Abstract
It has been demonstrated that the degree of agonist-induced desensitization of the β2-adrenoceptor is related to agonist efficacy (strength of signalling), whereby high-efficacy agonists (e.g. formoterol) cause more phosphorylation and internalization of the receptor than low-efficacy agonists (e.g. salmeterol). These early studies, however, used a protocol where agonists were matched for receptor occupancy rather than functional effect. In this issue of the BJP, Duringer and colleagues have extended these studies to compare the ability of agonists to cause desensitization at equi-effective (cAMP signalling) concentrations rather than equal occupancy. Their data and conclusions are quite different from those previously described. After prolonged exposure, all the agonists caused a similar degree of desensitization, whereas a pulse protocol uncovered a greater loss of responsiveness with the low-efficacy ligands. This is consistent with the notion that high-efficacy agonists have ‘spare receptors’, and are therefore less sensitive to loss of receptors through desensitization. It also reflects experience in the clinic, where both formoterol and salmeterol show a similar early decline in bronchoprotection, after which their effects remain stable. These findings challenge the notion that high-efficacy ligands always cause more functional desensitization.
Keywords: drug receptor mechanisms, molecular pharmacology, pulmonary and respiratory pharmacology, adrenaline/noradrenaline, receptors, antiasthma/copd drugs
Inhaled β2-adrenoceptor agonists are highly effective bronchodilating drugs used in the treatment of chronic obstructive pulmonary disease and asthma, where they are often combined with inhaled corticosteroids (Giembycz et al., 2008; Knox and Mortimer, 2008). In patients with mild disease, short-acting agonists are recommended, but for patients in whom these are not effective, longer-acting β2-adrenoceptor agonists (LABAs) have been developed that more effectively maintain airway tone over a longer period of time (Sutherland, 2004). One risk of long-term exposure of a G protein-coupled receptor (GPCR) agonist is desensitization of the response, most commonly via receptor phosphorylation and internalization (Johnson, 2006). Two LABAs are currently available in the clinic, formoterol and salmeterol. Pharmacologically, the clearest distinguishing feature between these drugs is the extent to which they activate the receptor, termed intrinsic efficacy. Formoterol demonstrates high intrinsic efficacy when stimulating cyclic adenosine monophosphate (cAMP) generation, whereas salmeterol has a much lower intrinsic efficacy, appearing as a partial agonist in all but the most highly expressed recombinant systems (McDonnell et al., 1998). This distinction has led several groups to investigate the influence of efficacy on desensitization of the β2-adrenoceptor.
Early studies showed a clear relationship between efficacy and desensitization, with the partial agonist salmeterol causing less receptor phosphorylation and internalization than the full agonist formoterol (January et al., 1997; 1998;). This relationship between efficacy and desensitization has now become a widely accepted phenomenon in GPCR biology, and the statement that partial agonists cause less desensitization is often quoted in the literature (Johnson, 1998; Clark et al., 1999; Hanania et al., 2002; Bleecker et al., 2006; Moore et al., 2007).
It is important, however, to note the design of the experiments that resulted in these conclusions. Agonist concentrations were chosen based on binding affinity so that the receptor occupancy was matched for each ligand at around 80%. Low-efficacy ligands are less able to activate the receptor and may not be sufficient to generate a full response, even when bound to all available receptors. In contrast, high-efficacy agonists may only need to occupy a small percentage of receptors to generate a full response, therefore having ‘spare receptors’ (van Rossum and Ariens, 1962). By choosing a high level of occupancy for each ligand, it is perhaps not surprising that the high-efficacy ligands were more effective at causing receptor phosphorylation and internalization, as by definition, they would result in a higher number of activated receptors than the partial agonists. In effect, this means that any differences in receptor reserve for the agonists were negated in these studies. Perhaps more importantly, doses given to patients in the clinic are not chosen based on receptor occupancy, but rather on the functional effect, which is directly related to intrinsic efficacy.
In the current issue of the BJP, Düringer et al. (2009) have addressed this problem by comparing the desensitization profile at equi-effective concentrations rather than at equal occupancy, more closely mimicking the clinical situation. Importantly, their first finding was that after a 24 h continuous exposure, all agonists desensitized the response to a subsequent formoterol challenge to the same degree, irrespective of their intrinsic efficacy. In an effort to simulate the in vivo clearance of the drugs, the authors designed an alternative ‘pulse’ protocol where the agonists were added for 1 h, after which the cells were washed and left for a further 23 h prior to stimulation by formoterol. Using this protocol, differences between the agonists emerged, but instead of the high-efficacy ligands causing a greater reduction to the response elicited by a subsequent formoterol challenge, in general, the lower-efficacy agonists caused a greater loss of responsiveness. As discussed by the authors, this is likely to be due to the reduced wash out of the more lipophilic compounds, but there were exceptions to this pattern. Perhaps most strikingly, the novel ultra-LABA indacaterol that exhibits a mid-range efficacy (Battram et al., 2006) induced much less desensitization than would be expected from its large degree of retention in the cells.
So, with these different experimental designs giving such contrasting results, which is more representative of the clinical situation?
The loss of responsiveness to chronic LABA therapy has been widely studied in the clinic. In general, there appears to be rapid desensitization of the bronchoprotection afforded against an external challenge, be it indirect (exercise or allergen) or direct (methacholine or histamine) (Lipworth, 1997). This is likely due to tachyphylaxis of receptor signalling on inflammatory cells, particularly mast cells (Scola et al., 2004; 2009;). The bronchodilator properties of β2 agonists, however, are much more resilient to tolerance, demonstrating efficacy that is normally stable after the first few days (Haney and Hancox, 2005). This is perhaps due to reduced levels of key proteins required in the regulation of receptor signalling in airway smooth muscle cells (McGraw and Liggett, 1997), and/or a higher initial receptor reserve in these cells (Chong and Peachell, 1999). Conclusions from clinical studies investigating desensitization of high- and low-efficacy ligands are often contradictory, complicated by differences in design and interpretation. Several studies have shown that while formoterol induces desensitization to both bronchoprotection (Aziz et al., 1998) and bronchodilation (Newnham et al., 1995), salmeterol does not (Ullman et al., 1990; Hanania et al., 2005). Other studies show the opposite, with no tachyphylaxis to the brochoprotective (FitzGerald et al., 1999) or bronchodilator (Steffensen et al., 1995; Lipworth et al., 1998) effects of formoterol, and a clear loss of responsiveness to salmeterol for both bronchoprotection (Bhagat et al., 1995; Booth et al., 1996; Drotar et al., 1998; Giannini et al., 2001) and bronchodilation (Donohue et al., 2002; 2003; Tsagaraki et al., 2006).
Should we be surprised that all β2 agonists appear to induce a small degree of tolerance, irrespective of efficacy?
As discussed above, choosing agonist concentrations based on similar occupancy levels is not representative of the clinical situation. While Düringer et al. (2009) have avoided this issue by choosing equi-effective concentrations, they determined the degree of desensitization by re-challenging after each agonist incubation with the same high-efficacy agonist formoterol. This was presumably done as the subsequent secondary response to formoterol was robust, but it does not necessarily mimic the situation in the clinic where the same drug is administered in a chronic setting. This is important because although low-efficacy agonists may cause less receptor desensitization at equal occupancy, they require more receptors to generate a subsequent response so will be more sensitive to loss of functional receptors (Kenakin, 2006). High-efficacy agonists, in contrast, may cause a greater loss of receptors, but are more tolerant to this, as they have ‘spare receptors’, resulting in a loss in potency but not necessarily maximal effect. This is nicely exemplified by classic experiments with alkylating agents that irreversibly block the agonist binding site, permanently preventing them from being activated (Fisher and Snider, 1987).
The balance between receptor desensitization and spare receptors can be demonstrated using the Operational Model of receptor function (Black and Leff, 1983), shown in equation (1).
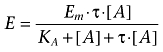 |
(1) |
In this model, the term τ is used as a composite for efficacy of an agonist in a particular tissue and can be further broken down to describe the influence of intrinsic efficacy (KE) and receptor concentration in the particular system ([RT]), as shown in equation (2).
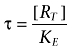 |
(2) |
Figure 1A,B shows a series of simulated concentration-response curves using the Operational Model. The two agonists have the same binding affinity (KA= 1 µM) but different efficacies [KE= 100 (low-efficacy agonist) in A or 1 (high-efficacy agonist) in B]. The family of curves illustrate the potency and maximal response at different receptor levels (RT). As can be seen, mimicking desensitization by reducing the receptor number reduces the response to both agonists, but to different degrees, with the partial (low-efficacy) agonist being more sensitive to loss of receptor than the high-efficacy agonist. This is shown more clearly in Figure 1C, where the response to an EC90 concentration for each agonist (determined at RT= 100, i.e. no loss of receptor) has been plotted against percentage of receptors lost. This mirrors the situation in the clinic where the dose is maintained for some time after the first administration. As can be seen, the response of an EC90 concentration reduces more rapidly with the partial agonist than with the high-efficacy agonist. For example, in order to achieve a 75% response, the partial agonist can only tolerate a loss of 26% receptors, whereas the high-efficacy agonist can lose up to 66% receptors. This difference is even more marked when one considers the effect on maximal response achievable by each agonist (Figure 1D). The maximal achievable response to the partial agonist reduces immediately upon loss of receptors, but the high-efficacy agonist can tolerate up to 90% loss of receptor before any effect is observed on the maximal response to the ligand. Hence, dose escalation with the high-efficacy agonist will result in a maintained response, even if 90% receptors are lost from the system. Increasing the dose of the low-efficacy agonist will not, however, gain any additional effect. While this is only a simulation, it demonstrates the balance between having sufficient efficacy to have spare receptors, but not so much that you drive excessive desensitization. So although there may be an optimal efficacy for airway smooth muscle cells, where the balance between internalization and receptor reserve is sufficient to limit functional tachyphylaxis, it is likely that the degree of desensitization and number of spare receptors will largely cancel each other out.
Figure 1.
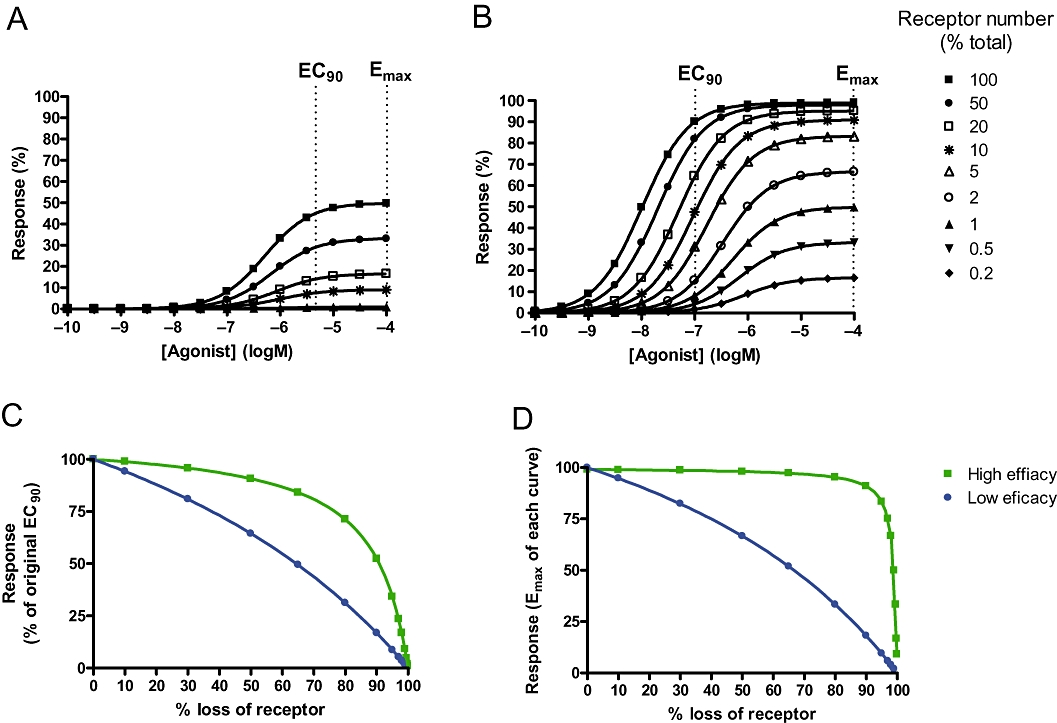
Simulating the loss of response to agonists of different efficacy as receptors are removed from the system. The operational model was used, KA was fixed at 1 µM and RT was reduced from 100 to 0.1. (A) Simulates a low efficacy agonist, with KE= 100. (B) Simulates a high-efficacy agonist with KE= 1. (C) The effect of an EC90 of each agonist (calculated where RT= 100) at different receptor numbers. Data were normalized to percentage of the response at the EC90 where RT= 100. (D) The influence of receptor loss on Emax.
There is growing evidence that some agonists are able to stabilize distinct receptor conformations that have different efficacies for downstream effectors (Kenakin, 2005). For example, an agonist may stabilize the receptor conformation that activates a G protein effector, but not the receptor form that is subject to phosphorylation or β-arrestin recruitment, critical steps in the desensitization process of the receptor. This is an attractive concept, as it opens up the potential for generating drugs that fully activate the required pathways but are not subject to the development of tolerance. It has recently been suggested that salmeterol acts in such a manner, being unable to induce the receptor state that recruits β-arrestin (Moore et al., 2007). Whether this represents true agonist-directed trafficking of receptor stimulus or just highlights the different degrees of receptor reserve for each pathway in a given tissue has yet to be proved, but until then, these new data from Düringer et al. (2009) suggest that high-efficacy agonists do not necessarily cause more functional desensitization as was once believed.
References
- Aziz I, Tan KS, Hall IP, Devlin MM, Lipworth BJ. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once-daily formoterol. Eur Respir J. 1998;12(3):580–584. doi: 10.1183/09031936.98.12030580. [DOI] [PubMed] [Google Scholar]
- Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Farr D, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317(2):762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest. 1995;108(5):1235–1239. doi: 10.1378/chest.108.5.1235. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Bleecker ER, Yancey SW, Baitinger LA, Edwards LD, Klotsman M, Anderson WH, et al. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006;118(4):809–816. doi: 10.1016/j.jaci.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Booth H, Bish R, Walters J, Whitehead F, Walters EH. Salmeterol tachyphylaxis in steroid treated asthmatic subjects. Thorax. 1996;51(11):1100–1104. doi: 10.1136/thx.51.11.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LK, Peachell PT. Beta-adrenoceptor reserve in human lung: a comparison between airway smooth muscle and mast cells. Eur J Pharmacol. 1999;378(1):115–122. doi: 10.1016/s0014-2999(99)00425-2. [DOI] [PubMed] [Google Scholar]
- Clark RB, Knoll BJ, Barber R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharmacol Sci. 1999;20(7):279–286. doi: 10.1016/s0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, Jr, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122(1):47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respir Med. 2003;97(9):1014–1020. doi: 10.1016/s0954-6111(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Drotar DE, Davis EE, Cockcroft DW. Tolerance to the bronchoprotective effect of salmeterol 12 h after starting twice daily treatment. Ann Allergy Asthma Immunol. 1998;80(1):31–34. doi: 10.1016/S1081-1206(10)62935-3. [DOI] [PubMed] [Google Scholar]
- Düringer C, Grundström G, Gürcan E, Dainty IA, Lawson M, Korn SH, et al. Agonist-specific patterns of (2-adrenoceptor responses in human airway cells during prolonged exposure. Br J Pharmacol. 2009;158:169–179. doi: 10.1111/j.1476-5381.2009.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SK, Snider RM. Differential receptor occupancy requirements for muscarinic cholinergic stimulation of inositol lipid hydrolysis in brain and in neuroblastomas. Mol Pharmacol. 1987;32:81–90. [PubMed] [Google Scholar]
- FitzGerald JM, Chapman KR, Della Cioppa G, Stubbing D, Fairbarn MS, Till MD, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. The Canadian FO/OD1 Study Group. J Allergy Clin Immunol. 1999;103(3)(1):427–435. doi: 10.1016/s0091-6749(99)70467-7. Pt. [DOI] [PubMed] [Google Scholar]
- Giannini D, Di Franco A, Bacci E, Dente FL, Bartoli ML, Vagaggini B, et al. Tolerance to the protective effect of salmeterol on allergen challenge can be partially restored by the withdrawal of salmeterol regular treatment. Chest. 2001;119:1671–1675. doi: 10.1378/chest.119.6.1671. [DOI] [PubMed] [Google Scholar]
- Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting beta2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol. 2008;153:1090–1104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania NA, Sharafkhaneh A, Barber R, Dickey BF. Beta-agonist intrinsic efficacy: measurement and clinical significance. Am J Respir Crit Care Med. 2002;165:1353–1358. doi: 10.1164/rccm.2109060. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Kalberg C, Yates J, Emmett A, Horstman D, Knobil K. The bronchodilator response to salmeterol is maintained with regular, long-term use in patients with COPD. Pulm Pharmacol Ther. 2005;18(1):19–22. doi: 10.1016/j.pupt.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Haney S, Hancox RJ. Rapid onset of tolerance to beta-agonist bronchodilation. Respir Med. 2005;99(5):566–571. doi: 10.1016/j.rmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, et al. beta2-adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem. 1997;272(38):23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- January B, Seibold A, Allal C, Whaley BS, Knoll BJ, Moore RH, et al. Salmeterol-induced desensitization, internalization and phosphorylation of the human beta2-adrenoceptor. Br J Pharmacol. 1998;123(4):701–711. doi: 10.1038/sj.bjp.0701658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med. 1998;158(5)(3):S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. Pt. [DOI] [PubMed] [Google Scholar]
- Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol. 2006;117(1):18–24. doi: 10.1016/j.jaci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4(11):919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Kenakin T. A Pharmacological Primer. Theory, Application and Methods. 2nd edn. Burlington, MA: Elsevier Inc; 2006. [Google Scholar]
- Knox AJ, Mortimer K. Combining inhaled glucocorticoids and long acting beta2-adrenoceptor agonists in asthma and COPD. Br J Pharmacol. 2008;153:1085–1086. doi: 10.1038/bjp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. Am J Med. 1998;104(5):431–438. doi: 10.1016/s0002-9343(98)00086-2. [DOI] [PubMed] [Google Scholar]
- Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists. Is there cause for concern? Drug Saf. 1997;16(5):295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- McDonnell J, Latif ML, Rees ES, Bevan NJ, Hill SJ. Influence of receptor number on the stimulation by salmeterol of gene transcription in CHO-K1 cells transfected with the human beta2-adrenoceptor. Br J Pharmacol. 1998;125(4):717–726. doi: 10.1038/sj.bjp.0702139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw DW, Liggett SB. Heterogeneity in beta-adrenergic receptor kinase expression in the lung accounts for cell-specific desensitization of the beta2-adrenergic receptor. J Biol Chem. 1997;272(11):7338–7344. doi: 10.1074/jbc.272.11.7338. [DOI] [PubMed] [Google Scholar]
- Moore RH, Millman EE, Godines V, Hanania NA, Tran TM, Peng H, et al. Salmeterol stimulation dissociates beta2-adrenergic receptor phosphorylation and internalization. Am J Respir Cell Mol Biol. 2007;36(2):254–261. doi: 10.1165/rcmb.2006-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50(5):497–504. doi: 10.1136/thx.50.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum J, Ariens EJ. Receptor-reserve and threshold-phenomena. II. Theories on drug-action and a quantitative approach to spare receptors and threshold values. Arch Int Pharmacodyn Ther. 1962;136:385–413. [PubMed] [Google Scholar]
- Scola AM, Chong LK, Suvarna SK, Chess-Williams R, Peachell PT. Desensitisation of mast cell beta2-adrenoceptor-mediated responses by salmeterol and formoterol. Br J Pharmacol. 2004;141(1):163–171. doi: 10.1038/sj.bjp.0705599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola AM, Loxham M, Charlton SJ, Peachell PT. The long-acting beta2-adrenoceptor agonist, indacaterol, inhibits IgE-dependent responses of human lung mast cells. Br J Pharmacol. 2009;158:267–276. doi: 10.1111/j.1476-5381.2009.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen I, Faurschou P, Riska H, Rostrup J, Wegener T. Inhaled formoterol dry powder in the treatment of patients with reversible obstructive airway disease. A 3-month, placebo-controlled comparison of the efficacy and safety of formoterol and salbutamol, followed by a 12-month trial with formoterol. Allergy. 1995;50(8):657–663. doi: 10.1111/j.1398-9995.1995.tb02582.x. [DOI] [PubMed] [Google Scholar]
- Sutherland ER. Outpatient treatment of chronic obstructive pulmonary disease: comparisons with asthma. J Allergy Clin Immunol. 2004;114:715–724. doi: 10.1016/j.jaci.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Tsagaraki V, Amfilochiou A, Markantonis SL. Evidence of tachyphylaxis associated with salmeterol treatment of chronic obstructive pulmonary disease patients. Int J Clin Pract. 2006;60(4):415–421. doi: 10.1111/j.1368-5031.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- Ullman A, Hedner J, Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. Am Rev Respir Dis. 1990;142(3):571–575. doi: 10.1164/ajrccm/142.3.571. [DOI] [PubMed] [Google Scholar]


