247–20250. doi: 10.1074/jbc.C000244200. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Furukawa C, Takano A, Ishikawa N, Kato T, Hayama S, et al. The neuromedin U-growth hormone secretagogue receptor 1b/neurotensin receptor 1 oncogenic signaling pathway as a therapeutic target for lung cancer. Cancer Res. 2006;66:9408–9419. doi: 10.1158/0008-5472.CAN-06-1349. [DOI] [PubMed] [Google Scholar]
- Tan CP, McKee KK, Liu QY, Palyha OC, Feighner SD, Hreniuk DL, et al. Cloning and characterization of a human and murine T-cell orphan G-protein-coupled receptor similar to the growth hormone secretagogue and neurotensin receptors. Genomics. 1998;52:223–229. doi: 10.1006/geno.1998.5441. [DOI] [PubMed] [Google Scholar]
- Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson HF. Reduced expression of metastasis suppressor RhoGD12 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res. 2004;10:3800–3806. doi: 10.1158/1078-0432.CCR-03-0653. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Murphy KG, Todd JF, Martin NM, Small CJ, Ghatei MA, et al. Chronic administration of NMU into the paraventricular nucleus stimulates the HPA axis but does not influence food intake or body weight. Biochem Biophys Res Commun. 2004;323:65–71. doi: 10.1016/j.bbrc.2004.08.058. [DOI] [PubMed] [Google Scholar]
- Torres R, Croll SD, Vercollone J, Reinhardt J, Griffiths J, Zabski S, et al. Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1, have impaired nociceptive responses. Pain. 2007;130:267–278. doi: 10.1016/j.pain.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Trejter M, Neri G, Rucinski M, Majchrzak M, Nussdorfer GG, Malendowicz LK. Neuromedin-U stimulates enucleation-induced adrenocortical regeneration in the rat. Int J Mol Med. 2008;21:683–687. [PubMed] [Google Scholar]
- Tsubota Y, Kakimoto N, Owada-Makabe K, Yukawa K, Liang XM, Mune M, et al. Hypotensive effects of neuromedin U microinjected into the cardiovascular-related region of the rat nucleus tractus solitarius. Neuroreport. 2003;14:2387–2390. doi: 10.1097/00001756-200312190-00020. [DOI] [PubMed] [Google Scholar]
- Vigo E, Roa J, Pineda R, Castellano JM, Navarro VM, Aguilar E, et al. Novel role of the anorexigenic peptide neuromedin U in the control of LH secretion and its regulation by gonadal hormones and photoperiod. Am J Physiol Endocrinol Metab. 2007a;293:E1265–E1273. doi: 10.1152/ajpendo.00425.2007. [DOI] [PubMed] [Google Scholar]
- Vigo E, Roa J, Lopez M, Castellano JM, Fernandez-Fernandez R, Navarro VM, et al. Neuromedin S as novel putative regulator of luteinizing hormone secretion. Endocrinology. 2007b;148:813–823. doi: 10.1210/en.2006-0636. [DOI] [PubMed] [Google Scholar]
- Westfall TD, McCafferty GP, Pullen M, Gruver S, Sulpizio AC, Aiyar VN, et al. Characterization of neuromedin U effects in canine smooth muscle. J Pharmacol Exp Ther. 2002;301:987–992. doi: 10.1124/jpet.301.3.987. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, et al. Hypothalamic actions of neuromedin U. Endocrinology. 2002;143:4227–4234. doi: 10.1210/en.2002-220308. [DOI] [PubMed] [Google Scholar]
- Wu Y, McRoberts K, Berr SS, Frierson HF, Conaway M, Theodorescu D. Neuromedin U is regulated by the metastasis suppressor RhoGDI2 and is a novel promoter of tumor formation, lung metastasis and cancer cachexia. Oncogene. 2007;26:765–773. doi: 10.1038/sj.onc.1209835. [DOI] [PubMed] [Google Scholar]
- Xia H, Liu L, Reinhart C, Michel H. Heterologous expression of human Neuromedin U receptor 1 and its subsequent solubilization and purification. Biochim Biophys Acta. 2008;1778:2203–2209. doi: 10.1016/j.bbamem.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Upadhyay S, Osada M, Hoque MO, Xiao Y, Mori M, et al. Pharmacologic unmasking of epigenetically silenced tumor suppressor genes in esophageal squamous cell carcinoma. Cancer Cell. 2002;2:485–495. doi: 10.1016/s1535-6108(02)00215-5. [DOI] [PubMed] [Google Scholar]
- Yokota M, Ozaki Y, Sakamoto F, Yamada S, Saito J, Fujihara H, et al. Fos expression in CRF-containing neurons in the rat paraventricular nucleus after central administration of neuromedin U. Stress. 2004;7:109–112. doi: 10.1080/10253890410001727370. [DOI] [PubMed] [Google Scholar]
- Yu XH, Cao CQ, Mennicken F, Puma C, Dray A, O'Donnell D, et al. Pro-nociceptive effects of neuromedin U in rat. Neuroscience. 2003;120:467–474. doi: 10.1016/s0306-4522(03)00300-2. [DOI] [PubMed] [Google Scholar]
- Zeng HK, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, et al. Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol. 2006;26:9352–9363. doi: 10.1128/MCB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Hu YH, Liu JH, Ouyang KQ. Screening of active compounds as neuromedin U2 receptor agonist from natural products. Bioorg Med Chem Lett. 2005;15:4531–4535. doi: 10.1016/j.bmcl.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ziolkowska A, Macchi C, Trejter M, Rucinski M, Nowak M, Nussdorfer GG, et al. Effects of neuromedin-U on immature rat adrenocortical cells: in vitro and in vivo studies. Int J Mol Med. 2008;21:303–307. [PubMed] [Google Scholar]


