Abstract
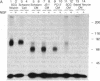
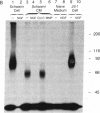
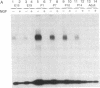
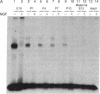
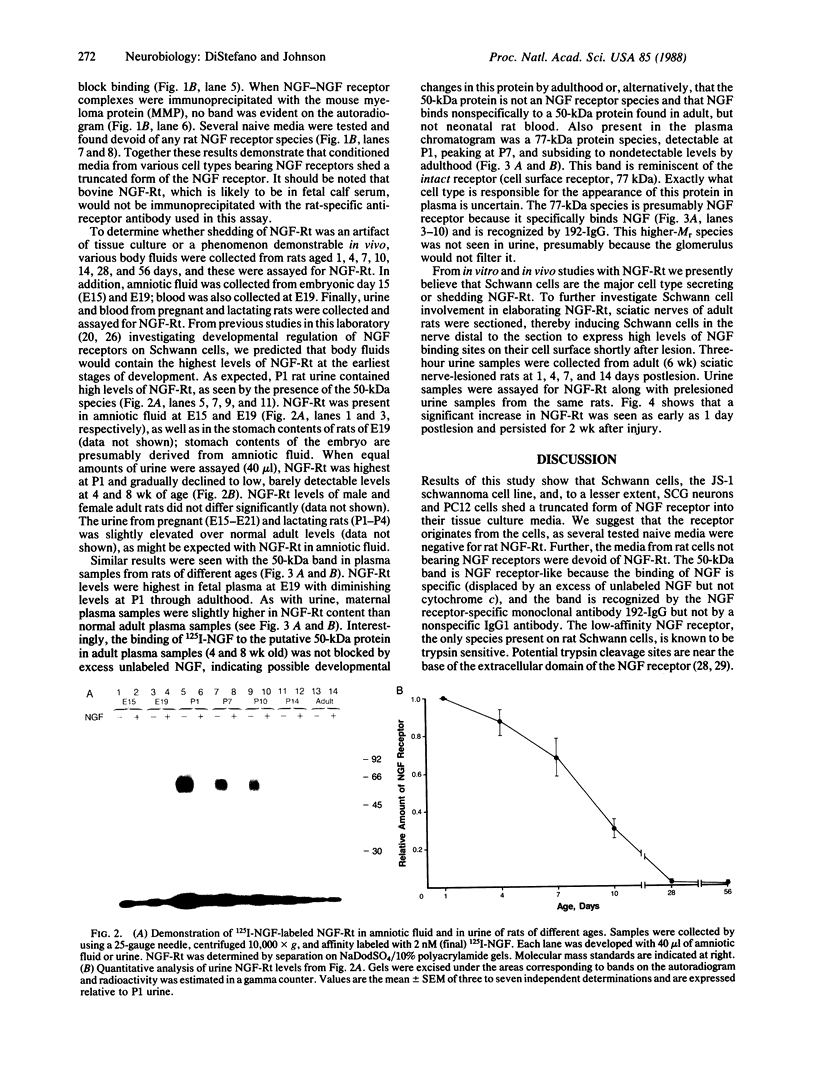
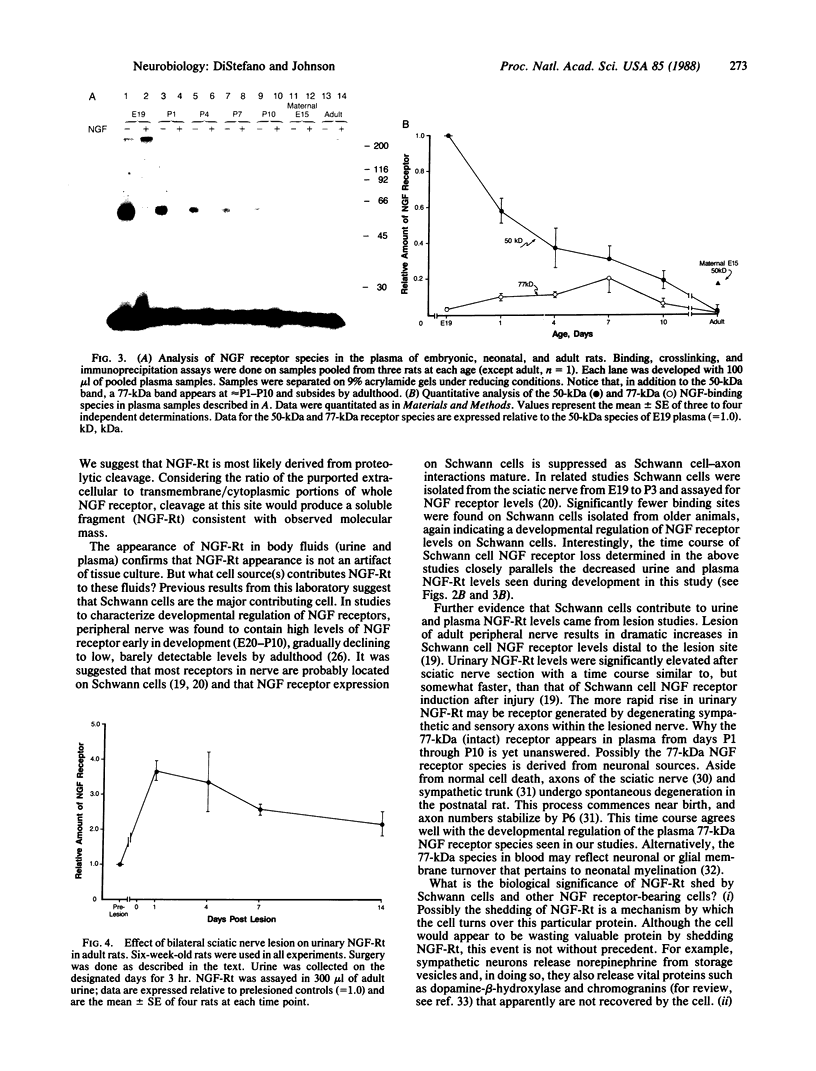
Schwann cells express growth factor (NGF) receptors on their cell surface in response to axotomy, a phenomenon that can be demonstrated both in vivo and in vitro. The predominant form of the NGF receptor on Schwann cells exists as an approximately equal to 80-kDa band, as determined by NaDodSO4/PAGE. We demonstrate that cultured Schwann cells shed a truncated (50-kDa) form of the NGF receptor (NGF-Rt) into their medium. Other cell types that shed the NGF-Rt into medium include a rat schwannoma and, to a lesser extent, PC12 cells and superior cervical ganglion neurons. NGF-Rt was not found in media conditioned by mixed neuron/glia cultures from various brain regions, or anterior pituitary cells derived from rat. In vivo, NGF-Rt was present in neonatal rat urine, and its presence was developmentally regulated: levels were high in postnatal day-1 rat urine and declined to low, but detectable, levels by weeks 4 and 8. NGF-Rt was also found in amniotic fluid and in the stomach contents of fetal rats. Maternal urine (pre- and postnatal) had slightly elevated NGF-Rt levels over normal adult urine. NGF-Rt was detected in rat plasma and showed developmental regulation similar to that found for urine. In addition, a 77-kDa receptor species was detected in plasma during early development. Finally, NGF-Rt was significantly elevated in the urine of adult rats with bilateral sciatic nerve lesions. These findings suggest that the developmentally regulated release of NGF-Rt, present in plasma and other body fluids, plays a regulatory role in nervous system development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Terry L. C., Bray G. M. Spontaneous loss of axons in sympathetic unmyelinated nerve fibers of the rat during development. Brain Res. 1973 May 17;54:360–364. doi: 10.1016/0006-8993(73)90061-9. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Dopamine- -hydroxylase: regulation of its synthesis and release from nerve terminals. Pharmacol Rev. 1972 Jun;24(2):233–243. [PubMed] [Google Scholar]
- Bernd P., Greene L. A. Association of 125I-nerve growth factor with PC12 pheochromocytoma cells. Evidence for internalization via high-affinity receptors only and for long-term regulation by nerve growth factor of both high- and low-affinity receptors. J Biol Chem. 1984 Dec 25;259(24):15509–15516. [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Burgess S. K., Jacobs S., Cuatrecasas P., Sahyoun N. Characterization of a neuronal subtype of insulin-like growth factor I receptor. J Biol Chem. 1987 Feb 5;262(4):1618–1622. [PubMed] [Google Scholar]
- Carbonetto S., Stach R. W. Localization of nerve growth factor bound to neurons growing nerve fibers in culture. Brain Res. 1982 Mar;255(3):463–473. doi: 10.1016/0165-3806(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Chandler C. E., Parsons L. M., Hosang M., Shooter E. M. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984 Jun 10;259(11):6882–6889. [PubMed] [Google Scholar]
- DiStefano P. S., Schweitzer J. B., Taniuchi M., Johnson E. M., Jr Selective destruction of nerve growth factor receptor-bearing cells in vitro using a hybrid toxin composed of ricin A chain and a monoclonal antibody against the nerve growth factor receptor. J Cell Biol. 1985 Sep;101(3):1107–1114. doi: 10.1083/jcb.101.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Green S. H., Rydel R. E., Connolly J. L., Greene L. A. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986 Mar;102(3):830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger P., Lenoir D. Nerve growth factor (NGF) stimulation of cholinergic telencephalic neurons in aggregating cell cultures. Brain Res. 1982 Feb;255(2):229–238. doi: 10.1016/0165-3806(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy U., Basu A., Rodeck U., Herlyn M., Ross A. H., Das M. Binding of an antagonistic monoclonal antibody to an intact and fragmented EGF-receptor polypeptide. Arch Biochem Biophys. 1987 Feb 1;252(2):549–560. doi: 10.1016/0003-9861(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Olender E. J., Stach R. W. Sequestration of 125I-labeled beta nerve growth factor by sympathetic neurons. J Biol Chem. 1980 Oct 10;255(19):9338–9343. [PubMed] [Google Scholar]
- PETERS A., MUIR A. R. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):117–130. doi: 10.1113/expphysiol.1959.sp001366. [DOI] [PubMed] [Google Scholar]
- Peterfreund R. A., Vale W. High molecular weight somatostatin secretion by cultured rat brain cells. Brain Res. 1982 May 13;239(2):463–477. doi: 10.1016/0006-8993(82)90522-4. [DOI] [PubMed] [Google Scholar]
- Radeke M. J., Misko T. P., Hsu C., Herzenberg L. A., Shooter E. M. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987 Feb 12;325(6105):593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Reier P. J., Hughes A. Evidence for spontaneous axon degeneration during peripheral nerve maturation. Am J Anat. 1972 Sep;135(1):147–152. doi: 10.1002/aja.1001350113. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Roles of extracellular matrix in neural development. Annu Rev Physiol. 1983;45:581–600. doi: 10.1146/annurev.ph.45.030183.003053. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Otten U., Agid Y., Thoenen H. Nerve growth factor (NGF) in the rat CNS: absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979 Jun 8;168(3):473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Seiler M., Schwab M. E. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984 May 21;300(1):33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld K. H., Ishii D. N. Fast and slow nerve growth factor binding sites in human neuroblastoma and rat pheochromocytoma cell lines: relationship of sites to each other and to neurite formation. J Neurosci. 1985 Jul;5(7):1717–1728. doi: 10.1523/JNEUROSCI.05-07-01717.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld K. H., Ishii D. N. Nerve growth factor effects and receptors in cultured human neuroblastoma cell lines. J Neurosci Res. 1982;8(2-3):375–391. doi: 10.1002/jnr.490080226. [DOI] [PubMed] [Google Scholar]
- Stach R. W., Wagner B. J. Decrease in the number of lower affinity (type II) nerve growth factor receptors on embryonic sensory neurons does not affect fiber outgrowth. J Neurosci Res. 1982;7(2):103–110. doi: 10.1002/jnr.490070202. [DOI] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Schweitzer J. B., Johnson E. M., Jr Nerve growth factor receptor molecules in rat brain. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1950–1954. doi: 10.1073/pnas.83.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Yan Q., Johnson E. M., Jr A quantitative study of the developmental expression of nerve growth factor (NGF) receptor in rats. Dev Biol. 1987 May;121(1):139–148. doi: 10.1016/0012-1606(87)90147-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Sutter A. beta-Nerve growth factor (beta NGF) receptors on glial cells. Cell-cell interaction between neurones and Schwann cells in cultures of chick sensory ganglia. EMBO J. 1983;2(6):879–885. doi: 10.1002/j.1460-2075.1983.tb01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]