Abstract
The G12 subfamily of heterotrimeric guanine nucleotide-binding proteins consists of two α subunits, Gα12 and Gα13. These proteins mediate signalling via G protein-coupled receptors and have been implicated in various physiological and pathophysiological processes. A number of direct and indirect effectors of Gα12 and Gα13 have been identified that mediate, or have been proposed to mediate, the diverse cellular responses accompanying activation of G12 proteins. This review describes the signalling pathways and cellular events stimulated by G12 proteins, with a particular emphasis on processes that are important in regulating cell migration and invasion, and could potentially be involved in the pathophysiology of cancer metastasis. Experimental findings directly implicating G12 proteins in the spread of metastatic disease are also summarized, indicating the importance of targeted inhibition of G12 signalling as a potential therapeutic option for locally advanced and metastatic disease.
Keywords: G proteins, G12 subfamily, oncogenic transformation, migration, invasion, metastasis
Heterotrimeric guanine nucleotide-binding proteins (G proteins) mediate extracellular signals from transmembrane G protein-coupled receptors (GPCRs) to engage intracellular effector pathways leading to a variety of cellular responses (Marinissen and Gutkind, 2001; Pierce et al., 2002; Oldham and Hamm, 2007). G proteins consist of a guanine nucleotide-binding α subunit and a βγ subunit dimer. In its inactive state, the α subunit binds a GDP molecule. Upon receptor activation by an agonist, the engagement of the liganded receptor with the G protein triggers a conformational change in the α subunit that leads to the exchange of GDP for GTP, and dissociation of the α subunit from the βγ dimer, both of which can then signal to their downstream effectors (Fields and Casey, 1997).
Heterotrimeric G proteins are classified into four subfamilies based on the sequence similarity of the α subunits: Gs, Gi, Gq and G12. The G12 subfamily consists of two α subunits, Gα12 and Gα13. Activation of the G12 proteins impacts on several signalling pathways including those linking G proteins to monomeric GTPases, mitogen-activated protein kinases (MAPKs) and non-receptor tyrosine kinases (non-RTKs), among others. Thus, G12 proteins have been implicated in several physiological and pathophysiological processes (Dhanasekaran et al., 1998; Rohrer and Kobilka, 1998; Offermanns, 2001; Dorsam and Gutkind, 2007). This review details our current understanding of how G12 proteins regulate some of these cellular events, in particular signalling pathways impacting oncogenesis and metastasis.
Biological roles of G12 proteins
Gα12 and Gα13 are expressed in virtually every tissue in the body (Milligan et al., 1992; Spicher et al., 1994). Activation of these proteins impacts such cellular processes as growth and proliferation, cytoskeleton rearrangement, cell polarity, paracellular permeability, cell–cell adhesion, migration and invasion (Kurose, 2003; Kelly et al., 2007). Several direct binding partners as well as indirect downstream effectors of Gα12/13 have been identified that have been implicated, and in some cases directly shown to be involved, in these and other biological events mediated by the G12 subfamily (Kelly et al., 2007). Many of the effects of G12/13 signalling are mediated by the monomeric GTPase Rho. Gα12 and Gα13 activate Rho principally through direct interaction of the activated Gα subunit with Rho-specific guanine nucleotide exchange factors (RhoGEFs) (see Figure 1), which include p115RhoGEF (Hart et al., 1998; Kozasa et al., 1998), PDZ-RhoGEF (Fukuhara et al., 1999) and leukemia-associated RhoGEF (LARG) (Fukuhara et al., 2000a). Other proteins that directly interact with Gα12/13, and hence could serve as effectors include cadherins (Meigs et al., 2001), radixin of the ezrin/radixin/moesin protein family (Vaiskunaite et al., 2000; Liu and Voyno-Yasenetskaya, 2005), non-RTKs (Jiang et al., 1998; Mao et al., 1998; Shi et al., 2000), protein phosphatases (Yamaguchi et al., 2002; Zhu et al., 2004; Zhu et al., 2007), A-kinase anchoring proteins (AKAPs) (Diviani et al., 2001; Niu et al., 2001), the tight junction protein, zonula occludens-1 (Meyer et al., 2002; Sabath et al., 2008), Hsp90 (Vaiskunaite et al., 2001) and regulators of G protein signalling RGS1, RGS16 and axin (Moratz et al., 2000; Johnson et al., 2003; Stemmle et al., 2006), among others (Kurose, 2003; Kelly et al., 2007).
Figure 1.
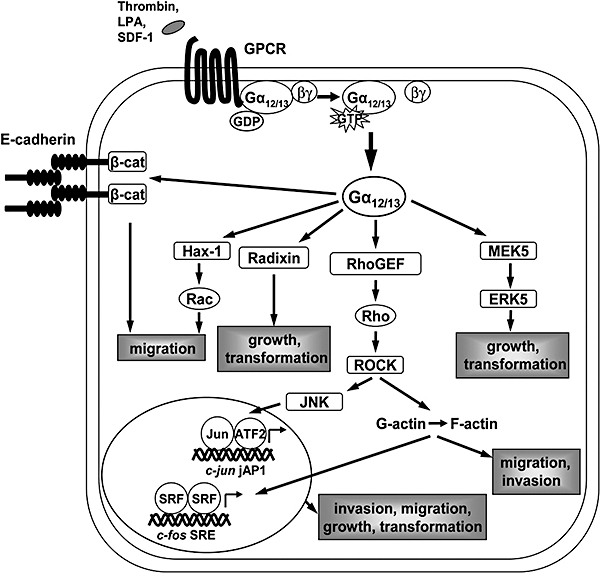
Schematic of Gα12/13 effectors and signalling pathways impacting cell growth and transformation, migration and invasion. β-cat, beta-catenin; ATF2, activating transcription factor-2; ERK, extracellular signal-regulated kinase; GPCR, G protein-coupled receptor; jAP1, c-jun AP1-like response element; JNK, c-Jun N-terminal kinase; LPA, lysophosphatidic acid; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; RhoGEF, Rho-specific guanine nucleotide exchange factor; ROCK, Rho kinase; SDF-1, CXC chemokine stromal cell-derived factor-1α; SRE, serum response element; SRF, serum response factor.
Perhaps the most extensively studied roles of G12/13 are in cell growth and proliferation, and cell migration (described later). The G12 proteins also influence several other important cellular functions. G12/13 signalling is required for agonist-induced smooth muscle contraction (Gohla et al., 2000; Hersch et al., 2004). GPCRs that mediate vasoconstriction such as angiotensin II, endothelin-1 and thromboxane A2 couple to both the Gq/11 and G12/13 subfamilies to stimulate myosin light chain (MLC) phosphorylation via Ca2+-dependent activation of MLC kinase, and Rho/Rho kinase-mediated inhibition of myosin phosphatase, respectively (Gohla et al., 2000). Phosphorylation of MLC leads to its interaction with actin and the generation of contractile force. A recent study utilizing conditional knockout of Gα12/13 in smooth muscle cells in mice demonstrated that G12/13 signalling is required for the development of salt-induced hypertension but not for the maintenance of basal blood pressure (Wirth et al., 2008). G13-mediated signalling is important for platelet activation in hemostasis and thrombosis (Moers et al., 2003). Platelets lacking Gα13 show defective shape change and aggregation in vitro and these defects are accompanied by reduced activation of RhoA and subsequently decreased MLC phosphorylation. Selective deletion of Gα13 in mouse platelets in vivo results in severely increased bleeding times and lack of formation of arterial thrombi in an experimentally induced thrombosis model (Moers et al., 2003). In addition, G12/13 mediate receptor-induced cardiac hypertrophic responses by activating a Gα12/13-Rho-c-Jun N-terminal kinase (JNK) pathway (Maruyama et al., 2002). JNK is a MAPK that is an indirect effector of G12/13 signalling, usually activated downstream of Rho GTPases (Goldsmith and Dhanasekaran, 2007). The G12 proteins also mediate protease-activated receptor-1 (PAR-1)-induced vascular endothelial barrier permeability via Rho and Rho kinase (ROCK) activation (McLaughlin et al., 2005).
While the G12 proteins clearly play a role in cell growth and proliferation (discussed below), they have also been implicated in apoptotic pathways. Constitutively activated Gα13 triggers apoptosis via a pathway involving Rho activation (Althoefer et al., 1997). Gα12 appears to regulate apoptosis in epithelial cells by activating JNK and protein phosphatase 2A (PP2A) leading to loss of expression of the anti-apoptotic protein, Bcl-2 (Yanamadala et al., 2007). Gα12 and Gα13 have also been shown to stimulate apoptosis via two MAPK pathways, one by activating apoptosis signal regulating kinase-1 (ASK-1) and the other by activating MAPK kinase kinase 1 (MEKK1), both leading to JNK activation (Berestetskaya et al., 1998). Interestingly, Gα13 forms a complex with ASK-1 and regulates apoptosis by reducing the rate of ASK-1 degradation (Kutuzov et al., 2007). Formation of the Gα13-ASK-1 complex is enhanced by coexpression of JNK-interacting leucine zipper protein (JLP) suggesting that JLP may be acting as a scaffolding protein to form a macromolecular complex (Kutuzov et al., 2007). JLP was identified earlier as physically interacting with Gα13 and leading to increased Gα13-mediated JNK activation (Kashef et al., 2005). In this regard, it is also important to note that Gα13 signals via p115RhoGEF and activates JNK to regulate primitive endoderm formation in murine embryonic carcinoma cells (Jho et al., 1997; Lee et al., 2004). An interaction between Gα13 and JLP is required for this process (Kashef et al., 2006).
G12 proteins in physiological cell migration
The role of G12 proteins in regulating physiological cell migration was first identified in studies in Drosophila. Genetic ablation of concertina, the single ortholog of Gα12 and Gα13 in Drosophila, impairs cell shape changes that underlie mesoderm internalization during gastrulation in flies (Parks and Wieschaus, 1991). An essential role of Gα12/13 has also been demonstrated in cell shape changes and migration events that occur during gastrulation in zebrafish (Lin et al., 2005). In mice, deletion of Gα13 has been shown to disrupt organization of the vascular system, resulting in lethality at approximately day 10.5 of embryogenesis (Offermanns et al., 1997; Ruppel et al., 2005). Embryonic fibroblasts cultured from these mice display a reduced chemokinetic response to thrombin, the agonist for PARs. Thus, this defect in cell migration may be responsible for the failed angiogenesis (Offermanns et al., 1997). Quite recently, a role for Gα12/13 was demonstrated in the development of the central nervous system (Moers et al., 2008). Gα12/13 appear to mediate stop signals which are required for the proper positioning of migrating cortical plate neurons and Purkinje cells during development. Conditional knockout of Gα12 and Gα13 in the nervous system of mice results in neuronal ectopia of the cerebral and cerebellar cortices due to overmigration of these cells (Moers et al., 2008). Another study showed that the orphan GPCR, GPR56, is highly expressed in neural progenitor cells (NPCs) and negatively regulates NPC migration via a Gα12/13-Rho pathway (Iguchi et al., 2008). Interestingly, loss of the mouse GPR56 gene causes neuronal ectopia in the cerebral cortex (Li et al., 2008). Thus, GPR56 may couple to G12/13 to regulate the migration of cells during cortical development.
Changes in cytoskeletal dynamics required for cell migration are coordinated in large part by Rho GTPases (Ras, Rac, Cdc42 and Rho) (Raftopoulou and Hall, 2004), and signalling by G12 proteins is responsible for many of the effects on cell movement that accompany Rho activation. Polymerization of actin and assembly of focal adhesions are important for cell shape changes and cell contraction during migration, and Gα12 and Gα13 stimulate the formation of stress fibres and focal adhesions in a Rho-dependent manner (Buhl et al., 1995; Gohla et al., 1998; 1999;). Such Rho-dependent signalling pathways appear to be important during gastrulation in Drosophila, as noted above a process dependent on concertina function (Barrett et al., 1997; Nikolaidou and Barrett, 2004), and during the corresponding process in zebrafish (Lin et al., 2005; Solnica-Krezel, 2005). Rho activation by Gα12/13 is also required, as mentioned above, for regulating NPC migration (Iguchi et al., 2008).
Besides their role in regulating cell migration during embryonic development, the G12 subfamily is also involved in controlling the migration of lymphocytes, neutrophils and vascular smooth muscle cells (VSMCs) (Xu et al., 2003; Rieken et al., 2006; Tan et al., 2006; Takashima et al., 2008) (see Table 1). The CXC chemokine stromal cell-derived factor-1α (SDF-1) induces cell migration in T lymphocytes by binding to the GPCR, CXCR4, to activate a Gα13-Rho signalling axis (Tan et al., 2006). On the other hand, Gα12/13 likely regulate the number of splenic marginal zone B (MZB) cells, a specialized population of B lymphocytes, by exerting an inhibitory effect on sphingosine-1-phosphate (S1P)-induced migration of these cells. Mice that lack the Gα12/13 subunits show significantly reduced numbers of MZB cells suggesting that, besides affecting peripheral MZB cell maturation, loss of Gα12/13 causes disinhibition of S1P-induced promigratory signalling (Rieken et al., 2006). G12 proteins dictate morphologic polarity in neutrophils that is necessary for their migration in uniform concentrations of attractants. This function of G12 and G13 is mediated by activation of Rho with subsequent stimulation of the Rho-dependent protein kinase, p160-ROCK and myosin II to generate myosin-based contraction of the trailing edge of the migrating cell (Xu et al., 2003). Gα12/13 subunits also control cell polarity and directed migration of fibroblasts in a Rho-dependent manner (Goulimari et al., 2005; 2008;). In VSMCs, S1P activates G12/13 signalling via the S1P(2) receptor, leading to activation of Rho and inhibition of both platelet-derived growth factor (PDGF)-induced Rac activation and migration of VSMCs (Takashima et al., 2008). These disparate effects of Rho activation reflect the intricacy of Rho signalling mechanisms impacting on cell migration (Sahai and Marshall, 2002).
Table 1.
GPCRs that activate G12/13 signalling to regulate the migration and invasion of mammalian cells
| GPCR | Cell type | References |
|---|---|---|
| PAR-1 | Embryonic fibroblasts | Offermanns et al., 1997 |
| Breast cancer cells | Kelly et al., 2006a | |
| Prostate cancer cells | Kelly et al., 2006b | |
| LPA receptor | Fibroblasts | Goulimari et al., 2005 |
| Goulimari et al., 2008 | ||
| Ovarian cancer cells | Bian et al., 2006 | |
| Embryonic cortical neurons | Moers et al., 2008 | |
| CXCR4 | T lymphocytes | Tan et al., 2006 |
| S1P receptor | MZB cells | Rieken et al., 2006 |
| VSMCs | Takashima et al., 2008 | |
| Glioblastoma cells | Malchinkhuu et al., 2008 | |
| Embryonic cortical neurons | Moers et al., 2008 | |
| Thromboxane A2 receptor | Breast cancer cells | Kelly et al., 2006a |
| Prostate cancer cells | Kelly et al., 2006b | |
| GPR56 | Neural progenitor cells | Iguchi et al., 2008 |
GPCR, G protein-coupled receptor; LPA, lysophosphatidic acid; MZB, splenic marginal zone B; PAR-1, protease-activated receptor-1; S1P, sphingosine-1-phosphate; VSMC, vascular smooth muscle cell.
Cell migration is a complex process (Friedl and Wolf, 2003; Raftopoulou and Hall, 2004; Van Haastert and Devreotes, 2004), and while in some cell types G12 proteins regulate migration via stimulation of Rho, in other cell types they promote migration via processes independent of Rho signalling (Meigs et al., 2002; Radhika et al., 2004) (Figure 1). In fibroblasts, Gα13-stimulated migration requires interaction of the Gα subunit with Hax-1, a cytoskeleton-associated, cortactin-interacting protein. Coexpression of Hax-1 with constitutively active Gα13 attenuates the ability of Gα13 to stimulate Rho activity and leads to a significant reduction in stress fibres, while at the same time potentiating Rac activity (Radhika et al., 2004). In another example, the ability of Gα12 to negatively regulate the adhesive function of cadherin enhances cell migration, and this function of Gα12 is independent of Rho activation (Meigs et al., 2002). The primary mechanism for the increased migration in this instance appears to be due to the ability of the Gα12-E-cadherin interaction to trigger the release of β-catenin from the cytoplasmic tail of E-cadherin and thereby reverse E-cadherin-mediated suppression of migration (Meigs et al., 2002).
In addition to mediating signals from GPCRs to impact on cell migration, in some cellular contexts G12 proteins may mediate signals from RTKs to regulate migration. An intriguing recent study demonstrated that Gα13 is required for RTK-induced migration of fibroblast and endothelial cells, and this function of Gα13 is independent of GPCR signalling (Shan et al., 2006). The mechanism through which Gα13 might be involved in this process without involvement of a GPCR is still unclear, but the finding suggests that G12 proteins may be coordinating promigratory signals from GPCRs and RTKs.
G12 proteins in oncogenic transformation and cancer
Soon after their discovery, several studies established a role for both Gα12 and Gα13 in oncogenic transformation (Chan et al., 1993; Xu et al., 1993). Interestingly, the wild-type form of Gα12 was identified as a transforming oncogene due to its ability to promote focus formation in NIH3T3 mouse fibroblasts, revealing the G12 subfamily as the only class of heterotrimeric G proteins that is transforming when over-expressed as a wild-type form (Chan et al., 1993). Subsequent studies utilizing overexpression or mutationally activated forms of Gα12/13 have confirmed the ability of these proteins to transform fibroblasts (Jiang et al., 1993; Xu et al., 1993; Vara Prasad et al., 1994; Voyno-Yasenetskaya et al., 1994). Furthermore, overexpression of GPCRs that couple to G12 proteins such as PAR-1 (Martin et al., 2001) and M1 muscarinic acetylcholine receptor (Fromm et al., 1997), or stimulation of GPCRs by agonists (Aragay et al., 1995; Marinissen et al., 2003; Radhika et al., 2005) has been shown to promote cell growth and transformation through endogenous G12 signalling. These studies led to the hypothesis that GPCRs may signal through G12 proteins to promote tumorigenesis and tumour cell growth (Radhika and Dhanasekaran, 2001). Recently, it was demonstrated that the expression of G12 proteins themselves is significantly upregulated in tissue specimens from patients with adenocarcinoma of the breast and prostate (Kelly et al., 2006a,b;). Interestingly, a growth-promoting effect of G12 proteins was not observed in any of the breast and prostate cancer cell lines examined in this study (Kelly et al., 2006a,b;), suggesting that G12-mediated effects on cell proliferation may be cell type-specific, and much more pronounced in fibroblasts than in epithelial-derived cells.
Importantly, the levels of several G12-coupled receptors are elevated in various cancers and contribute to tumour cell growth and metastasis when activated by circulating or locally produced agonists (Dorsam and Gutkind, 2007). The G12/13-coupled protease-activated receptor, PAR-1, is overexpressed in highly invasive breast carcinoma cell lines and tissue specimens (Even-Ram et al., 1998), and in advanced-stage prostate cancer patient samples (Daaka, 2004). Thrombin stimulates invasion of cancer cells in vitro (Henrikson et al., 1999; Shi et al., 2004; Kelly et al., 2006a,b;), and antisense-mediated downregulation of PAR-1 blocks cancer cell invasion (Even-Ram et al., 1998). Receptors for bio-active lipids, such as lysophosphatidic acid (LPA) and S1P, are involved in cancer cell proliferation and migration (Dolezalova et al., 2003; Mills and Moolenaar, 2003). LPA is secreted by ovarian cancer cells and, by acting on the LPA receptors, sets up an autocrine loop that promotes both growth and survival of the cancer cells (Mills and Moolenaar, 2003). LPA receptors couple to G12/13 (Riobo and Manning, 2005), and it was recently shown that G12 proteins are critical regulators of LPA-stimulated migration of ovarian cancer cells (Bian et al., 2006). In another human cancer cell line, a positive feedback mechanism of Rho/ROCK activation mediated via the Rho effector, Dia1 (a Diaphanous-related formin) and the RhoGEF, LARG, appears to be important for LPA-stimulated invasion (Kitzing et al., 2007). The G12/13-coupled chemokine receptor, CXCR4, is aberrantly overexpressed in many malignant tumours including breast, prostate and lung cancer (Balkwill, 2004). CXCR4 binds to and is activated only by SDF-1. Interestingly, the expression of SDF-1 has been observed to be high in the organs where these tumour cells most frequently metastasize (Muller et al., 2001; Balkwill, 2004). Thus, activation of G12/13 signalling by several GPCRs likely plays an important role in tumour progression. As mentioned earlier, Gα13 has been implicated in transducing promigratory signals from RTKs (Shan et al., 2006). Interestingly, these RTKs, viz, platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) are overexpressed in highly metastatic cancers (Ferrara et al., 2003; Jechlinger et al., 2006; Normanno et al., 2006).
The ability of G12 proteins to transform fibroblasts appears to be primarily dependent on the activation of Rho proteins (Fromm et al., 1997; Martin et al., 2001; Kumar et al., 2006a), although in certain contexts other G12 effectors may be involved (see below; Figure 1). Besides their importance in controlling actin cytoskeleton rearrangement and cell polarity, as noted above, Rho GTPases also regulate microtubule dynamics, transcription factor activity and aspects of cell growth (Etienne-Manneville and Hall, 2002). Stimulation of Rho GTPases by G12 proteins impacts on several signalling pathways that include MAPK signalling cascades, and G12 proteins coordinate the activation and/or inhibition of different MAPKs (Collins et al., 1996; Voyno-Yasenetskaya et al., 1996; Nagao et al., 1999; Arai et al., 2003; Dermott et al., 2004). Gα12 and Gα13 stimulate the activity of the MAPK, JNK (Goldsmith and Dhanasekaran, 2007); a major outcome of JNK activation in cells is the phosphorylation of the transcription factor c-Jun, leading to increased transcription of genes involved in proliferation and survival, cell motility and invasion, among others (Karin et al., 1997). Thus, many of the biological consequences of Gα12/13 activation, including growth, differentiation and cellular transformation, could potentially be due to an impact on c-Jun activity (Jho et al., 1997; Marinissen et al., 2003; Radhika et al., 2005) (Figure 1). Stimulation of Rho by G12 proteins has also been shown to lead to cellular transformation via other signalling pathways such as activation of PDGFα receptor and STAT3 (Kumar et al., 2006a,b;), induction of transcription of COX2 (Dermott et al., 1999; Slice et al., 1999) and Egr-1 (Vara Prasad et al., 1994; Vara Prasad and Dhanasekaran, 1999), and by regulation of transcription from serum response element (SRE) (Fromm et al., 1997). There is some evidence that effector pathways other than Rho GTPases, such as extracellular signal-regulated kinase 5 (ERK5) activation (Fukuhara et al., 2000b), may contribute to the transforming potential of G12 proteins. This is particularly important as Gα12 and Gα13 are more potent stimulators of cellular transformation than overexpressed or mutationally activated RhoA (Fromm et al., 1997). In this regard, activation of Rac is required for Gα13-mediated SRE-dependent gene transcription induced by radixin (Liu and Voyno-Yasenetskaya, 2005). Radixin is involved in cross-linking of the actin cytoskeleton to the plasma membrane, and it has been shown to interact with Gα13 and to play a role in Gα13-induced transformation of Rat-1 fibroblasts (Vaiskunaite et al., 2000). Thus, other G12-effector signalling axes besides the signalling pathways leading to Rho activation may be required for G12-stimulated oncogenic transformation and is an important area for further study.
G12 proteins in cancer invasion and metastasis
G protein-coupled receptors that couple to Gα12/13 as well as downstream effectors of Gα12/13 have been implicated in tumorigenesis and cancer progression (Sahai and Marshall, 2002; Dorsam and Gutkind, 2007). The findings noted above indicating the importance of G12 proteins in cell migration during development, and the fact that G12 protein expression is upregulated in some human cancers, provided hints that the G12 subfamily of heterotrimeric G proteins may have a role in metastasis. Indeed, a direct role of G12 proteins in cancer invasion and metastasis has been recently demonstrated (Kelly et al., 2006a,b;). Expression of constitutively activated Gα12 and Gα13, or activation of Gα12/13 signalling by PAR-1 as well as thromboxane A2 receptor, induces a striking increase in breast and prostate cancer cell invasion in vitro (Kelly et al., 2006a,b;) (Table 1). Furthermore, blocking downstream signalling via G12 reduces breast cancer metastasis in vivo and results in a significant increase in metastasis-free survival of mice (Kelly et al., 2006a). In these studies 4T1 mouse mammary carcinoma cells were employed; when implanted in the mammary fat pad of recipient mice these cells form tumours and metastasize in a manner similar to human breast cancer (Aslakson and Miller, 1992; Smith et al., 2004). Inhibition of G12 signalling in the 4T1 cells through expression of a dominant-negative form of p115RhoGEF reduces the rate of metastatic dissemination of the cells from the primary tumour following their implantation in the mouse mammary fat pad. Interestingly, when the same cells are introduced directly into the bloodstream, inhibition of G12 signalling has no effect on the ability of these cells to metastasize (Kelly et al., 2006a). These findings indicate that G12 signalling is important in the early steps of the metastasis process and appears to promote cancer metastasis by stimulating tumour cell invasion and entry into the bloodstream.
As noted above, many of the effects of G12 proteins on cell growth, transformation and migration are mediated by Rho proteins which have a well-described role in tumorigenesis and metastasis. RhoA and RhoC are overexpressed in several tumours including colon, breast, lung and pancreas (Fritz et al., 1999; Sahai and Marshall, 2002) and their expression levels correlate positively with the progression of the tumour (Suwa et al., 1998; Fritz et al., 1999). Thus, activation of Rho by G12 proteins appears to be a crucial signalling mechanism for regulating cancer metastasis. In fact, it has been demonstrated that G12 signalling leading to invasion in several breast and prostate cancer cell lines requires activation of Rho and its downstream effector ROCK (Kelly et al., 2006a,b; unpublished observations) (see Figure 1). Similarly, G12-mediated migration of ovarian cancer cells also depends on activation of Rho/ROCK signalling (Bian et al., 2006). On the other hand, the G12/13-Rho signalling pathway mediates S1P-induced inhibition of migration of glioblastoma cells (Malchinkhuu et al., 2008), revealing the complexity of cell invasion and migration and the different mechanisms by which Rho can regulate these processes.
The involvement of the two subtypes of G12 proteins in cancer cell invasion may be complicated and cell type-specific. For example, activated Gα12 inhibits, rather than promotes, the invasion of the inflammatory breast cancer cell line SUM149 (Patrick Kelly, unpublished observations). Also in this regard, a recent study showed that expression of constitutively active Gα13 or activation of G13-coupled receptors by lysophosphatidylcholine inhibits chemokine-stimulated invasion of melanoma cells in vitro and impairs their metastasis in mice. This effect of activated Gα13 in melanoma cells appears to be mediated by a reduction in the levels of Rho-GTP due to high p190RhoGAP activity (Bartolome et al., 2008). In addition, G12 signalling independent of Rho activation may also potentially lead to invasion and cancer progression. In the T47D breast cancer cell line, expression of a Gα12 mutant that is uncoupled from Rho-mediated signalling (Meigs et al., 2005) induces a small but significant increase in invasion (Kelly et al., 2006a). This finding suggests that, while Rho activation by G12 proteins is necessary for stimulating cancer cell invasion, other effectors of Gα12/13 may also be required for this function. As mentioned above, Gα12 and Gα13 interact with members of the cadherin superfamily of cell adhesion proteins, most notably E-cadherin, and negatively regulate its adhesive function (Meigs et al., 2002). E-cadherin is required for cells to maintain their epithelial character and several studies have shown that loss of E-cadherin function in cancer is associated with a transition to a more aggressive, mesenchymal phenotype (Conacci-Sorrell et al., 2002; Thiery, 2002). Thus, G12 signalling could promote cancer metastasis by inhibiting E-cadherin function. Taken together, these studies suggest that the G12 proteins possibly regulate cancer cell invasion and migration via several mechanisms.
Gα12 and Gα13 appear to similarly affect the migration and invasion of several cancer cell types (Bian et al., 2006; Kelly et al., 2006a,b; Bartolome et al., 2008; Malchinkhuu et al., 2008), suggesting that they have overlapping functions. For example, the effect of separately blocking signalling via G12 and G13 leads to a similar degree of inhibition of LPA-induced migration of ovarian cancer cells as that observed upon expression of dominant-negative p115RhoGEF that blocks downstream signalling by both G12 proteins. (Bian et al., 2006). The functions of G12 and G13, however, are not completely redundant during embryonic development. Gα13-deficient mice are embryonic lethal at around E10, where as Gα12-deficient mice are apparently normal (Gu et al., 2002). Thus, embryos are able to develop normally in the presence of a full complement of wild-type Gα13 alleles even if both Gα12 alleles are disrupted. Embryos lacking both Gα12 and Gα13 die between E8 and E8.5. Although having a single allele each of Gα12 and Gα13 is sufficient for survival, in the absence of a Gα12 allele one allele of Gα13 is not enough for the embryos to survive beyond E9.5 (Gu et al., 2002). This suggests that there is some functional overlap between Gα12 and Gα13 during early development. This is interesting, both in terms of understanding fully the roles of G12 and G13 signalling in cancer, and for designing drug targets to inhibit metastasis.
Concluding remarks
The G12 subfamily of heterotrimeric G proteins impacts a variety of cellular functions and physiological processes. The dysregulation of some of these processes clearly underlies the pathophysiology of tumour development and progression. The studies highlighted in this review provide compelling evidence that Gα12 and Gα13 play pivotal roles in many aspects of cancer invasion and metastasis. Various downstream effectors that are important in G12/13-induced cell growth and transformation, migration and invasion have been identified. Rho GTPase, in particular, appears to be a principle downstream target of G12/13 signalling leading to these cellular responses, but other effectors of G12 proteins are likely important for many of the consequences of their activation. A complete understanding of the signalling pathways triggered by G12/13 requires the elucidation of specific effectors that directly interact with the Gα proteins and the downstream molecules that these effectors engage. Although Gα12 and Gα13 affect many similar biologies, they may do so via different signalling mechanisms. For example, Hax-1 only interacts with Gα13 and not Gα12, and, as discussed earlier, this interaction is important for Gα13-stimulated migration of fibroblasts (Radhika et al., 2004). In this regard, the identification of mutational variants of Gα12/13 that are selectively uncoupled from specific effectors (Meigs et al., 2005) provides valuable tools to decipher the importance of specific downstream pathways in particular G12-mediated biologies; such information will be required to fully understand the role of G12 proteins in migration and invasion.
Although in vitro experiments provide information about the mechanism and the molecular players involved in the various signalling pathways triggered by G12/13, in vivo experiments are required for accurate modeling of the impact of activation of G12 proteins on physiological and pathophysiological processes. The importance of such studies has been recently demonstrated with the development of Gα12/13 conditional knockout mice (Ruppel et al., 2005; Moers et al., 2008; Wirth et al., 2008), and with the use of the dominant-negative RhoGEF construct in vivo (Kelly et al., 2006a). With these advances in experimental techniques and the application of siRNA technology to G12 signalling (Andreeva et al., 2006; Bartolome et al., 2008), new and more complex functions of G12/13 signalling have begun to emerge. These approaches will also aid in understanding further the role of G12 proteins in oncogenesis and metastasis.
From a clinical perspective, pharmacologic inhibition of G12 signalling could be an effective therapeutic option for controlling cancer metastases. This may be achieved by targeting a specific G12/GPCR interaction in a particular type of cancer, or by inhibiting specific downstream signalling pathway(s) activated by Gα12/13. Therefore, a complete understanding of the biological role of G12 proteins in cell growth, invasion and cancer progression is imperative and will constitute a major aspect of G12 research in the years to come.
Glossary
Abbreviations:
- ASK-1
apoptosis signal regulating kinase-1
- G protein
heterotrimeric guanine nucleotide-binding protein
- GPCR
G protein-coupled receptor
- JLP
JNK-interacting leucine zipper protein
- JNK
c-Jun N-terminal kinase
- LPA
lysophosphatidic acid
- MAPK
mitogen-activated protein kinase
- MLC
myosin light chain
- MZB
splenic marginal zone B
- NPC
neural progenitor cell
- non-RTK
non-receptor tyrosine kinase
- PAR-1
protease-activated receptor-1
- PDGF
platelet-derived growth factor
- RGS
regulator of G protein signalling
- RhoGEF
Rho-specific guanine nucleotide exchange factor
- ROCK
Rho kinase
- RTK
receptor tyrosine kinase
- S1P
sphingosine-1-phosphate
- SDF-1
CXC chemokine stromal cell-derived factor-1α
- SRE
serum response element
- VSMC
vascular smooth muscle cell
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoefer H, Eversole-Cire P, Simon MI. Constitutively active Galphaq and Galpha13 trigger apoptosis through different pathways. J Biol Chem. 1997;272(39):24380–24386. doi: 10.1074/jbc.272.39.24380. [DOI] [PubMed] [Google Scholar]
- Andreeva AV, Vaiskunaite R, Kutuzov MA, Profirovic J, Skidgel RA, Voyno-Yasenetskaya T. Novel mechanisms of G protein-dependent regulation of endothelial nitric-oxide synthase. Mol Pharmacol. 2006;69(3):975–982. doi: 10.1124/mol.105.018846. [DOI] [PubMed] [Google Scholar]
- Aragay AM, Collins LR, Post GR, Watson AJ, Feramisco JR, Brown JH, et al. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J Biol Chem. 1995;270(34):20073–20077. doi: 10.1074/jbc.270.34.20073. [DOI] [PubMed] [Google Scholar]
- Arai K, Maruyama Y, Nishida M, Tanabe S, Takagahara S, Kozasa T, et al. Differential requirement of G alpha12, G alpha13, G alphaq, and G beta gamma for endothelin-1-induced c-Jun NH2-terminal kinase and extracellular signal-regulated kinase activation. Mol Pharmacol. 2003;63(3):478–488. doi: 10.1124/mol.63.3.478. [DOI] [PubMed] [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91(7):905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Bartolome RA, Wright N, Molina-Ortiz I, Sanchez-Luque FJ, Teixido J. Activated G(alpha)13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity. Cancer Res. 2008;68(20):8221–8230. doi: 10.1158/0008-5472.CAN-08-0561. [DOI] [PubMed] [Google Scholar]
- Berestetskaya YV, Faure MP, Ichijo H, Voyno-Yasenetskaya TA. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J Biol Chem. 1998;273(43):27816–27823. doi: 10.1074/jbc.273.43.27816. [DOI] [PubMed] [Google Scholar]
- Bian D, Mahanivong C, Yu J, Frisch SM, Pan ZK, Ye RD, et al. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25(15):2234–2244. doi: 10.1038/sj.onc.1209261. [DOI] [PubMed] [Google Scholar]
- Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270(42):24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, Aaronson SA. Expression cDNA cloning of a transforming gene encoding the wild-type G alpha 12 gene product. Mol Cell Biol. 1993;13(2):762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LR, Minden A, Karin M, Brown JH. Galpha12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J Biol Chem. 1996;271(29):17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109(8):987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y. G proteins in cancer: the prostate cancer paradigm. Sci STKE. 2004;2004(216):re2. doi: 10.1126/stke.2162004re2. [DOI] [PubMed] [Google Scholar]
- Dermott JM, Reddy MR, Onesime D, Reddy EP, Dhanasekaran N. Oncogenic mutant of Galpha12 stimulates cell proliferation through cycloxygenase-2 signaling pathway. Oncogene. 1999;18(51):7185–7189. doi: 10.1038/sj.onc.1203345. [DOI] [PubMed] [Google Scholar]
- Dermott JM, Ha JH, Lee CH, Dhanasekaran N. Differential regulation of Jun N-terminal kinase and p38MAP kinase by Galpha12. Oncogene. 2004;23(1):226–232. doi: 10.1038/sj.onc.1207009. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran N, Tsim ST, Dermott JM, Onesime D. Regulation of cell proliferation by G proteins. Oncogene. 1998;17:1383–1394. doi: 10.1038/sj.onc.1202242. 11 Reviews. [DOI] [PubMed] [Google Scholar]
- Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276(47):44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- Dolezalova H, Shankar G, Huang MC, Bikle DD, Goetzl EJ. Biochemical regulation of breast cancer cell expression of S1P2 (Edg-5) and S1P3 (Edg-3) G protein-coupled receptors for sphingosine 1-phosphate. J Cell Biochem. 2003;88(4):732–743. doi: 10.1002/jcb.10394. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4(8):909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fields TA, Casey PJ. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem J. 1997;321:561–571. doi: 10.1042/bj3210561. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81(5):682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Fromm C, Coso OA, Montaner S, Xu N, Gutkind JS. The small GTP-binding protein Rho links G protein-coupled receptors and Galpha12 to the serum response element and to cellular transformation. Proc Natl Acad Sci USA. 1997;94(19):10098–10103. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274(9):5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000a;485(2–3):183–188. doi: 10.1016/s0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Marinissen MJ, Chiariello M, Gutkind JS. Signaling from G protein-coupled receptors to ERK5/Big MAPK 1 involves Galpha q and Galpha 12/13 families of heterotrimeric G proteins. Evidence for the existence of a novel Ras AND Rho-independent pathway. J Biol Chem. 2000b;275(28):21730–21736. doi: 10.1074/jbc.M002410200. [DOI] [PubMed] [Google Scholar]
- Gohla A, Harhammer R, Schultz G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J Biol Chem. 1998;273(8):4653–4659. doi: 10.1074/jbc.273.8.4653. [DOI] [PubMed] [Google Scholar]
- Gohla A, Offermanns S, Wilkie TM, Schultz G. Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J Biol Chem. 1999;274(25):17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87(3):221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26(22):3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- Goulimari P, Kitzing TM, Knieling H, Brandt DT, Offermanns S, Grosse R. Galpha12/13 is essential for directed cell migration and localized Rho-Dia1 function. J Biol Chem. 2005;280(51):42242–42251. doi: 10.1074/jbc.M508690200. [DOI] [PubMed] [Google Scholar]
- Goulimari P, Knieling H, Engel U, Grosse R. LARG and mDia1 Link G{alpha}12/13 to Cell Polarity and Microtubule Dynamics. Mol Biol Cell. 2008;19(1):30–40. doi: 10.1091/mbc.E06-11-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JL, Muller S, Mancino V, Offermanns S, Simon MI. Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc Natl Acad Sci USA. 2002;99(14):9352–9357. doi: 10.1073/pnas.102291599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280(5372):2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Henrikson KP, Salazar SL, Fenton JW, II, Pentecost BT. Role of thrombin receptor in breast cancer invasiveness. Br J Cancer. 1999;79(3–4):401–406. doi: 10.1038/sj.bjc.6690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch E, Huang J, Grider JR, Murthy KS. Gq/G13 signaling by ET-1 in smooth muscle: MYPT1 phosphorylation via ETA and CPI-17 dephosphorylation via ETB. Am J Physiol Cell Physiol. 2004;287(5):C1209–C1218. doi: 10.1152/ajpcell.00198.2004. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008;283(21):14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116(6):1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Davis RJ, Malbon CC. c-Jun amino-terminal kinase is regulated by Galpha12/Galpha13 and obligate for differentiation of P19 embryonal carcinoma cells by retinoic acid. J Biol Chem. 1997;272(39):24468–24474. doi: 10.1074/jbc.272.39.24468. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wu D, Simon MI. The transforming activity of activated G alpha 12. FEBS Lett. 1993;330(3):319–322. doi: 10.1016/0014-5793(93)80896-3. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ma W, Wan Y, Kozasa T, Hattori S, Huang XY. The G protein G alpha12 stimulates Bruton's tyrosine kinase and a rasGAP through a conserved PH/BM domain. Nature. 1998;395(6704):808–813. doi: 10.1038/27454. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Seasholtz TM, Waheed AA, Kreutz B, Suzuki N, Kozasa T, et al. RGS16 inhibits signalling through the G alpha 13-Rho axis. Nat Cell Biol. 2003;5(12):1095–1103. doi: 10.1038/ncb1065. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9(2):240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kashef K, Lee CM, Ha JH, Reddy EP, Dhanasekaran DN. JNK-interacting leucine zipper protein is a novel scaffolding protein in the Galpha13 signaling pathway. Biochemistry. 2005;44(43):14090–14096. doi: 10.1021/bi050604l. [DOI] [PubMed] [Google Scholar]
- Kashef K, Xu H, Reddy EP, Dhanasekaran DN. Endodermal differentiation of murine embryonic carcinoma cells by retinoic acid requires JLP, a JNK-scaffolding protein. J Cell Biochem. 2006;98(4):715–722. doi: 10.1002/jcb.20930. [DOI] [PubMed] [Google Scholar]
- Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2006a;103(21):8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem. 2006b;281(36):26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- Kelly P, Casey PJ, Meigs TE. Biologic functions of the G12 subfamily of heterotrimeric g proteins: growth, migration, and metastasis. Biochemistry. 2007;46(23):6677–6687. doi: 10.1021/bi700235f. [DOI] [PubMed] [Google Scholar]
- Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, et al. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21(12):1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280(5372):2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Kumar RN, Ha JH, Radhakrishnan R, Dhanasekaran DN. Transactivation of platelet-derived growth factor receptor alpha by the GTPase-deficient activated mutant of Galpha12. Mol Cell Biol. 2006a;26(1):50–62. doi: 10.1128/MCB.26.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RN, Shore SK, Dhanasekaran N. Neoplastic transformation by the gep oncogene, Galpha12, involves signaling by STAT3. Oncogene. 2006b;25(6):899–906. doi: 10.1038/sj.onc.1209132. [DOI] [PubMed] [Google Scholar]
- Kurose H. Galpha12 and Galpha13 as key regulatory mediator in signal transduction. Life Sci. 2003;74(2–3):155–161. doi: 10.1016/j.lfs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kutuzov MA, Andreeva AV, Voyno-Yasenetskaya TA. Regulation of apoptosis signal-regulating kinase 1 degradation by G alpha13. FASEB J. 2007;21(13):3727–3736. doi: 10.1096/fj.06-8029com. [DOI] [PubMed] [Google Scholar]
- Lee YN, Malbon CC, Wang HY. G alpha 13 signals via p115RhoGEF cascades regulating JNK1 and primitive endoderm formation. J Biol Chem. 2004;279(52):54896–54904. doi: 10.1074/jbc.M407581200. [DOI] [PubMed] [Google Scholar]
- Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 2008;28(22):5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Sepich DS, Chen S, Topczewski J, Yin C, Solnica-Krezel L, et al. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J Cell Biol. 2005;169(5):777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Voyno-Yasenetskaya TA. Radixin stimulates Rac1 and Ca2+/calmodulin-dependent kinase, CaMKII: cross-talk with Galpha13 signaling. J Biol Chem. 2005;280(47):39042–39049. doi: 10.1074/jbc.M504341200. [DOI] [PubMed] [Google Scholar]
- McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280(26):25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- Malchinkhuu E, Sato K, Maehama T, Mogi C, Tomura H, Ishiuchi S, et al. S1P(2) receptors mediate inhibition of glioma cell migration through Rho signaling pathways independent of PTEN. Biochem Biophys Res Commun. 2008;366(4):963–968. doi: 10.1016/j.bbrc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Mao J, Xie W, Yuan H, Simon MI, Mano H, Wu D. Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by Galpha12/13. EMBO J. 1998;17(19):5638–5646. doi: 10.1093/emboj/17.19.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22(7):368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Servitja JM, Offermanns S, Simon MI, Gutkind JS. Thrombin protease-activated receptor-1 signals through Gq- and G13-initiated MAPK cascades regulating c-Jun expression to induce cell transformation. J Biol Chem. 2003;278(47):46814–46825. doi: 10.1074/jbc.M305709200. [DOI] [PubMed] [Google Scholar]
- Martin CB, Mahon GM, Klinger MB, Kay RJ, Symons M, Der CJ, et al. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene. 2001;20(16):1953–1963. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Nishida M, Sugimoto Y, Tanabe S, Turner JH, Kozasa T, et al. Galpha(12/13) mediates alpha(1)-adrenergic receptor-induced cardiac hypertrophy. Circ Res. 2002;91(10):961–969. doi: 10.1161/01.res.0000043282.39776.7c. [DOI] [PubMed] [Google Scholar]
- Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta -catenin release. Proc Natl Acad Sci USA. 2001;98(2):519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs TE, Fedor-Chaiken M, Kaplan DD, Brackenbury R, Casey PJ. Galpha12 and Galpha13 negatively regulate the adhesive functions of cadherin. J Biol Chem. 2002;277(27):24594–24600. doi: 10.1074/jbc.M201984200. [DOI] [PubMed] [Google Scholar]
- Meigs TE, Juneja J, DeMarco CT, Stemmle LN, Kaplan DD, Casey PJ. Selective uncoupling of G alpha 12 from Rho-mediated signaling. J Biol Chem. 2005;280(18):18049–18055. doi: 10.1074/jbc.M500445200. [DOI] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Denker BM. Zonula occludens-1 is a scaffolding protein for signaling molecules. Galpha(12) directly binds to the Src homology 3 domain and regulates paracellular permeability in epithelial cells. J Biol Chem. 2002;277(28):24855–24858. doi: 10.1074/jbc.C200240200. [DOI] [PubMed] [Google Scholar]
- Milligan G, Mullaney I, Mitchell FM. Immunological identification of the alpha subunit of G13, a novel guanine nucleotide binding protein. FEBS Lett. 1992;297(1–2):186–188. doi: 10.1016/0014-5793(92)80357-m. [DOI] [PubMed] [Google Scholar]
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3(8):582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9(11):1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- Moers A, Nurnberg A, Goebbels S, Wettschureck N, Offermanns S. Galpha12/Galpha13 deficiency causes localized overmigration of neurons in the developing cerebral and cerebellar cortices. Mol Cell Biol. 2008;28(5):1480–1488. doi: 10.1128/MCB.00651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratz C, Kang VH, Druey KM, Shi CS, Scheschonka A, Murphy PM, et al. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J Immunol. 2000;164(4):1829–1838. doi: 10.4049/jimmunol.164.4.1829. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nagao M, Kaziro Y, Itoh H. The Src family tyrosine kinase is involved in Rho-dependent activation of c-Jun N-terminal kinase by Galpha12. Oncogene. 1999;18(31):4425–4434. doi: 10.1038/sj.onc.1202832. [DOI] [PubMed] [Google Scholar]
- Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol. 2004;14(20):1822–1826. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Niu J, Vaiskunaite R, Suzuki N, Kozasa T, Carr DW, Dulin N, et al. Interaction of heterotrimeric G13 protein with an A-kinase-anchoring protein 110 (AKAP110) mediates cAMP-independent PKA activation. Curr Biol. 2001;11(21):1686–1690. doi: 10.1016/s0960-9822(01)00530-9. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Offermanns S. In vivo functions of heterotrimeric G-proteins: studies in Galpha-deficient mice. Oncogene. 2001;20(13):1635–1642. doi: 10.1038/sj.onc.1204189. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science. 1997;275(5299):533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. How do receptors activate G proteins? Adv Protein Chem. 2007;74:67–93. doi: 10.1016/S0065-3233(07)74002-0. [DOI] [PubMed] [Google Scholar]
- Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991;64(2):447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Radhika V, Dhanasekaran N. Transforming G proteins. Oncogene. 2001;20(13):1607–1614. doi: 10.1038/sj.onc.1204274. [DOI] [PubMed] [Google Scholar]
- Radhika V, Hee Ha J, Jayaraman M, Tsim ST, Dhanasekaran N. Mitogenic signaling by lysophosphatidic acid (LPA) involves Galpha12. Oncogene. 2005;24(28):4597–4603. doi: 10.1038/sj.onc.1208665. [DOI] [PubMed] [Google Scholar]
- Radhika V, Onesime D, Ha JH, Dhanasekaran N. Galpha13 stimulates cell migration through cortactin-interacting protein Hax-1. J Biol Chem. 2004;279(47):49406–49413. doi: 10.1074/jbc.M408836200. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265(1):23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Rieken S, Sassmann A, Herroeder S, Wallenwein B, Moers A, Offermanns S, et al. G12/G13 family G proteins regulate marginal zone B cell maturation, migration, and polarization. J Immunol. 2006;177(5):2985–2993. doi: 10.4049/jimmunol.177.5.2985. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci. 2005;26(3):146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Kobilka BK. G protein-coupled receptors: functional and mechanistic insights through altered gene expression. Physiol Rev. 1998;78(1):35–52. doi: 10.1152/physrev.1998.78.1.35. [DOI] [PubMed] [Google Scholar]
- Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, et al. Essential role for Galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci USA. 2005;102(23):8281–8286. doi: 10.1073/pnas.0503326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath E, Negoro H, Beaudry S, Paniagua M, Angelow S, Shah J, et al. Galpha12 regulates protein interactions within the MDCK cell tight junction and inhibits tight-junction assembly. J Cell Sci. 2008;121:814–824. doi: 10.1242/jcs.014878. Pt 6. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY. The G protein G alpha(13) is required for growth factor-induced cell migration. Dev Cell. 2006;10(6):707–718. doi: 10.1016/j.devcel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Shi CS, Sinnarajah S, Cho H, Kozasa T, Kehrl JH. G13alpha-mediated PYK2 activation. PYK2 is a mediator of G13alpha -induced serum response element-dependent transcription. J Biol Chem. 2000;275(32):24470–24476. doi: 10.1074/jbc.M908449199. [DOI] [PubMed] [Google Scholar]
- Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2(7):395–402. [PubMed] [Google Scholar]
- Slice LW, Walsh JH, Rozengurt E. Galpha(13) stimulates Rho-dependent activation of the cyclooxygenase-2 promoter. J Biol Chem. 1999;274(39):27562–27566. doi: 10.1074/jbc.274.39.27562. [DOI] [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64(23):8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15(6):R213–R228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Spicher K, Kalkbrenner F, Zobel A, Harhammer R, Nurnberg B, Soling A, et al. G12 and G13 alpha-subunits are immunochemically detectable in most membranes of various mammalian cells and tissues. Biochem Biophys Res Commun. 1994;198(3):906–914. doi: 10.1006/bbrc.1994.1129. [DOI] [PubMed] [Google Scholar]
- Stemmle LN, Fields TA, Casey PJ. The regulator of G protein signaling domain of axin selectively interacts with Galpha12 but not Galpha13. Mol Pharmacol. 2006;70(4):1461–1468. doi: 10.1124/mol.106.023705. [DOI] [PubMed] [Google Scholar]
- Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77(1):147–152. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, et al. G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc Res. 2008;79(4):689–697. doi: 10.1093/cvr/cvn118. [DOI] [PubMed] [Google Scholar]
- Tan W, Martin D, Gutkind JS. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J Biol Chem. 2006;281(51):39542–39549. doi: 10.1074/jbc.M609062200. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, Adarichev V, Furthmayr H, Kozasa T, Gudkov A, Voyno-Yasenetskaya TA. Conformational activation of radixin by G13 protein alpha subunit. J Biol Chem. 2000;275(34):26206–26212. doi: 10.1074/jbc.M001863200. [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, Kozasa T, Voyno-Yasenetskaya TA. Interaction between the G alpha subunit of heterotrimeric G(12) protein and Hsp90 is required for G alpha(12) signaling. J Biol Chem. 2001;276(49):46088–46093. doi: 10.1074/jbc.M108711200. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5(8):626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- Vara Prasad MV, Dhanasekaran N. GTPase deficient mutant of G(alpha13) regulates the expression of Egr-1 through the small GTPase Rho. Oncogene. 1999;18(8):1639–1642. doi: 10.1038/sj.onc.1202461. [DOI] [PubMed] [Google Scholar]
- Vara Prasad MV, Shore SK, Dhanasekaran N. Activated mutant of G alpha 13 induces Egr-1, c-fos, and transformation in NIH 3T3 cells. Oncogene. 1994;9(8):2425–2429. [PubMed] [Google Scholar]
- Voyno-Yasenetskaya TA, Pace AM, Bourne HR. Mutant alpha subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene. 1994;9(9):2559–2565. [PubMed] [Google Scholar]
- Voyno-Yasenetskaya TA, Faure MP, Ahn NG, Bourne HR. Galpha12 and Galpha13 regulate extracellular signal-regulated kinase and c-Jun kinase pathways by different mechanisms in COS-7 cells. J Biol Chem. 1996;271(35):21081–21087. doi: 10.1074/jbc.271.35.21081. [DOI] [PubMed] [Google Scholar]
- Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14(1):64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114(2):201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Xu N, Bradley L, Ambdukar I, Gutkind JS. A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad Sci USA. 1993;90(14):6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Mori K, Negishi M. Galpha(12) and Galpha(13) interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol. 2002;12(15):1353–1358. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- Yanamadala V, Negoro H, Gunaratnam L, Kong T, Denker BM. Galpha12 stimulates apoptosis in epithelial cells through JNK1-mediated Bcl-2 degradation and up-regulation of IkappaBalpha. J Biol Chem. 2007;282(33):24352–24363. doi: 10.1074/jbc.M702804200. [DOI] [PubMed] [Google Scholar]
- Zhu D, Kosik KS, Meigs TE, Yanamadala V, Denker BM. Galpha12 directly interacts with PP2A: evidence FOR Galpha12-stimulated PP2A phosphatase activity and dephosphorylation of microtubule-associated protein, tau. J Biol Chem. 2004;279(53):54983–54986. doi: 10.1074/jbc.C400508200. [DOI] [PubMed] [Google Scholar]
- Zhu D, Tate RI, Ruediger R, Meigs TE, Denker BM. Domains necessary for Galpha12 binding and stimulation of protein phosphatase-2A (PP2A): Is Galpha12 a novel regulatory subunit of PP2A? Mol Pharmacol. 2007;71(5):1268–1276. doi: 10.1124/mol.106.033555. [DOI] [PubMed] [Google Scholar]


