Abstract
Background and purpose:
It has been demonstrated that cannabinoids evoke the release of endogenous opioids to produce antinociception; however, no information exists regarding the participation of cannabinoids in the antinociceptive mechanisms of opioids. The aim of the present study was to determine whether endocannabinoids are involved in central antinociception induced by activation of µ-, δ- and κ-opioid receptors.
Experimental approach:
Nociceptive threshold to thermal stimulation was measured according to the tail-flick test in Swiss mice. Morphine (5 µg), SNC80 (4 µg), bremazocine (4 µg), AM251 (2 and 4 µg), AM630 (2 and 4 µg) and MAFP (0.1 and 0.4 µg) were administered by the intracerebroventricular route.
Key results:
The CB1-selective cannabinoid receptor antagonist AM251 completely reversed the central antinociception induced by morphine in a dose-dependent manner. In contrast, the CB2-selective cannabinoid receptor antagonist AM630 did not antagonize this effect. Additionally, the administration of the anandamide amidase inhibitor, MAFP, significantly enhanced the antinociception induced by morphine. In contrast, the antinociceptive effects of δ- and κ-opioid receptor agonists were not affected by the cannabinoid antagonists. The antagonists alone caused no hyperalgesic or antinociceptive effects.
Conclusions and implications:
The results provide evidence for the involvement of cannabinoid CB1 receptors in the central antinociception induced by activation of µ-opioid receptors by the agonist morphine. The release of endocannabinoids appears not to be involved in central antinociception induced by activation of κ- and δ-opioid receptors.
Keywords: morphine, SNC80, bremazocine, CB1 receptor, CB2 receptor, central antinociception
Introduction
Cannabinoids and opioids are two separate groups of psychoactive drugs that exhibit several similar pharmacological effects, including analgesia, sedation, hypothermia and inhibition of motor activity (Manzaneres et al., 1999; Massi et al., 2001; Varvel et al., 2004). In addition, receptors for both drugs are coupled to similar intracellular signalling mechanisms, mainly to a decrease in cAMP production through the activation of Gi proteins (Bidaut-Russell et al., 1990; Childers, 1991).
Opioids produce their pharmacological effects by acting mainly through three types of opioid receptors, namely µ, δ and κ (Singh et al., 1997). Two types of cannabinoid receptors have been identified. CB1 receptors are expressed primarily in central and peripheral neurones, and CB2 receptors, mainly in immune cells (Pertwee, 2001; Howlett et al., 2002). CB2 expression in rat microglial cells (Carrier et al., 2004), cerebral granule cells (Skaper et al., 1996), mast cells (Samson et al., 2003) and adult rat retina (Lu et al., 2000) has also been demonstrated.
In recent years, the interaction between cannabinoid and opioid systems in nociceptive effects has been the focus of much attention (Welch and Eads, 1999; Finn et al., 2004). For example, a greater-than-additive interaction between Δ9-tetrahydrocannabinol (Δ9-THC) and morphine administered intravenously has been demonstrated, because inactive doses of the drugs in combination produced a potent analgesic effect. This combination of drugs produced effects through pathways mediated by both µ-opioid receptors and CB1 cannabinoid receptors, because potentiation was completely blocked by selective CB1 and µ receptor antagonists (Reche et al., 1996). Additionally, other studies have shown that Δ9-THC significantly enhanced the potency of morphine and codeine in the tail-flick test (Smith et al., 1998; Cichewicz et al., 1999). It has been suggested that endogenous opioids might be involved in the regulation of pain control by cannabinoids. Intrathecally administered Δ9-THC has been shown to release endogenous opioid peptides (Pugh et al., 1996). Cannabinoids Δ9-THC and levonantradol appear to enhance the antinociceptive effect of morphine by releasing dynorphin A and dynorphin B, respectively (Welch and Eads, 1999). This hypothesis is supported by a number of studies indicating that opioid receptor antagonists might block cannabinoid-induced antinociception (Cox and Welch, 2004). According to some authors, the influence of Δ9-THC on endogenous opioid effects depends on basal endogenous opioid tone. For example, the induction of the diabetic state in rats decreases the antinociceptive effect of morphine, an effect temporally related to decreased release of specific endogenous opioids. Conversely, Δ9-THC retains the ability to release endogenous opioids in diabetic rats, and maintains significant antinociception. Similarly, Δ9-THC was more active in the tail-flick test in diabetic than in non-diabetic mice (Williams et al., 2008). In addition, the effects of endocannabinoids have been recently reviewed (Maione et al., 2006; Degroot and Nomikos, 2007) indicating a significant role for the endocannabinoid system in various pain states (La Rana et al., 2006; Palazzo et al., 2006).
Anandamide, an endocannabinoid, is produced following intracellular cleavage of N-arachidonyl-phosphatidylethanolamine by phospholipase D, and shows preferential affinity for CB1 receptors (Howlett et al., 2002). It is synthesized on demand instead of being stored in synaptic vesicles, and is hydrolysed into arachidonic acid and ethanolamine by a membrane-bound enzyme called fatty acid amide hydrolase (FAAH) (Hohmann and Suplita, 2006). Research has shown that mice lacking the FAAH gene exhibited enhanced antinociceptive behaviour following exogenous administration of anandamide (Cravatt et al., 2001). Some inhibitors of FAAH have been described, such as MAFP. MAFP reacts irreversibly with FAAH (Deutsch et al., 1997), and thus causes potentiation of the responses induced by endocannabinoids (Ho and Randall, 2007).
The participation of opioids in antinociception induced by cannabinoids has been observed, and our group previously demonstrated, for the first time, the participation of cannabinoids in the peripheral antinociception induced by opioids (Pacheco et al., 2008). Therefore, the aim of the present study was to determine whether endogenous cannabinoids are also involved in central antinociception induced by activation of µ-, δ- and κ-opioid receptors.
Methods
Animals
The experiments were performed on 25–30 g male Swiss mice (n= 5 per group) from the CEBIO-UFMG (The Animal Centre of the University of Minas Gerais). The mice were housed in a temperature-controlled room (23 ± 1°C) on an automatic 12 h light/dark cycle (0600–1800 h light phase). All testing was performed during the light phase (0800–1500 h). Food and water were freely available until the onset of the experiments. The algesimetric protocol was approved by the Ethics Committee on Animal Experimentation (CETEA) of the Federal University of Minas Gerais (UFMG).
Algesimetric method
The tail-flick test used in the present study was a slight modification of the procedure described by D'Amour and Smith (1941). In brief, a heat source was applied to the tail of the mouse 2 cm from the tip, and the time (s) taken for the mouse to withdraw its tail from the heat source was described as the tail-flick latency. Heat intensity was adjusted so that the baseline latencies were between 3 and 4 s. To avoid tissue damage, a cut-off time was established at 9 s. The baseline latency was obtained for each mouse before drug administration (zero time) and determined from the average of three consecutive trials. To reduce stress, the mice were habituated to the apparatus 1 day prior to the experiments.
i.c.v. Injections
The mice were restrained by a special device, and the tops of their heads were then shaved. Next, drugs were injected into the lateral ventricle by i.c.v. route using a 5 µL Hamilton syringe. The injection site was 1 mm either side of the midline on a line drawn through the anterior base of the ears (modified from Haley and McCormick, 1957). The syringe was inserted perpendicularly through the skull into the brain to a depth of 2 mm, and 2 µL of solution was injected. To ascertain the areas in the brain ventricular system into which the drugs penetrated, the drugs were diluted in 0.5% Evans blue, and the brains were sectioned for confirmation after completion of the experiments.
Experimental protocol
SNC80 (δ-opioid agonist), morphine (µ-opioid agonist) and bremazocine (κ-opioid agonist) were administered i.c.v. into the lateral ventricle. Dose–response curves were obtained for all opioid receptor agonists to determine effective doses for this study (data not shown). AM251 (CB1 receptor antagonist) and AM630 (CB2 receptor antagonist) were injected i.c.v. 1 min prior to the opioid agonists. MAFP (anandamide amidase inhibitor) was administered by the i.c.v. route 1 min prior to the morphine.
The nociceptive threshold was always measured in the tail of the mouse. The protocol was assessed in pilot experiments to determine the best moment for the injection of each substance. In such experiments, the animals were measured at intervals of 5 min until the optimum effect for the test substance was observed.
Statistical analysis
Data were analysed statistically by one-way analysis of variance with post hoc Bonferroni test for multiple comparisons. Probabilities of less than 5% (P < 0.05) were considered to be statistically significant.
Chemicals
The following drugs and chemicals were used: morphine (Merck, Darmstadt, Germany); SNC80 (Tocris, Ellisville, MO, USA); bremazocine (RBI, Natick, MA, USA); AM251 (Tocris); AM630 (Tocris); and MAFP (Tocris). The drugs were dissolved as follows: morphine and bremazocine (saline); SNC80, AM251 and AM630 (20% DMSO in saline); MAFP (10% DMSO in saline), and injected in a volume of 2 µL into the lateral ventricle. The saline used for dilution of all drugs contained 0.5% Evans blue.
Results
Antagonism of morphine-induced antinociception by AM251
Intracerebroventricular administration of the CB1 receptor antagonist AM251 (2 and 4 µg) inhibited the morphine-induced central antinociception (5 µg) in a dose-dependent manner (Figure 1). The highest dose of AM251 did not alter the tail-flick latency.
Figure 1.
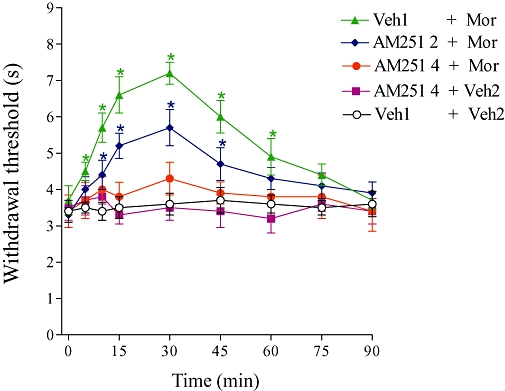
Antagonism induced by i.c.v. administration of AM251 on the central antinociception produced by morphine. AM251 (2 and 4 µg) was administered 1 min prior to morphine (Mor, 5 µg). This antagonist did not significantly modify the nociceptive threshold in control mice. Each point represents the mean ± SEM for five mice per group. *Indicates a significant difference compared to Veh1 + Veh2-injected group (analysis of variance + Bonferroni test, P < 0.05). Veh1, vehicle1 (20% DMSO in saline); Veh2, vehicle2 (saline).
Effect of AM630 on morphine-induced antinociception
The CB2 receptor antagonist AM630 (2 and 4 µg) did not modify the central antinociception of morphine (5 µg; Figure 2). In addition, this drug had no significant effect on the nociceptive threshold in control mice.
Figure 2.
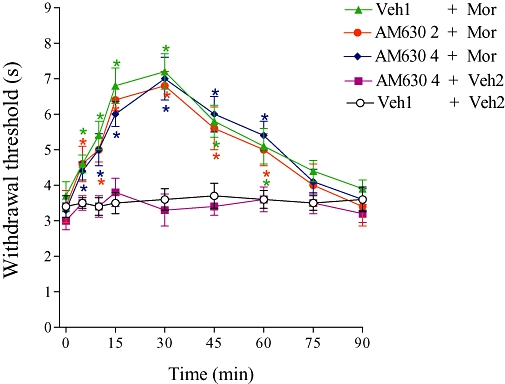
Effect of i.c.v. administration of AM630 on the central antinociception produced by morphine. AM630 (2 and 4 µg) was administered 1 min prior to morphine (Mor, 5 µg). Each point represents the mean ± SEM for five mice per group. *Indicates a significant difference compared to Veh1 + Veh2-injected group (analysis of variance + Bonferroni test, P < 0.05). Veh1, vehicle1 (20% DMSO in saline); Veh2, vehicle2 (saline).
Effect of AM251 and AM630 on antinociception induced by SNC80 or bremazocine
Neither AM251 (4 µg) nor AM630 (4 µg) reduced the central antinociceptive effect of SNC80 (4 µg; Figure 3A). In addition, AM251 (4 µg) and AM630 (4 µg) had no effect on the central antinociception induced by bremazocine (4 µg; Figure 3B).
Figure 3.
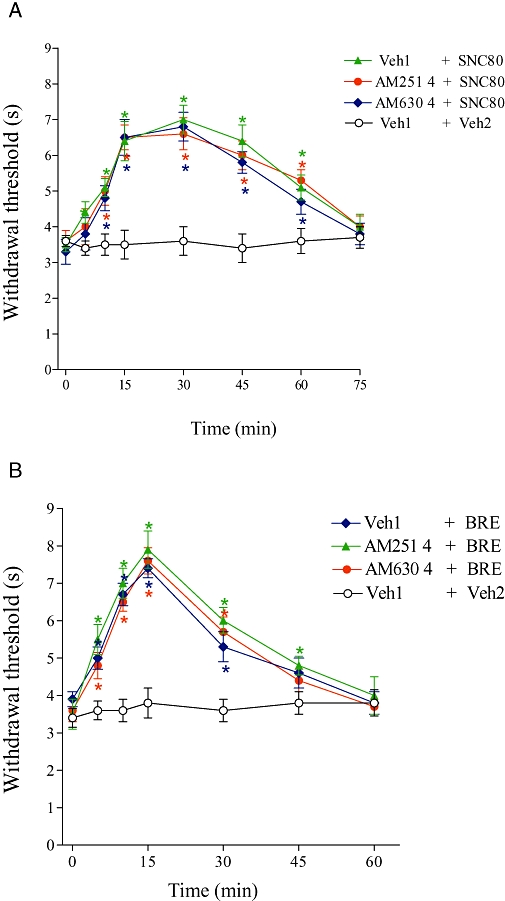
Effect of i.c.v. administration of AM251 and AM630 on the central antinociception produced by SNC80 (A) or bremazocine (B). AM251 (4 µg) or AM630 (4 µg) was administered 1 min prior to SNC80 (4 µg) or bremazocine (BRE; 4 µg). Each point represents the mean ± SEM for five mice per group. *Indicates a significant difference compared to Veh1 + Veh2-injected group (analysis of variance + Bonferroni test, P < 0.05). Veh1, vehicle1 (20% DMSO in saline); Veh2, vehicle2 [20% DMSO in saline (A) or saline (B)].
Increase of morphine-induced antinociception by MAFP
MAFP (0.1 and 0.2 µg) administration progressively enhanced the antinociception induced by a low dose of morphine (2.5 µg; Figure 4). MAFP alone had no antinociceptive effect.
Figure 4.
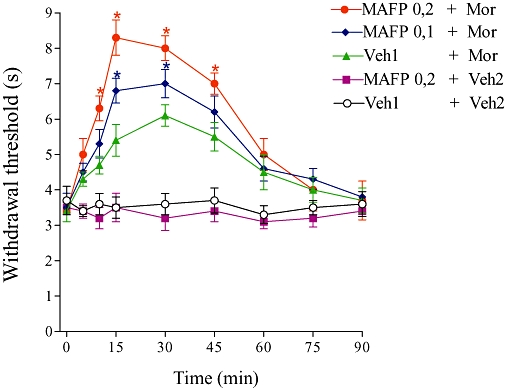
Potentiation of morphine-induced antinociception by MAFP. The MAFP (0.1 and 0.2 µg) was administered 1 min prior to morphine (2.5 µg). This drug alone (0.2 µg) did not induce any effect. Each point represents the mean ± SEM for five mice per group. *Indicates a significant difference compared to Veh1 + Veh2-injected group (analysis of variance + Bonferroni test, P < 0.05). Veh1, vehicle1 (10% DMSO in saline); Veh2, vehicle 2 (saline).
Discussion
Recent papers have suggested the reciprocal importance of endogenous opioid and cannabinoid systems in both the acute and chronic effects of these two systems. Evidence exists that cannabinoid-induced antinociception may depend, to some extent, on the release of opioid peptides (Reche et al., 1996). Given the lack of information regarding the participation of endogenous cannabinoids in the analgesic mechanism of opioids, the present work used AM251 (CB1 receptor antagonist) and AM630 (CB2 receptor antagonist) to characterize the role of endocannabinoids in the central antinociception induced by opioids.
Initially, the ability of the µ-, δ- and κ-opioid receptor agonists morphine, SNC80 and bremazocine, respectively, to induce central antinociception in the tail-flick test was investigated. Analysis of the results showed that the agonists produced a central antinociceptive effect in a dose-dependent manner (data not shown), with doses of 5, 4 and 4 µg of morphine, SNC80 and bremazocine, respectively, chosen for the present study.
AM251 was able to prevent the central antinociception induced by morphine, in a dose-dependent manner. Likewise, our group previously demonstrated that AM251 reversed the peripheral antinociception induced by morphine (Pacheco et al., 2008). AM251 is a potent CB1 receptor antagonist, 306-fold selective over CB2 receptors (Gatley et al., 1997; Lan et al., 1999). High levels of the CB1 receptor are expressed in the central nervous system (CNS), where cannabinoids act at pre-synaptic CB1 receptors to elicit changes in the synaptic efficacy of neuronal circuits (Freund et al., 2003). It has been verified that the activation of CB1 receptors present at peripheral, spinal and supraspinal sites produced antinociceptive effects (Hohmann, 2002; Hohmann and Suplita, 2006). Other CB1 antagonist compounds include SR141716A, AM281 and LY320135. It is noteworthy that although AM251 and AM281 are structurally very similar to SR141716A, and share its ability to block CB1 receptors and to produce inverse cannabimimetic effects, several pharmacological differences between SR141716A and either or both AM251 and AM281 have unexpectedly been detected in vitro, for example in experiments with cardiovascular tissue (Pertwee, 2004) and with rat hippocampal slices (Hájos and Freund, 2002). In addition, SR141716A was reported to increase the synaptic concentration of biogenic amines, that is, enhance the synaptic availability of the monoamine neurotransmitters noradrenalin, 5-hydroxytryptamine and dopamine in the brain (Witkin et al., 2005). AM251, in contrast to SR141716A, is devoid of vanilloid activity and is more selective for the CB1 receptor [Ki (CB1 vs. CB2) = 7.5 nM vs. >2 µM] than SR141716A [Ki (CB1 vs. CB2) = 5.6 nM vs. >1 µM] (Hohmann and Suplita, 2006).
The interaction between cannabinoids and opioids has been extensively studied, and numerous authors have reported that cannabinoids enhance the antinociception of morphine through the release of opioid peptides, for example: the cannabinoid Δ9-THC produced an increase in morphine antinociception by dynorphin A release (Welch and Eads, 1999); another study demonstrated that naloxone blocked the synergistic antinociception produced by low oral doses of Δ9-THC and morphine, indicating the involvement of the µ receptor in this effect (Cichewicz et al., 1999); and the potentiation between morphine and Δ9-THC was reversed by the µ-opioid receptor antagonist β-funaltrexamine, administered by i.c.v. route. The authors of the last study speculated that both cannabinoid and µ-supraspinal opioid receptors activate similar descending inhibitory pathways regulating the release of neurotransmitters involved in nociceptive transmission at a spinal level. Thus, a combination of both Δ9-THC and morphine may result in the sequential activation of spinal and supraspinal mechanisms, leading to antinociception (Reche et al., 1996). It has also been found that pretreatment with cannabinoids enhances the antinociceptive effect of a micro-injection of morphine into the ventral PAG, suggesting that alternating opioid and cannabinoid treatment could be therapeutically advantageous by preventing the development of tolerance and enhancing morphine antinociception (Wilson et al., 2008). Recently, it was suggested that CB1 and µ-opioid receptors form heterodimers (Rios et al., 2006). Heterodimer formation is needed for the function of certain G-protein-coupled receptors, such as the GABAB receptor (Ong and Kerr, 2000).
The CB2 receptor antagonist AM630 did not block the central antinociception induced by morphine. AM630 is a CB2 ligand 165-fold selective over CB1 receptors (Ross et al., 1999). The CB2 receptor is primarily located on immune cells in the periphery (Galiègue et al., 1995), although studies have demonstrated the presence of CB2 receptors in a number of brain regions, contrary to the prevailing view that they are restricted to peripheral tissues (Sickle et al., 2005; Gong et al., 2006; Onaivi et al., 2006). It has also been suggested that supraspinal CB2 in the thalamus may contribute to the modulation of neuropathic pain responses (Jhaveri et al., 2006). However, the CB2 receptor protein has not been located on central neurones, and the effects of endocannabinoids in the brain have always been attributed to an action at CB1 receptors. Moreover, many studies have shown that CB2 receptor-selective agonists produce peripheral antinociception, but do not cause CNS effects produced by non-selective cannabinoid receptor agonists, suggesting that selective activation of CB2 receptors may achieve the goal of peripheral pain relief without CNS effects (Malan et al., 2001). These receptors have not been found on peripheral neurones, suggesting that the activation of CB2 receptors produces antinociception indirectly, by causing the release of mediators from non-neuronal cells that alter the responsiveness of primary afferent neurones to noxious stimuli. One cell type that might mediate the actions of CB2 receptor-selective agonists is the keratinocytes, which have been reported to express CB2 receptors (Casanova et al., 2003) and to contain endogenous opioid peptides (Kauser et al., 2003). It has been demonstrated that antinociception produced by CB2 receptor-selective agonists may be mediated by the stimulation of β-endorphin release from cells expressing CB2 receptors. The β-endorphin released thus appears to act at µ-opioid receptors, probably on the terminals of primary afferent neurones, to produce peripheral antinociception (Ibrahim et al., 2005).
Several putative endocannabinoids have been isolated in the brain, including anandamide, 2-arachidonoylglycerol (2-AG), noladin ether, virodhamine and N-arachidonoyl dopamine (NADA). Three of these five putative endocannabinoids, anandamide, 2-AG and NADA, are susceptible to degradation by FAAH. In order to confirm the participation of endocannabinoids in the central antinociceptive effects of morphine, MAFP was used, an irreversible inhibitor of FAAH. Additionally, the compound MAFP, which is commonly used to inhibit FAAH, has been found to be a potent inhibitor of monoacylglycerol lipase (MGL) activity (Dinh et al., 2002). The crucial role of FAAH and MGL in the inactivation of anandamide suggests that inhibitors of these enzymes could be used to enhance endocannabinoid activity (Ho and Raldall, 2007). It was demonstrated that the combination of URB597 (inhibitor of FAAH) and anandamide produced maximal antinociception in the mouse tail-flick test versus either substance alone. The combination of URB597/anandamide was not active in CB1−/− knock-out mice, but retained activity in MOR−/− knock-out mice. These data are the first to demonstrate that anandamide, if protected from degradation, acts via the CB1 receptor to interact with the κ-opioid receptors to induce opioid-mediated analgesia (Haller et al., 2008). On the other hand, the present results demonstrated that MAFP administration increased the central antinociception produced by a low dose of morphine (2.5 µg), suggesting that the activation of µ-opioid receptors releases cannabinoids. Anandamide is an agonist at CB1 and CB2 receptors, but presents greater affinity for CB1 receptors (Howlett et al., 2002), and the present work showed that the antinociceptive effect of morphine was completely reversed by the CB1 receptor antagonist AM251. Only a few investigators have evaluated the role of the endocannabinoid systems in modulating opioid systems in behavioural studies. Cannabinoid receptor CB1−/− knock-out mice are unable to learn to self-administer morphine, suggesting a reduction in morphine's reinforcing property in these mice (Cossu et al., 2001). Morphine-induced place preference is also abolished in cannabinoid CB1−/− receptor knock-out mice (Martin et al., 2000).
Currently, the identification of endocannabinoids involved in pain modulation is obtained directly by microdialysis, and liquid and/or gas chromatography mass spectrometry (Cravatt et al., 2001; Cravatt and Lichtman, 2002), and indirectly by administration of pharmacological agents that regulate endocannabinoid uptake or degradation (Hohmann and Suplita, 2006). The present study focused on the indirect approach.
In contrast to morphine, AM251 and AM630 did not exert an effect on the central antinociception induced by SNC80 or bremazocine at doses effective on morphine. Also, higher doses were tested without success. However, some studies have demonstrated that intrathecally administered cannabinoids evoke the release of endogenous opioids that stimulate δ- and κ-opioid receptors to produce antinociception (Welch, 1993; Pugh et al., 1996). Other studies have shown that µ- and, particularly, κ-, but not δ-receptors, are involved in the antinociceptive action of Δ9-THC (Reche et al., 1996). At present, no studies showing the participation of cannabinoids in the activation of κ- and δ-opioid receptors exist.
In conclusion, the results presented in this paper do not show directly that µ-opioid receptor activation releases endocannabinoids, but the blockade of morphine-induced antinociception by CB1 receptor antagonist and its potentiation by protection of anandamide degradation are strong evidence that the central antinociceptive effect of morphine is mediated by a cannabinoid system.
Acknowledgments
Fellowships were awarded to the authors by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and the Conselho Nacional de Pesquisa.
Glossary
Abbreviations:
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM630
6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone
- FAAH
fatty acid amide hydrolase
- MAFP
methyl arachidonyl fluorophosphonate/(5Z,8Z,11Z,14Z)-5,8,11,14-eicosatetraenyl-methyl ester phosphonofluoridic acid
- SNC80
(+)-4-[(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide
- Δ9-THC
Δ9-tetrahydrocannabinol
Conflict of interest
The authors state no conflict of interest.
References
- Bidaut-Russell M, Devane WA, Howlett AC. Cannabinoid receptors and modulation of cyclic AMP accumulation in the rat brain. J Neurochem. 1990;55:21–26. doi: 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Casanova ML, Blázquez C, Martínez-Palacio J, Villanueva C, Fernández-Aceñero MJ, Huffman JW, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR. Opioid receptor-coupled second messengers. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement of µ opioid antinociception by oral Δ9-tetrahydrocannabinol: dose–response analysis and receptor identification. J Pharmacol Exp Ther. 1999;289:859–867. [PubMed] [Google Scholar]
- Cossu G, Ledent C, Parmentier M, Maldonado R, Valverde O. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Cox ML, Welch SP. The antinociceptive effect of Δ9-tetrahydrocannabinol in the arthritic rat. Eur J Pharmacol. 2004;493:65–74. doi: 10.1016/j.ejphar.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids. 2002;121:135–148. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DQ, Martin DR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Degroot A, Nomikos GG. In vivo neurochemical effects induced by changes in endocannabinoid neurotransmission. Curr Opin Pharmacol. 2007;7:62–68. doi: 10.1016/j.coph.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Omeir R, Arreaza G, Salehani D, Prestwich GD, Huang Z, et al. Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase. Biochem Pharmacol. 1997;53:255–260. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, et al. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci. 2004;19:678–686. doi: 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:191–197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Hájos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Haley TJ, McCormick WG. Pharmacological effects produced by intracerebroventricular injection of drugs in the conscious mouse. Br J Pharmac Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller VL, Stevens DL, Welch SP. Modulation of opioids via protection of anandamida degradation by fatty acid amide hydrolase. Eur J Pharmacol. 2008;600:50–58. doi: 10.1016/j.ejphar.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Ho WSV, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8:693–708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht J, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barret DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauser S, Schallreuter KU, Thody AJ, Gummer C, Tobin DJ. Regulation of human epidermal melanocyte biology by beta-endorphin. J Invest Dermatol. 2003;120:1073–1080. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- La Rana G, Russo R, Campolongo P, Bortolato M, Mangieri RA, Cuomo V, et al. Modulation of neuropathic and inflammatory pain by the endocannabinoid transport inhibitor AM404 [N-(4-hydroxyphenyl)-eicosa-5,8,11,14-tetraenamide. J Pharmacol Exp Ther. 2006;317:1365–1371. doi: 10.1124/jpet.105.100792. [DOI] [PubMed] [Google Scholar]
- Lu Q, Straiker A, Lu Q, Maguire G. Expression of CB2 cannabinoid receptor mRNA in adult rat retina. Vis Neurosci. 2000;17:91–95. doi: 10.1017/s0952523800171093. [DOI] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Manzaneres J, Corchero J, Romero JJ, Fernandez-Ruiz JA, Ramos JÁ, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabionoids. Trends Pharmacol Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Romorini S, Parolaro D. Comparative characterization in the rat of the interaction between cannabinoids and opioids for their immunosopressive and analgesic effects. J Neuroimmunol. 2001;117:116–124. doi: 10.1016/s0165-5728(01)00323-x. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Ong J, Kerr DI. Recent advances in GABAB receptors: from pharmacology to molecular biology. Acta Pharmacol Sin. 2000;21:111–123. [PubMed] [Google Scholar]
- Pacheco DF, Klein A, Perez AC, Pacheco CMF, Francischi JN, Duarte ID. The mu-opioid receptor agonist morphine, but not agonists at delta- or kappa-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br J Pharmacol. 2008;154:1143–1149. doi: 10.1038/bjp.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Petrosino S, Marabese I, Vita D, Giordano C, et al. Neuropathic pain and the endocannabinoid system in the dorsal raphe: pharmacological treatment and interactions with the serotonergic system. Eur J Neurosci. 2006;24:2011–2020. doi: 10.1111/j.1460-9568.2006.05086.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Novel pharmacological targets for cannabinoids. Curr Neuropharmacol. 2004;2:9–29. [Google Scholar]
- Pugh G, Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–616. [PubMed] [Google Scholar]
- Reche I, Fuentes JA, Ruiz-Gaio M. Potentiation of Δ9-tetrahydrocannabinol-induced analgesia by morphine in mice: involvement of µ and κ opioid receptors. Eur J Pharmacol. 1996;318:11–16. doi: 10.1016/s0014-2999(96)00752-2. [DOI] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson MT, Small-Howard A, Shimoda LM, Koblan-Huberson M, Stokes AJ, Turner H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J Immunol. 2003;170:4953–4962. doi: 10.4049/jimmunol.170.10.4953. [DOI] [PubMed] [Google Scholar]
- Sickle MDV, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Singh VK, Bajpai K, Biswas S, Haq W, Khan MY, Mathur KB. Molecular biology of opioid receptors: recent advances. Neuroimmunomodulation. 1997;4:285–297. doi: 10.1159/000097349. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, et al. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebral granule neurons. Proc Natl Acad Sci USA. 1996;93:3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Cichewiez D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;60:559–566. doi: 10.1016/s0091-3057(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Cishewicz DL, Lichtman AH. Interactions between cannabinoids and opioids. In: Wenger Tibor., editor. Recent Advances in Pharmacology and Physiology of Cannabinoids. Cochin: Kerala Press; 2004. pp. 157–182. [Google Scholar]
- Welch SP. Blockade of cannabinoid-induced antinociception by norbinaltorphimine, but not N,N-diallyl-tyrosine-Aib-phenylalanine-leucine, ICI 174864 or naloxone in mice. J Pharmacol Exp Ther. 1993;265:633–640. [PubMed] [Google Scholar]
- Welch SP, Eads M. Synergistic interactions of endogenous opioids and cannabinoids systems. Brain Res. 1999;848:183–190. doi: 10.1016/s0006-8993(99)01908-3. [DOI] [PubMed] [Google Scholar]
- Williams J, Haller VL, Stevens DL, Welch SP. Decreased basal endogenous opioid levels in diabetic rodents: effects on morphine and delta-9-tetrahydrocannabinoid-induced antinociception. Eur J Pharmacol. 2008;584:78–86. doi: 10.1016/j.ejphar.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Wilson AR, Maher L, Morgan MM. Repeat cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology. 2008;55:1219–1225. doi: 10.1016/j.neuropharm.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]


