Abstract
Background and purpose:
β2-Adrenoceptor agonists (β2-agonists) are important bronchodilators used in the treatment of asthma and chronic obstructive pulmonary disease. At the molecular level, β2-adrenergic agonist stimulation induces desensitization of the β2-adrenoceptor. In this study, we have examined the relationships between initial effect and subsequent reduction of responsiveness to restimulation for a panel of β2-agonists in cellular and in vitro tissue models.
Experimental approach:
β2-Adrenoceptor-induced responses and subsequent loss of receptor responsiveness were studied in primary human airway smooth muscle cells and bronchial epithelial cells by measuring cAMP production. Receptor responsiveness was compared at equi-effective concentrations, either after continuous incubation for 24 h or after a 1 h pulse exposure followed by a 23 h washout. Key findings were confirmed in guinea pig tracheal preparations in vitro.
Key results:
There were differences in the reduction of receptor responsiveness in human airway cells and in vitro guinea pig trachea by a panel of β2-agonists. When restimulation occurred immediately after continuous incubation, loss of responsiveness correlated with initial effect for all agonists. After the 1 h pulse exposure, differences between agonists emerged, for example isoprenaline and formoterol induced the least reduction of responsiveness. High lipophilicity was, to some extent, predictive of loss of responsiveness, but other factors appeared to be involved in determining the relationships between effect and subsequent loss of responsiveness for individual agonists.
Conclusions and implications:
There were clear differences in the ability of different β2 agonists to induce loss of receptor responsiveness at equi-effective concentrations.
Keywords: β2-adrenoceptor agonists, airway smooth muscle cells, receptor desensitization
Introduction
Agonists of β2-adrenoceptors (β2-agonists) are highly effective bronchodilators and important therapeutic agents in the treatment of asthma and chronic obstructive pulmonary disease. The β2-agonists available for clinical use vary widely in potency and intrinsic activity and, as a consequence, produce the desired efficacy at varying levels of receptor occupancy. As β2-agonists are generally administered to achieve similar levels of efficacy, it is important, when making relevant comparisons of receptor desensitization by these agents, to consider them at equi-effective doses. Upon stimulation, the β2-adrenoceptor is desensitized by mechanisms that involve both agonist-independent effects following phosphorylation of the receptor by (cAMP)-activated protein kinase A and agonist-dependent effects such as phosphorylation by G-protein regulatory kinases and binding of β-arrestin (see McGraw and Liggett, 2005). Under experimental conditions when panels of β2-agonists are compared at high receptor occupancy (>90 and 88% respectively), the compounds of greater intrinsic activity induce greater levels of reduced responsiveness (January et al., 1997; Scola et al., 2004). In essence, reduced responsiveness is linked quantitatively to signal strength. Recent studies suggest, however, that different agonists may couple in very different ways to underlying signal transduction processes, resulting in unique signalling profiles of β2-agonists (Swift et al., 2007). Thus, it becomes important to ask whether there are qualitative differences between beneficial effects and desensitization for various β2-agonists, that is, whether there are differences in the loss of receptor responsiveness between β2-agonists at equi-effective concentrations. A recent study (Moore et al., 2007) suggests that such a qualitative difference may exist, as salmeterol and ephedrine were found to have a reduced ability to stimulate β2-adrenoceptor internalization and degradation compared with formoterol, adrenaline and salbutamol although all agonists stimulated phosphorylation of the β2-adrenoceptor by G-protein regulatory kinases.
In this study, we have examined the relationships between initial effect and subsequent reduction of responsiveness to restimulation for a panel of β2-agonists that vary in a number of properties, such as potency, duration of action, intrinsic efficacy and lipophilicity. The work was carried out in two relevant cell types from the human airway: primary human airway smooth muscle cells (HASMC) and bronchial epithelial cells, with cAMP production as the primary indicator of responsiveness. The chief findings were as follows: (i) Under conditions of continuous stimulation, reduction in receptor responsiveness was proportional to the initial effect and similar for all agonists studied. (ii) Under conditions of pulse exposure followed by removal of β2-agonist, there were marked differences in the relationship between effect and loss of responsiveness of the agonists at equi-effective concentrations. These findings obtained in primary human cells were consistent with results from in vitro studies in guinea pig isolated trachea.
Methods
Determination of β2-agonist affinity at the β2-adrenoceptor
Membranes from HEK293 cells stably transfected with the coding sequence of the human β2-adrenoceptor (Genbank:NM000024) (Cat#: RBHBE2M, PerkinElmer, Waltham, MA, USA) were used. Binding of [125I]-iodocyanopindolol and competition of compounds were performed for 2 h at room temperature in 50 mmol·L−1 HEPES buffer (pH 7.4) containing EDTA (1 mmol·L−1), NaCl (120 mmol·L−1), gelatine (1%, w/v) and 1% (v/v) dimethyl sulphoxide (DMSO). The concentration of [125I]-iodocyanopindolol used was 0.09 nmol·L−1, equivalent to the KD for [125I]-iodocyanopindolol at this receptor. Non-specific binding was determined by using 10 µmol·L−1 propranolol. Membrane-bound [125I]-iodocyanopindolol was separated from [125I]-iodocyanopindolol in solution by filtration onto PEI-coated GF/B filter plates (PerkinElmer, Waltham, MA, USA) presoaked for 1 h in assay buffer, using a 96-well plate cell harvester (Nunc, Thermo Fischer Scientific, MA, USA). Five washes with 50 mmol·L−1 HEPES buffer (pH 7.4) containing EDTA (1 mmol·L−1) and NaCl (120 mmol·L−1) were performed at 4°C to remove unbound radioactivity. The plates were dried for at least 2 h at 50°C or overnight at room temperature. Filter-bound radioactivity was measured with a scintillation counter (TopCount, PerkinElmer, Waltham, MA, USA). Total specific binding was determined by subtracting the non-specific binding from the mean total binding, and concentration-dependent inhibition of [125I]-iodocyanopindolol binding by compounds was calculated as pIC50 values, which were converted to KD values by using the Cheng–Prusoff correction (Cheng and Prusoff, 1973).
Determination of β2-agonist lipophilicity
The distribution coefficient between 1-octanol and aqueous buffer (logD) at pH 7.4 was used to measure the lipophilicity of the β2-agonists. An amount of 700 µL of octanol saturated with 0.1 mol·L−1 phosphate buffer pH 7.4 was added to 1–2 mg of compound in a 96-well plate. The 96-well plate was then shaken overnight by using an orbital shaker set at 450 rpm followed by centrifugation at 365×g for 30 min to sediment any undissolved compounds to the bottom of each vial. An amount of 200 µL of each sample was then transferred to a custom-built 24-well plate containing glass vials. An amount of 2 mL of octanol saturated with 0.1 mol·L−1 pH 7.4 phosphate buffer was then added to each vial in the 24-well plate. The plate was then shaken for 30 min at 20°C as described above followed by centrifugation for 30 min at 160×g. The octanol and aqueous layers were then separated, and the compound concentration in the two phases was measured by using high performance liquid chromatography (HPLC) with quantitative mass spectrometry. The distribution coefficient was expressed on the log scale as concentration of agonist in octanol/concentration of agonist in water.
Cell isolation and culture
HASMC were either purchased from Lonza (Basel, Switzerland) or isolated from biopsies from bronchi of patients undergoing lung resection at the Karolinska University Hospital in Solna, Sweden, or at Lund University Hospital in Lund, Sweden. Ethical approval was obtained from the local ethics committees in Stockholm (amendment to approval for Dnr 02-327) and Lund (LU-402-03/Dnr 178/2005), Sweden, and informed consent was given by the patients. The bronchial biopsy was washed several times in ice-cold Dulbecco's modified Eagle's medium (DMEM) with 4.5 g·L−1 D-glucose, glutamax and pyruvate to remove blood and mucus before epithelium, cartilage, adipose tissue and connective tissue were carefully removed from the smooth-muscle tissue. The smooth muscle was cut into smaller explants that were cultured in 60 mm tissue culture plates in DMEM with 4.5 g·L−1 D-glucose, glutamax and pyruvate supplemented with 10% heat-inactivated foetal bovine serum (FBS), penicillin (25 U·mL−1), streptomycin (25 µg·mL−1), Fungizone (1 µg·mL−1), mL) and gentamicin (25 µg·mL−1), with the medium changed twice a week. Cells growing from the explants were detached with 0.05% trypsin-EDTA solution and were then cultured in the same medium as the explants, only without the gentamicin. The Lonza HASMC were expanded and frozen by using Lonza-recommended media, and reagents and thawed cells were then cultured in the same way as the HASMC grown from explants. During serum-free culture conditions and experimental incubations, the FBS was replaced with 1% insulin-transferrin-selenium A. HASMC were used after a limited number of passages (6–8) to preserve phenotype characteristics. The HASMC isolated from lung biopsies resembled smooth muscle cells, growing as elongated, spindle-shaped cells with a hill and valley appearance and stained positive by immunocytochemistry for antibodies to α-smooth muscle actin (Dako, Glostrup, Denmark), SM22-α (Abcam, Cambridge, MA, USA) and, to some extent, for prolyl-4-hydroxylase (Dako), an enzyme involved in collagen synthesis. HASMC from three donors were used in this study, two of our explant-cultured cells and one cell from the commercial source. Of the two tissue donors for explant-cultured cells, one was taking inhaled β2-agonists, and the other was taking a β2-blocker. We have no information on the medications of the commercial tissue donor.
Normal human bronchial epithelial cells (NHBEC, Lonza) were cultured in bronchial epithelial growth medium (BEGM, Lonza) without the GA-1000 additive. During experimental conditions, a reduced BEGM without the GA-1000, adrenaline, hydrocortisone and retinoic acid additives was used. Lonza-recommended reagents were used for subculturing. Passages 3–5 were used for experiments.
The human airway epithelial cell line NCI-H292 derived from a human mucoepidermoid pulmonary carcinoma (ATCC, Manassas, VA, USA) was cultured in RPMI 1640 medium with 10% heat inactivated FBS (PAA Laboratories, Pasching, Austria), and 2 mmol·L−1 L-glutamine. Accutase was used to detach confluent cell layers for subculturing.
All cells were grown in vented cap cell culture flasks in a humidified incubator at 37°C, 5% CO2.
cAMP measurements
HASMC were grown to confluence in 96-well cell culture plates coated with human fibronectin (10 µg·mL−1, Calbiochem, Merck, Darmstadt, Germany). The cells were washed with phosphate buffered saline (PBS) without CaCl2 and MgCl2 and cultured in serum-free medium for 24 h before being incubated with β2-agonists. DMEM used during β2-agonist exposure contained 0.3 mmol·L−1 ascorbic acid, and, during the 15 min cAMP stimulations, 0.5 mmol·L−1 of isobutyl methylxanthine (IBMX) was also added to prevent cAMP degradation by phosphodiesterases. Forskolin and β2-agonists were dissolved in DMSO, except for isoprenaline, which was dissolved in DMEM. Final DMSO concentration in the medium was 0.1–0.2%. After β2-agonist stimulations, the cells were washed with PBS and lysed by addition of 0.9% Tween-20, 0.1% (BSA), 5 mmol·L−1 HEPES and 1 mmol·L−1 IBMX, pH 7.4 followed by freezing at −80°C and thawing at room temperature. Concentrations of cAMP in lysates were determined with the AlphaScreen cAMP assay kit (PerkinElmer, Waltham, MA, USA). HASMCs from three donors were used, one was purchased from Lonza and two were isolated from bronchial biopsies. The cells showed different magnitudes of cAMP production in response to β2-adrenoceptor stimulation, but, when the responses were normalized to the maximal response obtained with isoprenaline in each cell, the relative responses were similar in the cells from the three donors. Results from the three donors have been pooled throughout.
The same method was used for measuring cAMP in NHBEC, with the following differences. These cells were grown to near confluence in 96-well plates and then cultured in the reduced BEGM for 24 h prior to the experiments. During β2-agonist treatments, the reduced BEGM was supplemented with 0.3 mmol·L−1 ascorbic acid, and, during the 15 min stimulations, 0.5 mmol·L−1 IBMX was added. Cells from two donors were used.
cAMP measurements in NCI-H292 cells followed a similar protocol. Cells were seeded in 96-well plates in RPMI 1640 medium (containing 10% FBS and 2 mmol·L−1 L-glutamine) at 104 cells per well, and the plates incubated overnight in a humidified incubator at 37°C, 5% CO2. RPMI 1640 medium used during β2-agonist exposure contained 0.3 mmol·L−1 ascorbic acid, and, during the 15 min cAMP stimulations, 0.5 mmol·L−1 of IBMX was also added. All of the agonists were dissolved in DMSO. Final DMSO concentration in the medium was 0.2–1.2%.
Investigation of reduced receptor responsiveness induced by β2-agonist stimulation
We have used two protocols to investigate reduced receptor responsiveness induced by stimulation with the β2-agonists in our panel. In the first protocol adapted from Scola et al. (Scola et al., 2004), HASMC and NCI-H292 cells were exposed continuously to a concentration range of β2-agonists for 24 h before the cells were washed three times with 200 µL medium (washes approximately 2 min long), and the cAMP response to a stimulation with 0.1 µmol·L−1 formoterol for 15 min was then measured in the presence of IBMX, as described in the ‘cAMP measurements’ section. In the second protocol, HASMC and NCI-H292 cells were exposed to a concentration range of β2-agonists for 1 h, washed as described above and incubated in β2-agonist-free medium for 23 h. The cAMP response to a stimulation with 0.1 µmol·L−1 formoterol for 15 min was then measured as described above.
Measurement of smooth muscle cell contraction on collagen gels
48-well tissue culture plates (Nunc) were coated with 1% BSA in PBS overnight at 4°C and then washed with sterile PBS. A collagen solution was made up of five parts 2× DMEM, one part 0.2 mol·L−1 HEPES pH 8 and four parts collagen type I (PureCol, 3 mg·mL−1, Nutacon, Leimuiden, the Netherlands). Gels, 200 µL per well, were allowed to form for 1 h at 37°C. Confluent HASMC were detached from culture flasks by using Accutase (Sigma Aldrich, St. Louis, MO, USA), washed in serum-free DMEM and resuspended at 500 000 cells·mL−1 in the same medium. Cells were carefully seeded on top of the gels at a density of 100 000 cells per gel and allowed to attach and spread at 37°C for 2.5 h. The gels were then detached from the wells by using a thin spatula, and spontaneous contraction was allowed to take place for 30 min at 37°C. β2-Agonists, dissolved in DMSO, were added 15 min prior to gel detachment, and equivalent amounts of DMSO were added in the controls. Thirty minutes after gel detachment, the plate containing the gels was placed on a transilluminator and photographed using a digital camera (Gel Photo system GFS1000, Techtum Lab, Umeå, Sweden). The diameter of each gel was measured from the image, and the gel area was calculated. The degree of contraction was calculated as the ratio between the gel area after 30 min and the original gel area (the area of the well). For each treatment, at least duplicate gels were measured.
Accumulation of β2-agonists on cells
Confluent HASMC in 6-well culture plates were exposed to approximate equi-effective concentrations of 3H-labelled formoterol (0.5 nmol·L−1), indacaterol (10 nmol·L−1), salbutamol (200 nmol·L−1) or salmeterol (0.5 nmol·L−1) for 1 h in experiment medium (see above). Cells were washed three times with 2 mL medium (washes approximately 2 min long) and then further incubated for 23 h in β2-agonist-free medium before being harvested by scraping. The remaining material in the wells was collected by ethanol extraction (99.5% ethanol for 5 min at room temperature). All incubation and washing media, harvested cells and ethanol extracts were saved, and radioactivity was determined by scintillation counting in a Packard 2200CA liquid scintillation analyser (Canberra-Packard International, Schwadorf, Austria).
Isometric force measurement in guinea pig isolated trachea
All animal care and experimental procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986 and subject to local ethical review. Male albino Dunkin Hartley guinea pigs (300–350 g) were killed by cervical dislocation, and the tracheas were removed. After the adherent connective tissue were removed, the trachea was cut into ring segments (each consisting of 2–3 cartilage rings). These were suspended in 10 mL organ baths containing a modified Krebs solution composition (mmol·L−1): NaCl, 117.56; KCI, 5.36; NaH2P04, 1.15; MgS04, 1.18; glucose, 11.10; NaHCO3, 25.00; and CaCI2, 2.55. The tracheal rings were maintained at 37°C and continually gassed with 5% CO2 in O2. Indomethacin (2.8 µmol·L−1), corticosterone (10 µmol·L−1), ascorbate (1 mmol·L−1), CGP20712A (1 µmol·L−1) and phentolamine (3 µmol·L−1) were added to the Krebs solution: indomethacin to prevent development of smooth-muscle tone due to the synthesis of cyclooxygenase products, corticosterone to inhibit postsynaptic uptake of β2-agonists, ascorbate to prevent oxidation of isoprenaline and CGP20712A and phentolamine to avoid any complicating effects of β1- and α-adrenoceptor activation respectively. Indomethacin was dissolved in 10% w/v Na2CO3, corticosterone 21-acetate in ethanol and isoprenaline and salmeterol in DMSO. All agonists were dissolved in DMSO at a concentration of 10 mmol·L−1. These were diluted in Krebs before being added to tissues, and the level of DMSO in the bath was <0.1%. The tracheal rings were suspended between two stainless steel hooks, one attached to an isometric force transducer and the other to a stationary support in the organ bath. Changes in isometric force were recorded.
At the beginning of each experiment, a force of 1.0 g.wt. was applied to the tissues, and this was reinstated over a 30 min equilibration period until it remained steady. Tissues were then exposed to 1 µmol·L−1 of a muscarinic agonist, methacholine, to assess tissue viability and contractility. Tissues were washed by changing the Krebs solution three times. After 30 min, the tissues were again contracted with 1 µmol·L−1 methacholine and a relaxant, cumulative concentration effect (E/[A]) curve constructed by cumulative additions of isoprenaline at 0.5 log10 unit increments (10−9 mol·L−1–10−5 mol·L−1). Tissues were again washed and, 30 min later, contracted with 1 µmol·L−1 methacholine. Either salmeterol or formoterol at equi-effective concentrations chosen to produce around 30, 50 or 70% relaxation of the induced tone (see Table 3) or buffer (control) was added when the contraction had reached a plateau and left for 60 min to define the full relaxation induced by the given concentration of agonist. The rings were again washed to remove methacholine and β2-agonist, and the same concentration of agonist or buffer control was re-added immediately. This was then left for a further 3 h before being washed and recontracted with 1 µmol·L−1 methacholine. When the contraction had reached a plateau, a final cumulative E/[A] curve was constructed by cumulative additions of isoprenaline.
Table 3.
Equi-effective concentrations of formoterol and salmeterol in the guinea pig trachea relaxation assay and the respective shift in concentration of the isoprenaline response curve
| Concentration (nmol·L−1) | Initial effect (% relaxation) | Shift of isoprenaline curve (log10 units) | |
|---|---|---|---|
| Formoterol | 0.6 | 39 ± 13 | 0.2 ± 0.2 |
| 1 | 52 ± 11 | 0.3 ± 0.2 | |
| 3 | 64 ± 10 | 0.6 ± 0.1 | |
| Salmeterol | 3 | 34 ± 11 | 0.5 ± 0.3 |
| 20 | 49 ± 8 | 1.4 ± 0.2 | |
| 80 | 66 ± 10 | 1.9 ± 0.2 |
Salmeterol and formoterol were preincubated at the concentrations shown to produce and initial relaxation effect as indicated. The agonist-induced shifts in the isoprenaline response curves were relative to the EC50 of isoprenaline in tissue without agonist preincubation.
Data were collected by using the ADInstruments Chart4 (ADInstruments, Bella Vista, Australia) for Windows software, which measured the maximum tension generated at each concentration of agonist. All responses were expressed as percentage of the methacholine-induced contraction, and comparisons were made between the final isoprenaline E/[A] curves to determine the effect of pre-incubation with salmeterol or formoterol.
Data analysis
Maximal responses (Emax) and potencies (pEC50) were calculated from individual non-linear regression fits of the data, using a four-parameter logistic function. The Excel-based utility XLfit4 was used for the modelling. Intrinsic activity was calculated as the Emax of an agonist relative to the Emax obtained with the reference full agonist isoprenaline.
Materials
All cell culture media and reagents were from Gibco, Invitrogen, Sweden, unless otherwise stated. Cell culture plastic was from Corning, the Netherlands. Isoprenaline, salbutamol and all other chemicals were purchased from Sigma Aldrich, St. Louis, MO, USA, unless otherwise stated. Formoterol, indacaterol, salmeterol, picumeterol, sibenadet and the aryl aniline AA1 8-hydroxy-5-((R)-1-hydroxy-2-{2-[4-(6-methoxybiphenyl-3-amino)phenyl]ethylamino}ethyl)-1H-quinolin-2-one (as disclosed in WO2004101525 and denoted here as AA1) as well as 3H-labelled formoterol, salmeterol, indacaterol and salbutamol were synthesized at AstraZeneca R&D (Lund, Sweden and Loughborough, UK). Drug and molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008).
Results
Pharmacological properties of β2-agonists in cells from the human respiratory tract – cAMP response and inhibition of smooth muscle contraction
A panel of eight short- and long-acting β2-agonists representing a range of pharmacological and physicochemical properties was studied (Table 1). Agonist-mediated cAMP responses were compared between HASMC, NHBEC and NCI-H292 cells, with isoprenaline as a reference full agonist (Figure 1A–C). The agonist AA1 was only studied in the HASMC. The agonists showed a wide range of potencies and intrinsic activities compared with isoprenaline, and the three cell types differed in their responsiveness to β2-agonist stimulation, with agonist potencies and intrinsic activities being highest in the NHBEC, followed by the NCI-H292 and HASMC (Table 2). For example, in NHBEC, indacaterol was a nearly full agonist, whereas, in the HASMC, it was a partial agonist.
Table 1.
Properties of a panel of β2-agonists
| Rank order of responsiveness to re-stimulation | logD | pKd | Chemical structure | |
|---|---|---|---|---|
| Isoprenaline | 1 | −0.7 | 6.2 ± 0.1 (n= 11) | 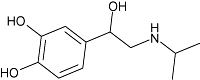 |
| Formoterol | 2 | 0.4 | 7.8 ± 0.0 (n= 6) | 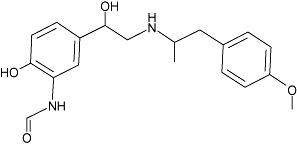 |
| Indacaterol | 3 | 2.7 | 7.5 ± 0.1 (n= 12) | 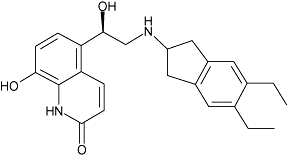 |
| Salbutamol | 4 | <−1.5 | 6.3 ± 0.1 (n= 11) | 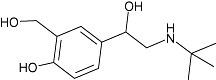 |
| Sibenadet* | 5 | 2.6 | 8.5 ± 0.1 (n= 5) | 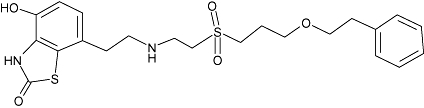 |
| AA1 | 6 | 3.1 | 8.1 ± 0.0 (n= 3) | 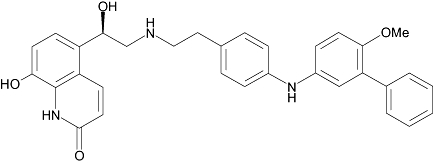 |
| Salmeterol | 7 | 1.9 | 8.9 ± 0.1 (n= 8) | 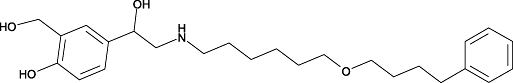 |
| Picumeterol | 8 | 1.8 | 9.0 ± 0.2 (n= 4) | 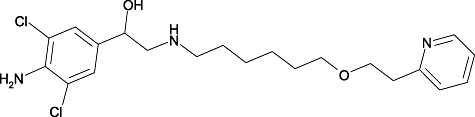 |
The table is arranged according to the rank order of responsiveness to re-stimulation with formoterol in HASMC after a 1 h pulse exposure to a β2-agonist concentration producing 20% of the maximal isoprenaline effect (Figure 3B, dotted line). pKD data are means ± SEM.
Dual β2-adrenoceptor and dopamine D2 receptor agonist (Dougall et al., 2003).
HASMC, human airway smooth muscle cells; IBMX, isobutyl methylxanthine; SEM, standard error of the mean.
Figure 1.
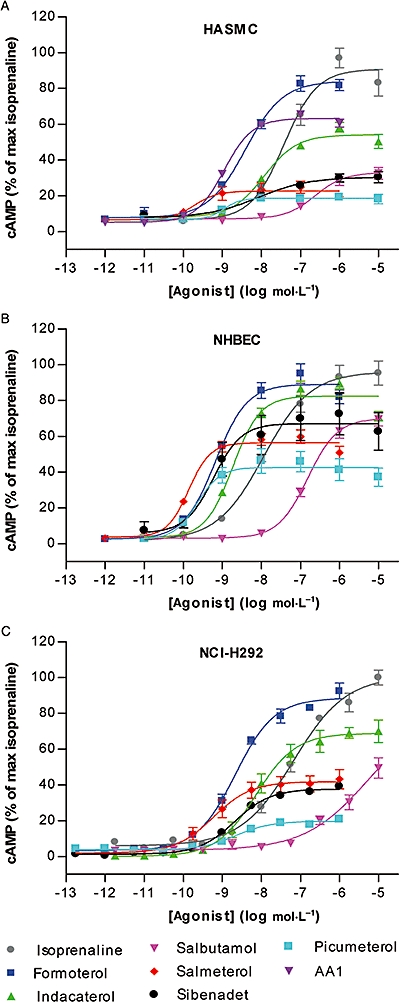
cAMP responses, over a range of concentrations, to isoprenaline, formoterol, indacaterol, salbutamol, sibenadet, salmeterol, picumeterol and AA1 during a 15 min stimulation in the presence of 0.5 mmol·L−1 IBMX in (A) primary HASMC (n= 6, cells from 3 donors), (B) NHBEC (n= 12 for isoprenaline, formoterol, indacaterol and salmeterol and n= 6 for salbutamol, sibenadet and picumeterol, cells from 2 donors) and (C) NCI-H292 cells (n= 6). Error bars represent SEM. cAMP levels are expressed relative to the maximal response obtained by isoprenaline stimulation. Potencies and intrinsic activities of the agonists are listed in Table 2. HASMC, human airway smooth muscle cells; IBMX, isobutyl methylxanthine; SEM, standard error of the mean.
Table 2.
Potencies and maximal responses for cAMP stimulation of seven β2-agonists in human primary airway smooth muscle cells (HASMC), normal human bronchial epithelial cells (NHBEC) and the human airway epithelial cell line NCI-H292
|
pEC50 |
Intrinsic activity |
|||||
|---|---|---|---|---|---|---|
| HASMC | NBHEC | NCI-H292 | HASMC | NBHEC | NCI-H292 | |
| Isoprenaline | 7.3 ± 0.1 | 7.9 ± 0.1 | 7.2 ± 0.1 | 1.00 ± 0.07 | 1.00 ± 0.07 | 1.00 ± 0.07 |
| Formoterol | 8.4 ± 0.1 | 9.2 ± 0.0 | 8.6 ± 0.1 | 0.93 ± 0.04 | 0.90 ± 0.04 | 0.88 ± 0.03 |
| Indacaterol | 7.9 ± 0.1 | 8.7 ± 0.0 | 8.1 ± 0.1 | 0.60 ± 0.03 | 0.83 ± 0.03 | 0.70 ± 0.05 |
| Salbutamol | 6.6 ± 0.1 | 6.8 ± 0.0 | – | 0.36 ± 0.04 | 0.71 ± 0.04 | 0.49 ± 0.06 |
| Salmeterol | 9.6 ± 0.1 | 9.9 ± 0.0 | 9.2 ± 0.1 | 0.25 ± 0.03 | 0.57 ± 0.03 | 0.42 ± 0.05 |
| Sibenadet | 8.2 ± 0.3 | 9.1 ± 0.2 | 8.7 ± 0.1 | 0.34 ± 0.03 | 0.70 ± 0.12 | 0.37 ± 0.01 |
| Picumeterol | 8.8 ± 0.2 | 9.7 ± 0.1 | 8.7 ± 0.0 | 0.21 ± 0.01 | 0.43 ± 0.06 | 0.20 ± 0.02 |
| AA1 | 8.9 ± 0.0 | 0.70 ± 0.02 | ||||
Intrinsic activity was calculated as Emax of the agonist/Emax of isoprenaline in each cell type. Data are means of 6–13 individual determinations ± SEM.
SEM, standard error of the mean.
The cAMP responses in HASMC for formoterol, indacaterol, salbutamol and salmeterol were compared with inhibition of contraction of the same cells on collagen type I gels. HASMC spontaneously contracted collagen gels to 28% [standard error of the mean (SEM) = 2.7%, n= 11] of the original area. Formoterol (pEC50= 8.9 ± 0.2, n= 5), indacaterol (pEC50= 8.8 ± 0.2, n= 3), salbutamol (pEC50= 6.8 ± 0.2, n= 4) and salmeterol (potency not possible to determine, n= 5) inhibited spontaneous HASMC-mediated gel contraction in a dose-dependent fashion (Figure 2). Intrinsic activities were consistent with observations made with cAMP responses in these cells, and the potency of formoterol, indacaterol and salbutamol to either generate cAMP or inhibit cell-mediated gel contraction was similar. In contrast, the potency of salmeterol to inhibit gel contraction was considerably diminished relative to the potency to generate cAMP.
Figure 2.
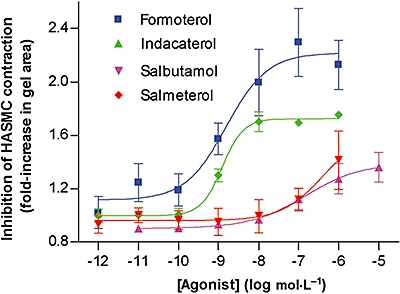
Inhibition of HASMC-mediated collagen gel contraction by formoterol, indacaterol, salbutamol and salmeterol. Cells from three donors were used and results are averages of 3–5 separate experiments with error bars representing SEM. HASMC, human airway smooth muscle cells; IBMX, isobutyl methylxanthine; SEM, standard error of the mean.
Differential mediation of reduced receptor responsiveness by β2-agonists at equi-effective concentrations
We devised two protocols to investigate reduced receptor responsiveness induced by stimulation with the β2-agonists in our panel. In the first protocol, HASMC and NCI-H292 cells were exposed continuously to a concentration range of β2-agonists for 24 h before the cells were washed and immediately restimulated with 0.1 µmol·L−1 formoterol for 15 min. The cAMP produced by restimulation with formoterol was plotted against the cAMP response produced by the β2-agonists during the 15 min initial stimulation described above (Figure 3A, plotted utilizing data from Figure 1). By plotting the data this way, we take into account the differences in potencies and intrinsic activities of the agonists, allowing interpretation of the level of reduced responsiveness induced at equi-effective concentrations.
Figure 3.
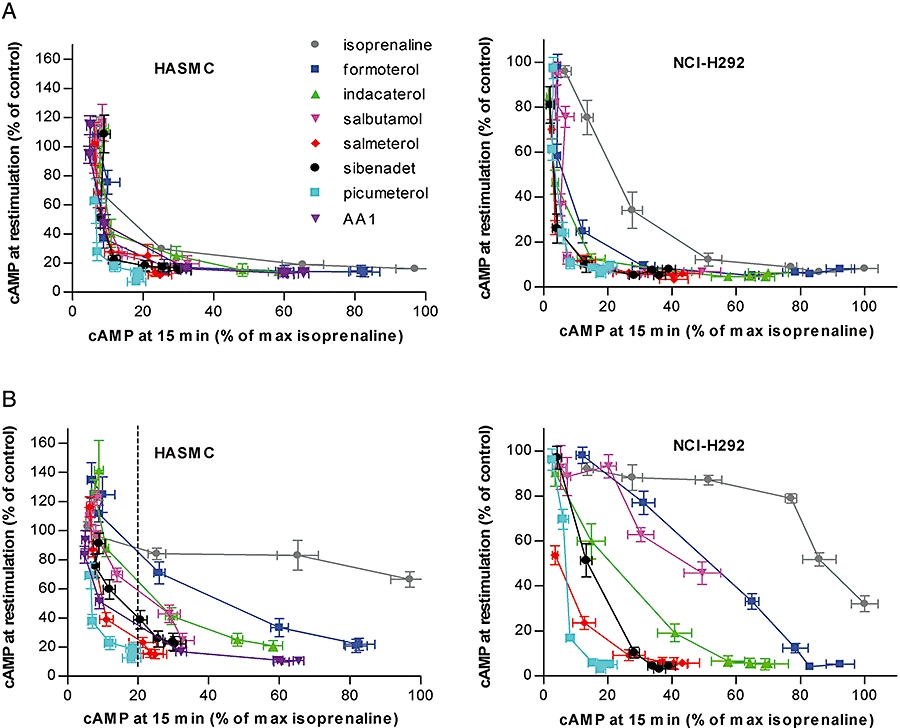
cAMP response to a 15 min stimulation with 0.1 µm formoterol in the presence of 0.5 mmol·L−1 IBMX after exposure to a range of β2-agonists in HASMC and NCI-H292 cells after 24 h continuous exposure (A) or 1 h pulse exposure followed by a 23 h incubation (B). The remaining cAMP response at re-stimulation on the Y-axis is expressed relative to the response of cells previously not exposed to any β2-agonist. n= 6, error bars represent SEM. Values on X-axes are the % initial cAMP response relative to isoprenaline as seen on the Y-axes in Figure 1A,C. The dotted line in b was used to rank the agonists according to the remaining responsiveness to re-stimulation with formoterol in HASMC after a 1 h pulse exposure to a β2-agonist concentration producing 20% of the maximal isoprenaline effect, as listed in Table 1. HASMC, human airway smooth muscle cells; IBMX, isobutyl methylxanthine; SEM, standard error of the mean.
The degree of reduced responsiveness was related and found to be very sensitive to the initial effect, with relatively large losses in the capacity for restimulation attributed to low levels of initial stimulation. The effect-responsiveness curves for most agonists were essentially superimposable in both cell types despite the wide range in intrinsic efficacy of the agonists tested. The only clear exception was isoprenaline, which, in the NCI-H292 cells, resulted in less reduction in responsiveness per unit effect than in the other agonists.
The second protocol was designed to investigate the effects of a 1 h pulse exposure followed by removal of agonist from cells. HASMC and NCI-H292 cells were exposed to a concentration range of β2-agonists for 1 h, then washed and incubated in β2-agonist-free medium for 23 h. Cellular functional responses were then investigated by restimulation with 0.1 µmol·L−1 formoterol for 15 min followed by quantification of cAMP. The residual functional activity was plotted against initial effect as described above (Figure 3B). There was a marked difference in the resulting β2-adrenoceptor-mediated response following a 1 h pulse exposure to the different β2-agonists. For rank order of reduction in responsiveness to be estimated, agonists were compared at an initial effect of 20% relative to the full agonist isoprenaline (Figure 3, dotted line and Table 1). Cells were least sensitive to loss of responsiveness by isoprenaline stimulation, followed in order of increased loss of responsiveness by formoterol, indacaterol, salbutamol, sibenadet, AA1, salmeterol and picumeterol (Table 1). Salmeterol, picumeterol and AA1 surprisingly induced a similar reduction of responsiveness both in the continuous and in the 1 h pulse exposure protocols in HASMC. The loss of responsiveness observed was not due to a generally decreased ability of the cells to produce cAMP, as responses to the adenylate cyclase stimulator forskolin was not affected by β2-agonist pretreatment, suggesting that the reduced responsiveness was receptor dependent (data not shown). Similar studies were performed with isoprenaline, formoterol, indacaterol and salmeterol in NHBEC, and the effect-response patterns exhibited under the two protocols were comparable with the HASMC (data not shown).
Differential retention of β2-agonists
The differences between the reduced receptor responsiveness in the continuous and 1 h pulse exposure protocols suggested that differences in retention of compounds in cells during the incubation and washing steps might contribute to loss of responsiveness. Indeed, other investigators have noted, for example, that salmeterol cannot easily be removed from cells in vitro by washing (January et al., 1998), an effect that may be associated with the physicochemical properties of the agonist. We measured the lipophilicity (logD) of the β2-agonists in our panel and found them to group roughly into hydrophilic (isoprenaline, salbutamol), intermediate (formoterol) and lipophilic (salmeterol, indacaterol, picumeterol, sibenadet and AA1) (Table 1). To understand how lipophilicity contributed to the observations in the HASMC in vitro system, we measured the retention of selected radiolabelled agonists with differing logD. HASMC were exposed to equi-effective concentrations (20% of max isoprenaline cAMP response) of 3H-labelled formoterol, indacaterol, salbutamol and salmeterol for 1 h then washed and incubated in agonist-free medium for 23 h. The cell-associated radioactivity expressed as the percentage of the total radioactivity added [standard deviation (±SD)] was 0.1 ± 0.1% for formoterol (n= 4), 10.1 ± 3.2% for indacaterol (n= 2), 0.0 ± 0.0% for salbutamol (n= 4) and 25.9 ± 10.7% for salmeterol (n= 4). The fractional recovery of radioactivity from all fractions (media, washes, cells, plastic extraction) ± SD was 99.2 ± 1.9% for formoterol, 78.2 ± 9.4% for indacaterol, 101.2 ± 2.5% for salbutamol and 79.8 ± 5.0% for salmeterol.
Effect-response coupling in tracheal preparations
We next tested whether the differences in effect-response coupling of the β2-agonists in the cells in vitro also could be observed in a tissue system. Formoterol and salmeterol were chosen to represent β2-agonists at opposite ends of the effect-response scale. Methacholine-contracted guinea pig trachea was exposed to three equi-effective concentrations of formoterol or salmeterol for a total of 4 h before the tissue was washed, contracted with methacholine and the relaxant response to isoprenaline was tested (Figure 4). Both agonists caused a rightward shift of the isoprenaline concentration response curve. At each equi-effective concentration, salmeterol produced a greater shift of the isoprenaline concentration response curve than formoterol (Table 3). The reduced responsiveness appeared to be receptor dependent because pretreatment with β2-agonist did not impair the ability of the neuropeptide, vasoactive intestinal peptide to relax tracheal preparations (data not shown).
Figure 4.
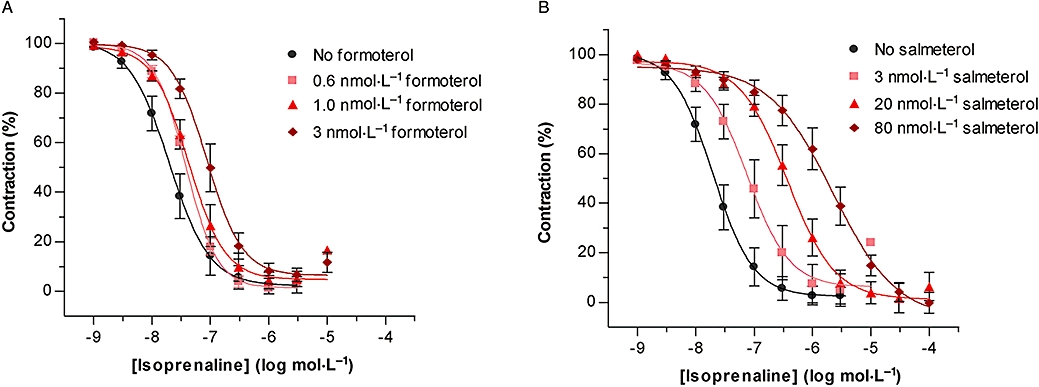
Relaxant responses in guinea pig isolated trachea to cumulative concentrations of isoprenaline following a 4 h exposure to equi-effective concentrations of formoterol (A) and salmeterol (B). Results are averages of 4–6 separate experiments and error bars represent SEM. SEM, standard error of the mean.
Discussion
Using HASMC and epithelial cells, we have addressed the relationship between prolonged stimulation of the β2-adrenoceptor and downstream responsiveness to a panel of β2-agonists. In this study, we based our conclusions on calculated equi-effective concentrations in order to understand differences that might exist between agonists. Other studies compared β2-agonists on the basis of equal concentrations or equal receptor occupancy (January et al., 1997; Clark et al., 1999; Scola et al., 2004). Such studies yield important information about comparative pharmacology but do not take into account the different efficacies of the agonists investigated, which, together with clearance and metabolism characteristics, govern the doses of the agonists used in clinical practice.
We have used intracellular increases in cAMP as the primary measure of β2-adrenoceptor activation in response to β2-agonists. It is known that a total cellular increase in cAMP is not always crucial for signalling but that transient increases in local cAMP play important roles in activating downstream effects (Cooper, 2005). For the cells used in this study, we detected robust increases of cAMP concentrations, which mostly correlated with inhibition of smooth muscle cell collagen gel contraction in vitro. The exception to this correlation was salmeterol, which showed more than a 100-fold drop in potency in the functional assay compared with increases in cAMP, for reasons that are not understood.
We found that, in cells continuously exposed to β2-agonists for 24 h, the suppression of the subsequent cAMP response was related directly to the magnitude of the initial effect for all β2-agonists tested regardless of intrinsic activity and lipophilicity. This result was comparable across the two cell types investigated. This reduction in receptor responsiveness was specific, in that the cAMP response to forskolin was not suppressed (data not shown).
In cells exposed to agonists for a 1 h pulse, washed, and incubated for an additional 23 h, differences emerged in the compound-specific effect-responsiveness curves. In vivo, clearance of drugs from cells and tissues contributes importantly to overall pharmacological properties, and our pulse incubation and washing protocol may model some aspects of the clearance process. With important exceptions, the more lipophilic compounds tended to drive greater downstream reduction of responsiveness to restimulation. For example, salmeterol, picumeterol and AA1 induced similar loss of responsiveness in the 1 h pulse and in the continuous exposure protocols. This effect could be the result of a high degree of cell retention, leaving the agonist in contact with cells for 24 h despite washing as in the 1 h pulse exposure protocol. Studies with radio-labelled compounds showed clear differences in the retention of agonists on cells following removal from the medium. Indeed, the high degree of adherence of salmeterol to cells demonstrated here with radio-ligand-binding studies has been previously described in cells and tissues by others (Ball et al., 1991; Nials et al., 1993; January et al., 1998). The reason for increased cell retention could be attributed, in part, to a higher degree of lipophilicity. It is important to note, however, that partitioning between octanol and water, as measured by logD, may not necessarily reflect partitioning into the cell membranes and lipids. Evidence presented previously suggests that salmeterol is distinct from other β2-agonists in that it is able to access an additional binding site in proximity to the receptor, the ‘exosite’ (Green et al., 1996). Binding to an exosite could make salmeterol less liable to removal by washing, as suggested by Green et al., (2001).
Prolonged retention or even accumulation of agonists in the vicinity of the receptor could lead to increased loss of responsiveness by causing a greater degree of receptor desensitization. In addition, retained partial agonists would attenuate restimulation by the full agonist formoterol by acting as partial antagonists at the receptor (Dougall et al., 1991). Thus, the rank order of remaining responsiveness is likely to reflect both efficacy and tissue retention.
High logD value in combination with low efficacy was, to some extent, predictive of the loss of responsiveness in the 1 h pulse exposure protocol. However, there were exceptions to this principle, and no single factor explained the rank order of reduced responsiveness to formoterol. For example, salbutamol, the most hydrophilic compound in the panel, led to greater loss of responsiveness than the slightly lipophilic formoterol, and the lipophilic indacaterol induced less loss of responsiveness than would be expected from its cellular retention. Thus, other structural features may contribute to the higher-than-expected suppression by salbutamol and the lower-than-expected suppression by indacaterol. It is well described that individual agonists may induce specific receptor conformations to produce unique signalling patterns (Kenakin, 1995; Berg and Clarke, 2006; Swift et al., 2007), suggesting the possibility for each agonist to display a unique effect due to both receptor antagonism and desensitization.
A recent study (Moore et al., 2007) suggested that, when compared with high intrinsic activity agonists such as formoterol, the salmeterol-stimulated phosphorylated receptors did not bind β-arrestin, whereas the formoterol-stimulated receptors did. It was suggested that salmeterol induces a receptor conformation that was less sensitive to desensitization. In our studies, we did not find evidence to support that conclusion. In particular, the reduced responsiveness did not correlate with high agonist intrinsic activity because the high-intrinsic-activity agonists isoprenaline and formoterol induced the least loss of responsiveness. Rather, our data suggest that the way that the agonist associates with the cell over time may be the critical factor determining the degree of downstream loss of responsiveness.
Selecting formoterol and salmeterol as compounds with distinctly different effect-responsiveness profiles in the 1 h pulse exposure protocol, we found that similar responses were obtained in in vitro experiments using guinea pig trachea. There was a much greater loss of subsequent relaxant response to isoprenaline after incubation with salmeterol than with formoterol. As in the cellular experiments, the higher level of reduced responsiveness induced by salmeterol in the tracheal preparations may be a result of its retention in the tissue even after washing, making it more prone to cause desensitization and, as a partial agonist, attenuating restimulation by the full agonist isoprenaline. Whatever the mechanism, whether by partial antagonism or by additional desensitization via some other mechanism, the tissue previously exposed to salmeterol was clearly less responsive to restimulation by isoprenaline than the tissue exposed to formoterol.
The relevance of in vitro findings for design of therapeutic compounds is always open to question. However, in the principal model used for these studies, the relevance comes chiefly from the use of HASMC, compared on the basis of effect, as would be carried out in a clinical study, and from stimulation over a prolonged period. Thus, our work demonstrates clear differences in the pharmacological profile of agonists with respect to downstream reduction of receptor responsiveness at equi-effective concentrations.
Acknowledgments
We thank Petter Sjölin, Jonas Bergare and David Wilkinson for providing the tritiated β2-agonists, Cecilia Kemi and Ulrika Zagai for assistance with smooth muscle cell culture and Rupert Austin for LogD measurements.
Glossary
Abbreviations:
- β2-agonists
β2-adrenoceptor agonists
- BEGM
bronchial epithelial growth medium
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulphoxide
- HASMC
human airway smooth-muscle cells
- IBMX
isobutyl methylxanthine
- NHBEC
normal human broncial epithelial cells
- PBS
phosphate buffered saline
Conflict of interest
C Düringer, E Gürcan, IA Dainty, M Lawson, H Falk Håkansson, SH Korn, A Jerre, E Wieslander, DJ Nicholls and DS Silberstein are all full-time employees of AstraZeneca. G Grundström and K Fredriksson are former employees of AstraZeneca. CG Löfdahl has received payments for lectures and participation in advisory boards as well as institutional grants from AstraZeneca, Boehringer, GlaxoSmithKline, MSD and Novartis. CM Sköld has received consultancy and Advisory Board fees from Boehringer-Ingelheim/Pfizer and GlaxoSmithKline and lecture fees from AstraZeneca, Boehringer-Ingelheim, Pfizer and GlaxoSmithKline. M Löfdahl states no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball DI, Brittain RT, Coleman RA, Denyer LH, Jack D, Johnson M, et al. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991;104:665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP. Development of functionally selective agonists as novel therapeutic agents. Drug Discov Today. 2006;3:421–428. [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clark RB, Knoll BJ, Barber R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharmacol Sci. 1999;20:279–286. doi: 10.1016/s0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- Cooper DM. Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans. 2005;33:1319–1322. doi: 10.1042/BST0331319. [DOI] [PubMed] [Google Scholar]
- Dougall IG, Harper D, Jackson DM, Leff P. Estimation of the efficacy and affinity of the beta 2-adrenoceptor agonist salmeterol in guinea-pig trachea. Br J Pharmacol. 1991;104:1057–1061. doi: 10.1111/j.1476-5381.1991.tb12549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall IG, Young A, Ince F, Jackson DM. Dual dopamine D2 receptor and beta2-adrenoceptor agonists for the treatment of chronic obstructive pulmonary disease: the pre-clinical rationale. Respir Med. 2003;97(Suppl. A):S3–S7. [PubMed] [Google Scholar]
- Green SA, Spasoff AP, Coleman RA, Johnson M, Liggett SB. Sustained activation of a G protein-coupled receptor via ‘anchored’ agonist binding. Molecular localization of the salmeterol exosite within the 2-adrenergic receptor. J Biol Chem. 1996;271:24029–24035. doi: 10.1074/jbc.271.39.24029. [DOI] [PubMed] [Google Scholar]
- Green SA, Rathz DA, Schuster AJ, Liggett SB. The Ile164 beta(2)-adrenoceptor polymorphism alters salmeterol exosite binding and conventional agonist coupling to G(s) Eur J Pharmacol. 2001;421:141–147. doi: 10.1016/s0014-2999(01)01049-4. [DOI] [PubMed] [Google Scholar]
- January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, et al. Beta2-adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem. 1997;272:23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- January B, Seibold A, Allal C, Whaley BS, Knoll BJ, Moore RH, et al. Salmeterol-induced desensitization, internalization and phosphorylation of the human beta2-adrenoceptor. Br J Pharmacol. 1998;123:701–711. doi: 10.1038/sj.bjp.0701658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. [see comment] Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Liggett SB. Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc Am Thorac Soc. 2005;2:292–296. 311–312. doi: 10.1513/pats.200504-027SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RH, Millman EE, Godines V, Hanania NA, Tran TM, Peng H, et al. Salmeterol stimulation dissociates beta2-adrenergic receptor phosphorylation and internalization. Am J Respir Cell Mol Biol. 2007;36:254–261. doi: 10.1165/rcmb.2006-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nials AT, Sumner MJ, Johnson M, Coleman RA. Investigations into factors determining the duration of action of the beta 2-adrenoceptor agonist, salmeterol. Br J Pharmacol. 1993;108:507–515. doi: 10.1111/j.1476-5381.1993.tb12833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola AM, Chong LK, Chess-Williams R, Peachell PT. Influence of agonist intrinsic activity on the desensitisation of beta2-adrenoceptor-mediated responses in mast cells. Br J Pharmacol. 2004;143:71–80. doi: 10.1038/sj.bjp.0705905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift SM, Schwarb MR, Mihlbachler KA, Liggett SB. Pleiotropic beta-agonist-promoted receptor conformations and signals independent of intrinsic activity. Am J Respir Cell Mol Biol. 2007;36:236–243. doi: 10.1165/rcmb.2006-0257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


