Abstract
Background and purpose:
The long-acting β2-adrenoceptor agonist, indacaterol, has been developed as a bronchodilator for the therapeutic management of respiratory diseases. The aim of the present study was to determine whether indacaterol has any anti-inflammatory activity. To this end, the effects of indacaterol on human lung mast cell responses were investigated.
Experimental approach:
The effects of indacaterol, and the alternative long-acting β-agonists formoterol and salmeterol, were investigated on the IgE-dependent release and generation of histamine, cysteinyl-leukotrienes and prostaglandin D2 from human lung mast cells. Moreover, the extent to which long-term (24–72 h) incubation of mast cells with long-acting β-agonists impaired the subsequent ability of β-agonists to inhibit mast cell responses was assessed.
Key results:
Indacaterol was as potent and as efficacious as the full agonist, isoprenaline (EC50, ∼4 nmol·L−1), at inhibiting the IgE-dependent release of histamine from mast cells. Formoterol was a full agonist whereas salmeterol was a partial agonist as inhibitors of histamine release. All three long-acting β-agonists were effective inhibitors of the IgE-dependent generation of cysteinyl-leukotrienes and prostaglandin D2. Long-term incubation of mast cells with long-acting β-agonists led to a reduction in the subsequent ability of β-agonists to stabilize mast cell responses. This tendency to induce functional desensitization was least evident for indacaterol.
Conclusions and implications:
Indacaterol is an effective inhibitor of the release of mediators from human lung mast cells. This suggests that, as well as bronchodilation, mast cell stabilization may constitute an additional therapeutic benefit of indacaterol.
Keywords: asthma, bronchodilators, β2-adrenoceptors, histamine
Introduction
Bronchodilator β2-adrenoceptor agonists continue to play an important role in the therapeutic management of respiratory diseases (Waldeck, 2002; Cazzola et al., 2005). In general terms, β-agonists can be classified as either short-acting (salbutamol, terbutaline) or long-acting (salmeterol, formoterol) with effects lasting for either 4 or 12 h respectively (Waldeck, 2002). An incremental advance on existing therapies is the development of even longer-acting β-agonists, such as indacaterol, whose therapeutic effects can last for 24 h (Battram et al., 2006; Beeh et al., 2007). An important attendant benefit of once-daily β-agonists is that patient compliance is less likely to be an issue (Cazzola et al., 2005).
While the principal effect of β-agonists is to relax airway smooth muscle, additional beneficial effects may include anti-inflammatory activity (Barnes, 1999). In this respect, the mast cell may be an important target. Previous in vitro studies have shown that β-agonists are very effective at preventing the release and generation of mediators from human lung mast cells that can cause bronchoconstriction and inflammation (Schild, 1937; Orange et al., 1971; Assem and Schild, 1973; Church and Hiroi, 1987; Butchers et al., 1991; Lau et al., 1994; Nials et al., 1994; Scola et al., 2004a). These studies are supported by in vivo findings in which stabilization of mast cells by β-agonists has been reported (Howarth et al., 1985; O'Connor et al., 1994; Taylor et al., 1997; Nightingale et al., 1999; Ketchell et al., 2002; Russo et al., 2005).
Although β-agonists are unquestionably useful, it is possible that the continued therapeutic utility of these drugs may be compromised by the development of tolerance. While it is uncertain to what extent tolerance is an issue in clinical practice, the development of tolerance to β-agonists has been shown to occur far more readily in the context of mast cell stabilization than for airway smooth muscle relaxation (Van der Heijden et al., 1984; O'Connor et al., 1992; Cockcroft et al., 1993; Chong and Peachell, 1999; Swystun et al., 2000; Jokic et al., 2001).
At the molecular level, tolerance probably reflects receptor desensitization. Agonist-driven receptor desensitization is recognized as a multi-step process involving receptor uncoupling, internalization and down-regulation (Su et al., 1980; Kohout and Lefkowitz, 2003). Previous studies of our own have demonstrated that long-term incubations of mast cells with β-agonists that lead to extensive levels of functional desensitization are not associated with proportionate levels of receptor loss suggesting that, in the mast cell, uncoupling and/or sequestration of the β2-adrenoceptor are more prominent processes contributing to desensitization (Chong et al., 2003; Scola et al., 2004a,b;).
The principal aim of the present paper was to evaluate the effects of indacaterol alongside other long-acting β-agonists on human lung mast cells and, thereby, to determine whether indacaterol displays any valuable anti-inflammatory activity. Moreover, an additional aim was to establish the extent to which indacaterol and other long-acting β-agonists induce tolerance to the mast cell stabilizing effects of β-agonists.
Methods
Buffers
Phosphate buffered saline (PBS) was employed in these studies. PBS contained (mmol·L−1): NaCl 137; Na2HPO4.12H2O 8; KCl 2.7; KH2PO4 1.5. PBS-FBS was PBS which additionally contained: CaCl2.2H2O 1 mmol·L−1; MgCl2.6H2O 1 mmol·L−1; glucose 5.6 mmol·L−1; foetal bovine serum (FBS) 2%; DNase 15 µg·mL−1. PBS-HSA was PBS additionally supplemented with: CaCl2.2H2O 1 mmol·L−1; MgCl2.6H2O 1 mmol·L−1; glucose 5.6 mmol·L−1; human serum albumin (HSA) 30 µg·mL−1. The pH of all PBS buffers was titrated to 7.3.
Krebs buffer contained (mmol·L−1): NaCl 118, NaHCO3 25, KCl 4.7, MgSO4.7H2O 0.6, KH2PO4 1.2, glucose 11.1, CaCl2.2H2O 1.3.
Preparation of inhibitors and stimuli
Indacaterol, salmeterol and formoterol were prepared as 10 mmol·L−1 stock solutions in dimethyl sulphoxide and stored at 4°C. Stock solutions of (-)-isoprenaline bitartrate (10 mmol·L−1) were prepared in 0.05% sodium metabisulphite (dissolved in 0.9% saline) on a fortnightly basis and stored at 4°C. ICI118551 was prepared as a stock solution (10 mmol·L−1) in distilled water and stored at 4°C. Lyophilized polyclonal goat anti-human IgE antibody, was reconstituted in distilled water and stored at 4°C. The drugs were diluted to the desired concentration in buffer just prior to use. Preliminary experiments indicated that the vehicles used to prepare the drugs had no effect on mediator release assays.
Lung tissue
Human lung tissue was obtained from surgical resections of patients following surgery with the approval of the Local Research Ethics Committee. Most of the patients were undergoing surgery for carcinoma. The majority of the patients were Caucasian (90%), and there was an approximate 1:1 split between men and women.
Cell isolation
Mast cells were isolated from human lung tissue by a modification of the method described by Ali and Pearce (1985). Macroscopically normal tissue from lung resections was chopped vigorously for 15 min with scissors in a small volume of PBS buffer. The chopped tissue was washed over a nylon mesh (100 µm pore size; Incamesh, Warrington, UK) with 0.5–1 L of PBS buffer to remove lung macrophages. The tissue was reconstituted in PBS-FBS [10 mL (g tissue)−1] containing collagenase Ia (350 units mL−1 of PBS-FBS) and agitated by using a water-driven magnetic stirrer immersed in a water bath set at 37°C. The supernatant (containing some mast cells) was separated from the tissue by filtration over nylon mesh. The collagenase-treated tissue was then reconstituted in a small volume of PBS-FBS buffer and disrupted mechanically with a syringe. The disrupted tissue was then washed over nylon gauze with PBS-FBS (300–600 mL). The pooled filtrates were sedimented (480× g, room temperature, 10 min), the supernatant discarded and the pellets reconstituted in PBS-FBS (100 mL). The pellet was washed twice further. Mast cells were visualized by microscopy using an Alcian Blue stain (Gilbert and Ornstein, 1975). Of the total cells, 3–13% were mast cells. This method generated approximately 6 × 105 mast cells (g tissue)−1. Mast cells prepared in this manner were used in mediator release experiments.
Mediator release
Mediator release experiments were performed in PBS-HSA buffer. Mast cells were incubated with or without a β-agonist for 10 min before challenge with a maximal releasing concentration of anti-IgE (1:300). In studies involving the β2-adrenoceptor selective antagonist ICI118551, mast cells were first incubated (45 min) with the antagonist and then together with agonist for a further 10 min before challenge with anti-IgE. Stimulus-induced secretion was allowed to proceed for 25 min at 37°C after which time the cells were pelleted by centrifugation (480× g, room temperature, 4 min). Histamine released into the supernatant was determined by the modified (Ennis, 1991) automated fluorometric method of Siraganian (1974). Total histamine content was determined by lysing aliquots of the cells with perchloric acid at a final concentration of 1.6%. Cells incubated in buffer alone served as a measure of spontaneous histamine release which ranged from 2% to 8% of the total histamine content. Histamine release was thus expressed as a percentage of the total histamine content after subtracting the spontaneous histamine release.
The amounts of eicosanoids [cysteinyl-leukotrienes (cys-LTs) and prostaglandin D2 (PGD2)] in the supernatants were determined using commercially available enzyme immunoassay kits (Cayman Chemical Company, Ann Arbor, MI, USA). All assays were performed in duplicate.
When long-term incubations were performed, RPMI 1640 buffer supplemented with penicillin (10 units mL−1), streptomycin (10 µg·mL−1), gentamicin (50 µg·mL−1) and FBS (2%) was employed. Cells were incubated (24–72 h at 37°C) at a density of 0.1 × 106 mast cells mL−1 in six well plates, with or without an agonist. After each 24 h period the cells were washed three times and either placed back in culture with or without an agonist, in supplemented RPMI 1640, or reconstituted in PBS-HSA for mediator release experiments as described above.
Preparation of human bronchial rings
Bronchi (≤3 mm diameter) were dissected free from parenchymal tissue and bronchial rings prepared. The rings were mounted under a resting tension of 1 g in 10 mL organ baths attached to force transducers for isometric tension. The rings were allowed to equilibrate in aspirated (O2 95%, CO2 5%) Krebs buffer with several washes over 1 h. The rings were then challenged with anti-IgE (1:300) in order to induce contraction. After contraction had plateaued (15 min), indacaterol or other β-agonists were added cumulatively to the bath. After the final cumulative addition of β-agonist, a high concentration of isoprenaline (30 µmol·L−1) was added to each organ bath and relaxations with β-agonists were calculated as a percentage of this relaxation with isoprenaline. On occasions, aminophylline (1 mmol·L−1) was added to all rings at the end of the experiment to ensure that the maximal attainable relaxation had been achieved.
Data analysis
Antagonist affinity was estimated using the following formula: pKB= log(dose ratio – 1) – log(antagonist concentration) where the dose ratio is the ratio of the EC50 values in the presence and absence of antagonist. Maximal responses (Emax) and potencies (pD2) were determined by non-linear regression analysis (GraphPad Prism, version 3.0a). To determine whether there was any difference in the responses after treatments with drugs, anova was performed followed by Dunnett or Tukey post tests as appropriate.
Materials
The following were purchased from the sources indicated: aminophylline, anti-human IgE, collagenase, dimethyl sulphoxide, DNase, FBS, HSA, (-)-isoprenaline (all Sigma, Poole, UK); calcium chloride and magnesium chloride (BDH, Poole, UK); gentamicin, penicillin/streptomycin, RPMI 1640 (Invitrogen, Paisley, UK); salmeterol and ICI118551 (Tocris, Bristol, UK).
Indacaterol maleate and formoterol fumarate were synthesized by the Department of Chemistry (Novartis, Horsham, UK).
Receptor nomenclature
Receptor nomenclature used in this manuscript conforms to published guidelines (Alexander et al., 2008).
Results
Indacaterol inhibits mediator release
The effects of the long-acting β-agonist, indacaterol, on the IgE-dependent release of histamine from human lung mast cells were determined. Two other long-acting β-agonists, formoterol and salmeterol, were studied in parallel (Figure 1). All three long-acting β-agonists inhibited IgE-mediated histamine release in a concentration-dependent manner and all were at least as potent as the full agonist, isoprenaline (Table 1). However, whereas indacaterol and formoterol were full agonists in the context of inhibiting histamine release, salmeterol was a partial agonist (Table 1). The intrinsic activity (Emax of a long-acting β-agonist/Emax of isoprenaline) of indacaterol, formoterol and salmeterol was 1.03 ± 0.06, 1.01 ± 0.08 and 0.50 ± 0.08 respectively (n= 18).
Figure 1.
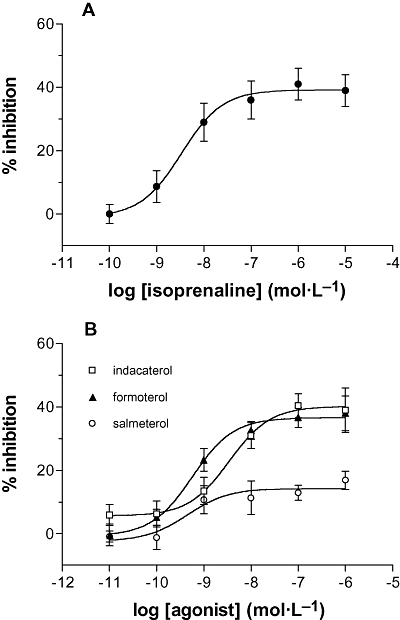
Effects of β-adrenoceptor agonists on mast cells. Cells were incubated without or with (A) isoprenaline or (B) indacaterol, formoterol or salmeterol for 10 min before challenge with a maximal releasing concentration of anti-IgE (1:300) for a further 25 min for histamine release. Results are expressed as the % inhibition of the control histamine release which was 30 ± 3%. Values are means ± SEM, n= 13–21.
Table 1.
pD2 and Emax values for the inhibition of histamine release by β-adrenoceptor agonists
| Isoprenaline | Indacaterol | Formoterol | Salmeterol | |
|---|---|---|---|---|
| pD2 | 8.4 ± 0.2 | 8.8 ± 0.2 | 9.3 ± 0.2 | 9.1 ± 0.3 |
| Emax (%) | 40 ± 5 | 43 ± 4 | 37 ± 3 | 19 ± 4 |
Values were calculated from the data used to generate Figure 1 and further relevant details can be found in the legend to that figure. Values are means ± SEM.
In further studies, the effects of the long-acting β-agonists (10−12–10−6 mol·L−1) indacaterol, formoterol and salmeterol together with isoprenaline (10−6 mol·L−1) on the IgE-dependent generation of eicosanoids from mast cells were investigated. Indacaterol was an effective inhibitor of the IgE-mediated generation of eicosanoids (cys-LT and PGD2) (Figure 2A). Formoterol (Figure 2B) and salmeterol (Figure 2C) also inhibited eicosanoid generation from mast cells (Table 2). Whereas both indacaterol and formoterol were full agonists as inhibitors of the release of histamine and eicosanoids from mast cells, salmeterol was a partial agonist for the inhibition of histamine (intrinsic activity, 0.44 ± 0.11) and PGD2 (0.73 ± 0.07) generation and a full agonist as an inhibitor of cys-LT (0.96 ± 0.10) generation (Figure 3).
Figure 2.
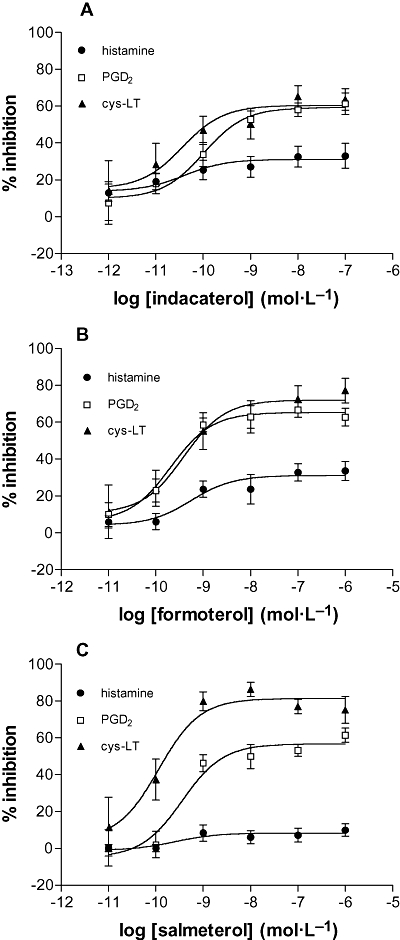
Effects of β-adrenoceptor agonists on eicosanoid generation. Mast cells were incubated without or with (A) indacaterol, (B) formoterol or (C) salmeterol for 10 min before challenge with a maximal releasing concentration of anti-IgE (1:300) for a further 25 min. The histamine, PGD2 and cys-LT content of supernatants were assessed. Results are expressed as the % inhibition of the control (unblocked) generation of a given mediator. Control releases were for histamine 27 ± 4%, for PGD2 generation 71 ± 19 ng (106 mast cells)−1 and for cys-LT generation 19 ± 5 ng (106 mast cells)−1. Values are means ± SEM, n= 4–11.
Table 2.
pD2 and Emax values for long-acting β-agonists for the inhibition of histamine, PGD2 and cys-LT generation from mast cells
|
Indacaterol |
Formoterol |
Salmeterol |
||||
|---|---|---|---|---|---|---|
| pD2 | Emax (%) | pD2 | Emax (%) | pD2 | Emax (%) | |
| Histamine | 9.6 ± 0.3 | 33 ± 6 | 9.0 ± 0.3 | 37 ± 6 | 9.6 ± 0.5 | 8 ± 3 |
| PGD2 | 9.8 ± 0.3 | 62 ± 4 | 9.8 ± 0.2 | 66 ± 5 | 9.4 ± 0.1 | 58 ± 5 |
| cys-LT | 10.2 ± 0.3 | 66 ± 7 | 9.1 ± 0.3 | 77 ± 7 | 10.2 ± 0.2 | 81 ± 4 |
Values were calculated from the data used to generate Figure 2 and further relevant details can be found in the legend to that figure. In the experiments described in Figure 3, a single concentration (10−6 mol·L−1) of isoprenaline was also studied for inhibitory effects on IgE-dependent mediator release. Isoprenaline (10−6 mol·L−1) inhibited histamine, PGD2 and cys-LT generation by 37 ± 4%, 67 ± 6% and 74 ± 5% respectively (n= 12). Values in the table are means ± SEM.
Figure 3.
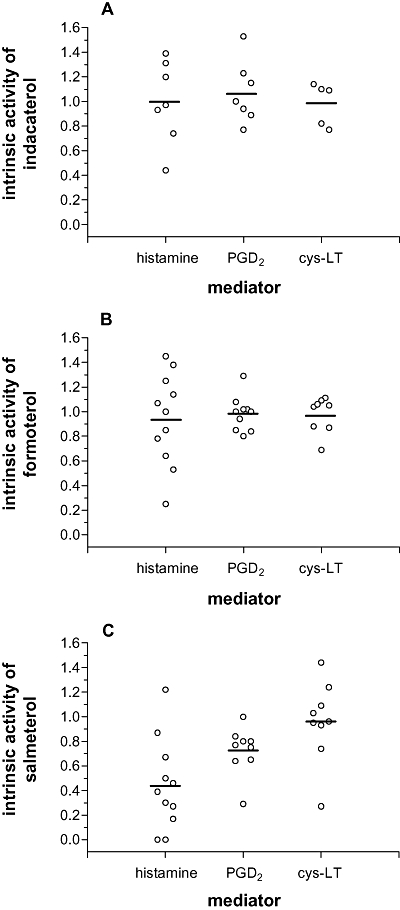
Intrinsic activities of long-acting β-agonists as inhibitors of histamine release and eicosanoid generation. In this figure, intrinsic activities were calculated by considering the Emax for inhibition by a long-acting β-agonist divided by inhibition by a maximally effective concentration (1 µmol·L−1) of the full agonist isoprenaline on IgE-dependent histamine release, PGD2 and cys-LT generation. Each point represents the intrinsic activity of (A) indacaterol (n= 7), (B) formoterol (n= 11) and (C) salmeterol (n= 11) in an individual experiment. Note that not all mast cell preparations generated cys-LTs following activation with anti-IgE. Solid horizontal bars represent the mean intrinsic activities.
Indacaterol relaxes human airway smooth muscle
The effects of indacaterol, salmeterol, formoterol and isoprenaline on pre-contracted bronchial rings were investigated (Figure 4). Bronchial rings were mounted in organ baths and challenged with anti-IgE (1:300). Once contraction had plateaued, β-agonists were added to the bath cumulatively. All the β-agonists relaxed bronchial ring contraction in a concentration-dependent fashion and isoprenaline, indacaterol, formoterol and salmeterol showed similar activity although salmeterol was not quite as efficacious (Table 3).
Figure 4.
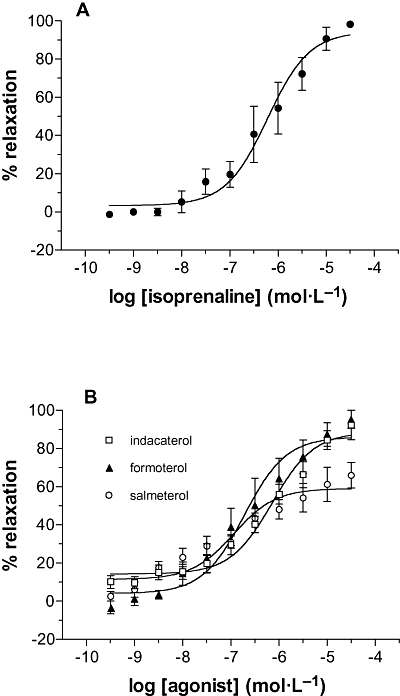
Relaxation of airway smooth muscle. Human bronchial rings were pre-contracted with anti-IgE (1:300) and once the contraction had plateaued, the effects of cumulative additions of (A) isoprenaline and (B) long-acting β-agonists on the contraction determined. The control contraction was 1.5 ± 0.3 g. Results are expressed as a percentage of the maximal relaxation observed with 30 µmol·L−1 isoprenaline. Values are means ± SEM, n= 4–5.
Table 3.
pD2 and Emax values for the relaxation of human bronchial rings by β-adrenoceptor agonists
| Isoprenaline | Indacaterol | Formoterol | Salmeterol | |
|---|---|---|---|---|
| pD2 | 6.2 ± 0.3 | 6.1 ± 0.2 | 6.6 ± 0.3 | 7.2 ± 0.4 |
| Emax (%) | 98 ± 3 | 90 ± 6 | 92 ± 5 | 62 ± 9 |
Values were calculated from the data used to generate Figure 4 and further relevant details can be found in the legend to that figure. Values are means ± SEM.
Indacaterol interacts with β2-adrenoceptors
In order to confirm that these effects of indacaterol were mediated by β2-adrenoceptors, the effects of the β2-adrenoceptor selective antagonist, ICI118551, on the indacaterol inhibition of histamine release from mast cells was determined. IC1118551 (30 nmol·L−1) caused a 50-fold rightward shift in the concentration-response curve for indacaterol (Figure 5A). ICI118551 behaved similarly when antagonizing the effects of formoterol (Figure 5B) although it was somewhat more effective at reversing the inhibitory effects of isoprenaline (Figure 5C). Calculated pKB values for ICI118551 were 9.2, 9.2 and 9.6 for the antagonism of indacaterol, formoterol and isoprenaline respectively.
Figure 5.
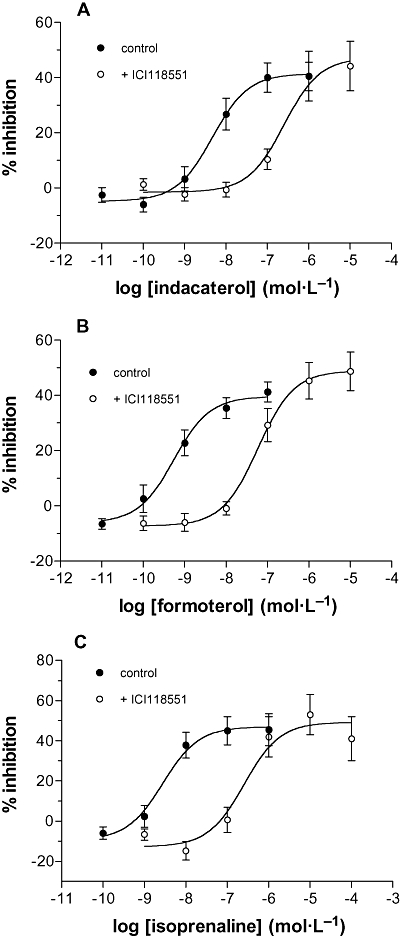
Antagonism of the inhibition by ICI118551. Cells were incubated with or without ICI118551 (30 nmol·L−1) for 45 min and then together with or without (A) indacaterol (B) formoterol or (C) isoprenaline for a further 10 min before challenge with anti-IgE (1:300) for a further 25 min. Results are expressed as the % inhibition of the control histamine releases which were for (A) 33 ± 3% and 32 ± 3% (control and ICI118551 respectively) for (B) 30 ± 4% and 33 ± 4% (control and ICI118551 respectively) and for (C) 30 ± 5% and 25 ± 4% (control and ICI118551 respectively). Values are means ± SEM, n= 12 for (A), n= 11 for (B) and n= 8 for (C).
Long-acting β-agonists induce functional desensitization
The effects of long-term exposure of mast cells to indacaterol, formoterol or salmeterol on the subsequent ability of β-agonists to inhibit histamine release induced by anti-IgE were evaluated. Mast cells were incubated (24 h) with indacaterol, formoterol or salmeterol at either a third or three times the calculated EC50 value for the inhibition of histamine release (Table 4). The cells were then washed extensively and the effectiveness of the same long-acting β-agonists and isoprenaline (all used at 10 times the EC50 value) to inhibit histamine release was evaluated. The data show that at the higher desensitizing concentration (three times the EC50) all three long-acting β-agonists caused extensive levels of functional desensitization (Figure 6). However, at the lower desensitizing concentration (third of the EC50) indacaterol did not impair the inhibitory effects of β-agonists to a statistically significant (P > 0.05) extent causing between 31% and 37% desensitization of full agonist responses {% desensitization calculated as, [1 − (inhibition by an agonist after desensitizing treatment/inhibition by an agonist) × 100]}. By contrast, pretreatment of mast cells with the lower desensitizing concentrations of formoterol and salmeterol caused a significant (P < 0.05) impairment in the effectiveness of full agonists to inhibit causing between 48% and 65% desensitization of the inhibitory responses.
Table 4.
Concentrations of agonists used for desensitization experiments
|
(nmol·L−1) |
||||
|---|---|---|---|---|
| EC50 | EC50/3 | 3×EC50 | 10×EC50 | |
| Indacaterol | 3.5 | 1.2 | 10.5 | 35 |
| Formoterol | 0.8 | 0.26 | 2.4 | 8 |
| Salmeterol | 0.6 | 0.2 | 1.8 | 6 |
| Isoprenaline | 3.2 | – | – | 32 |
EC50 values were calculated for each agonist using all the available concentration-response curves generated for this study. With reference to Figures 6 and 7, the Table further shows the concentrations of agonist used to induce desensitization (third and three times the EC50) as well as the concentrations of agonists used (10 times the EC50) to inhibit histamine release after the desensitizing treatment.
Figure 6.
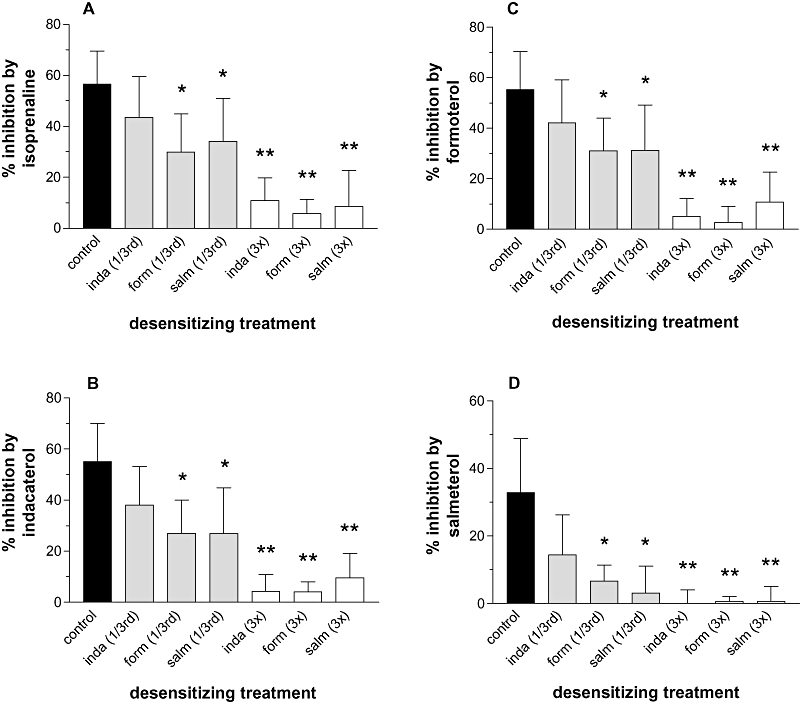
Functional desensitization induced by long-acting β-agonists. Mast cells were incubated (24 h) with or without (control) an agonist at a third or three times the EC50 of the given agonist (see Table 4) after which the cells were washed three times and the subsequent effectiveness of a given β-agonist, at 10 times its EC50, to inhibit histamine release induced by anti-IgE was assessed. The effects of these treatments on the inhibitory activity of (A) isoprenaline, (B) indacaterol (inda), (C) formoterol (form) and (D) salmeterol (salm) are shown. Results are expressed as the % inhibition of the control histamine releases which were 36 ± 6% after cells were incubated for 24 h in buffer or ranged from 37 ± 5% to 40 ± 3% following 24 h treatments with agonists. Values are means ± SEM, n= 4. Statistically significant reductions in inhibitory activity following treatments, compared with control are indicated; *P < 0.05, **P < 0.01.
In further studies, the effects of desensitization over 3 days were evaluated. Mast cells were incubated with or without a given long-acting β-agonist (third of the EC50) over 3 days and at each 24 h interval, the effects of the desensitizing treatment on the effectiveness of the same long-acting β-agonist (10 times the EC50) to inhibit IgE-dependent histamine release evaluated. The data show that after 3 days of desensitizing treatments there were significant (P < 0.05 at least) reductions in the effectiveness of the long-acting β-agonists to inhibit (Figure 7). Nonetheless, indacaterol was still able to inhibit histamine release to some extent (66 ± 7% and 21 ± 9% inhibition after 3 days with buffer and agonist respectively), formoterol inhibited to a very modest degree (63 ± 9% and 10 ± 9% inhibition) whereas the inhibitory effects of salmeterol were completely abolished (50 ± 9 and −4 ± 3% inhibition).
Figure 7.
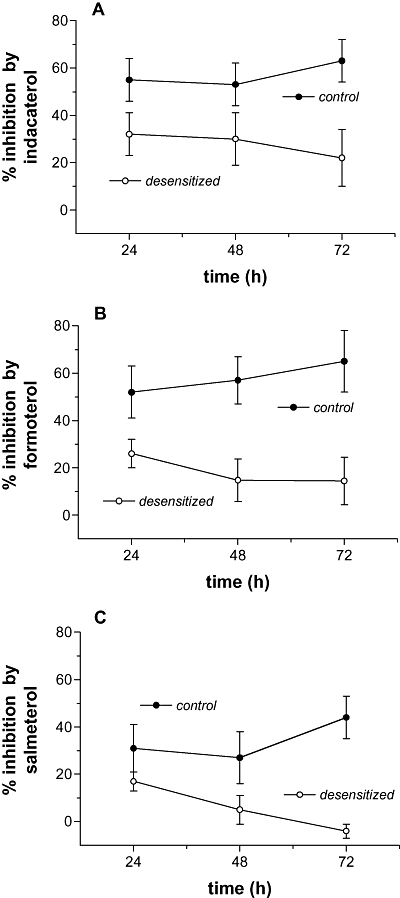
Time dependence of desensitization. Mast cells were incubated for 24, 48 or 72 h with or without (control) an agonist at a third of the EC50 of the given agonist (see Table 4) after which the cells were washed three times and the subsequent effectiveness of the same β-agonist, at 10 times its EC50, to inhibit histamine release induced by anti-IgE was assessed. The effects of these treatments on the inhibitory activity of (A) indacaterol, (B) formoterol and (C) salmeterol are shown. Results are expressed as the % inhibition of the control histamine releases which ranged from 33 ± 6% to 39 ± 6% following 24 h treatments with buffer or agonist, 32 ± 8% to 38 ± 8% following 48 h treatments and 31 ± 6% to 40 ± 9% following 72 h treatments. Values are means ± SEM, n= 4–5.
Discussion
The human lung mast cell has long been recognized as central to the mediation of asthma (Holgate et al., 1986; Bingham and Austen, 2000; Black, 2002). Activation of lung mast cells leads to the release and generation of a wide array of autacoids (Holgate et al., 1986; Bingham and Austen, 2000). Mast cell autacoids can be preformed and stored within granules such as histamine, or synthesized de novo following cell activation such as the eicosanoids, PGD2 and cys-LTs. These autacoids, along with other mast cell-derived mediators including cytokines, chemotactic factors and enzymes (Williams and Galli, 2000), can cause bronchoconstriction and promote inflammation. Over the longer term, mast cell-derived mediators may also contribute to airway remodelling (Sommerhoff, 2001; Holgate et al., 2003). Moreover, recent studies have shown that mast cells contribute to the airway hyperreactivity that is seen in people with asthma (Brightling et al., 2002).
Although the mast cell has a prominent role in asthma, current frontline therapies do not target the mast cell specifically. Perhaps surprisingly, anti-inflammatory steroids have no effect on the stimulated release of mediators from mast cells (Schleimer et al., 1983). By contrast, β-agonists, which act principally to relax airway smooth muscle, may also act to stabilize mast cells. Certainly, a large body of in vitro data spanning many decades demonstrates that β-agonists are effective inhibitors of mediator release from mast cells (Schild, 1937; Orange et al., 1971; Assem and Schild, 1973; Church and Hiroi, 1987; Butchers et al., 1991; Lau et al., 1994; Nials et al., 1994; Scola et al., 2004a) findings supported by in vivo studies from the more recent past (Howarth et al., 1985; O'Connor et al., 1994; Taylor et al., 1997; Nightingale et al., 1999; Ketchell et al., 2002).
The present study confirms our own work and that of others showing that the long-acting β-agonists, formoterol and salmeterol, inhibit the IgE-dependent release of histamine from mast cells (Butchers et al., 1991; Lau et al., 1994; Nials et al., 1994; Chong et al., 1998; Scola et al., 2004b). In this study, we have also shown that the long-acting β-agonist, indacaterol, is an effective inhibitor of histamine release. These inhibitory effects of indacaterol were reversed by the β2-adrenoceptor selective antagonist ICI118551 (pKB, 9.2) in a fashion consistent with an effect of indacaterol at mast cell β2-adrenoceptors (O'Donnell and Wanstall, 1980; Chong et al., 2002). Indacaterol and formoterol were at least as potent and as efficacious as the full agonist isoprenaline as inhibitors of IgE-dependent histamine release from mast cells. By contrast, salmeterol was a partial agonist as an inhibitor of histamine release being about half as efficacious as either formoterol or indacaterol. These data are supported to some degree by studies in vivo showing that salmeterol is not as effective as formoterol as a stabilizer of mast cells (Taylor et al., 1997; Proud et al., 1998; Ketchell et al., 2002).
While salmeterol acts as a partial agonist as an inhibitor of histamine release, it is of interest that it displays comparatively greater activity and is closer to being a full agonist in the context of relaxing pre-contracted bronchial rings. This highlights that the intrinsic activity of an agonist can be heavily influenced by the system being studied (Kenakin, 1984). These data extend previous observations showing that agonists that act as partial agonists as inhibitors of histamine release are closer to being full agonists as relaxants of pre-contracted bronchial rings (Chong and Peachell, 1999). Furthermore, these data suggest that there is a greater receptor reserve for the relaxation of airway smooth muscle by β-agonists than for the inhibition of histamine release (Van der Heijden et al., 1984; Chong and Peachell, 1999).
Although salmeterol has been shown to be a partial agonist as an inhibitor of the IgE-dependent release of histamine it is of particular interest that it is a more efficacious inhibitor of the generation of eicosanoids being at least as effective as indacaterol and formoterol in attenuating the generation of cys-LTs. These findings demonstrate that intrinsic activity is dependent not only on the system being studied but also on the output measured (Scott et al., 1999; Borgland et al., 2003). It is highly probable therefore that in human lung mast cells there is a higher receptor reserve for the inhibition by β-agonists of the de novo generation of eicosanoids than for the release of stored mediators (such as histamine and tryptase) from granules (Undem et al., 1988).
One factor that may limit the therapeutic effectiveness of β-agonists is the development of tolerance although the extent to which tolerance develops in a real-life context is hard to evaluate. Nonetheless, in controlled experiments, tolerance to β-agonists can be readily demonstrated suggesting that it could constitute an important therapeutic consideration (Salpeter et al., 2004). Moreover, tolerance to the mast cell stabilizing effects of β-agonists appears to occur more readily than tolerance to bronchodilation (O'Connor et al., 1992; Cockcroft et al., 1993; Swystun et al., 2000; Jokic et al., 2001), consistent with a lower receptor reserve in mast cells.
As a paradigm of tolerance, we have investigated whether long-term (24 h) treatment of mast cells with long-acting β-agonists leads to a subsequent reduction in the ability of β-agonists to prevent the IgE-dependent release of histamine from mast cells. Our studies demonstrated that indacaterol, salmeterol and formoterol were all capable of inducing extensive levels of functional desensitization when mast cells were incubated with a high concentration (three times the EC50) of agonist. However, at a lower concentration (third of the EC50), indacaterol did not induce as much desensitization as either formoterol or salmeterol. This observation may be quite surprising since an expectation, based on studies in other systems (Pittman et al., 1984; January et al., 1997; Benovic et al., 1998; Clark et al., 1999), might be that a partial agonist (salmeterol) would not cause as much tolerance as a full agonist (formoterol, indacaterol). However, our own previous studies in mast cells have shown that at low receptor occupancies there is no correlation between the intrinsic activity of agonists and the extent of tolerance induced (Scola et al., 2004a).
In further studies, the effects of a 3-day incubation with a β-agonist (third of the EC50) on the subsequent ability of the same agonist to inhibit IgE-dependent histamine release were determined. These experiments indicated that, over 3 days, any ability of salmeterol to prevent degranulation from mast cells is abolished, the effects of formoterol substantially compromised whereas those of indacaterol, while reduced, are still evident. Clearly, if similar processes were operative in vivo, this could affect how effectively individual long-acting β-agonists continue to stabilize mast cells.
In this study we have investigated the inhibitory effects of β-adrenoceptor agonists against IgE-dependent activation of human lung mast cells. While IgE-dependent activation is probably the most important mechanism by which mast cells are triggered, the mast cell may well be activated, in a pathophysiological context, by other stimuli such as neuropeptides, anaphylatoxins and stem cell factor (Lowman et al., 1988; Bischoff and Dahinden, 1992). Whether β-adrenoceptor agonists would behave similarly against mast cells activated by such non-IgE-dependent mechanisms has not been evaluated.
In summary, we have shown that indacaterol is an effective inhibitor of the IgE-dependent release of mediators from human lung mast cells. Moreover, in comparison to other long-acting β-agonists, indacaterol does not promote as much functional desensitization. These properties suggest that, in vivo, indacaterol may stabilize mast cells effectively and may be more likely to sustain this stabilization over time.
Acknowledgments
The authors are grateful to Mr J. Edwards, Mr J. Rao, Mr T. Locke, Mr G. Cooper and Mr D. Hopkinson (Cardiothoracic Surgery), Dr S.K. Suvarna, Dr P. Kitsanta and Dr C. Layton (Histopathology) at the Northern General Hospital, Sheffield for their invaluable help in providing lung tissue specimens.
Glossary
Abbreviations:
- cys-LTs
cysteinyl-leukotrienes
- FBS
foetal bovine serum
- HSA
human serum albumin
- PBS
phosphate buffered saline
- PGD2
prostaglandin D2
Conflict of interest
This work was supported by Novartis.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (3rd) 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. edn. 2008 revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H, Pearce FL. Isolation and properties of cardiac and other mast cells from the rat and guinea-pig. Agents Actions. 1985;16:138–140. doi: 10.1007/BF01983121. [DOI] [PubMed] [Google Scholar]
- Assem ESK, Schild HO. Beta-adrenergic receptors concerned with the anaphylactic mechanism. Int Archs Allergy Appl Immunol. 1973;45:62–69. doi: 10.1159/000231003. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Effect of β2-agonists on inflammatory cells. J Allergy Clin Immunol. 1999;104:S10–S17. doi: 10.1016/s0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Farr D, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol), a novel inhaled β2 adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Derom E, Kanniess F, Cameron R, Higgins M, van As A. Indacaterol, a novel inhaled β2-agonist, provides sustained 24-h bronchodilation in asthma. Eur Respir J. 2007;29:871–878. doi: 10.1183/09031936.00060006. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Staniszewski C, Mayor F, Caron MG, Lefkowitz RJ. β-adrenergic kinase. Activity of partial agonists for stimulation of adenylate cyclase correlates with ability to promote receptor phosphorylation. J Biol Chem. 1998;8:3893–3897. [PubMed] [Google Scholar]
- Bingham CO, Austen KF. Mast-cell responses in the development of asthma. J Allergy Clin Immunol. 2000;105:S527–S534. doi: 10.1016/s0091-6749(00)90056-3. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Dahinden CA. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. The role of mast cells in the pathophysiology of asthma. N Engl J Med. 2002;346:1742–1743. doi: 10.1056/NEJM200205303462212. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, MacDonald JC. Opiod agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opiod receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Brightling CJ, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- Butchers PR, Vardey CJ, Johnson M. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br J Pharmacol. 1991;104:672–676. doi: 10.1111/j.1476-5381.1991.tb12487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Matera MG, Lötvall J. Ultra long-acting β2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2005;14:775–783. doi: 10.1517/13543784.14.7.775. [DOI] [PubMed] [Google Scholar]
- Chong LK, Peachell PT. β-Adrenoceptor reserve in human lung: a comparison between airway smooth muscle and mast cells. Eur J Pharmacol. 1999;378:115–122. doi: 10.1016/s0014-2999(99)00425-2. [DOI] [PubMed] [Google Scholar]
- Chong LK, Cooper E, Vardey CJ, Peachell PT. Salmeterol inhibition of mediator release from human lung mast cells by β-adrenoceptor-dependent and independent mechanisms. Br J Pharmacol. 1998;123:1009–1015. doi: 10.1038/sj.bjp.0701703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LK, Chess-Williams R, Peachell PT. Pharmacological characterisation of the β-adrenoceptor expressed by human lung mast cells. Eur J Pharmacol. 2002;437:1–7. doi: 10.1016/s0014-2999(02)01263-3. [DOI] [PubMed] [Google Scholar]
- Chong LK, Suvarna K, Chess-Williams R, Peachell PT. Desensitization of β2-adrenoceptor-mediated responses by short-acting β2-adrenoceptor agonists in human lung mast cells. Br J Pharmacol. 2003;138:512–520. doi: 10.1038/sj.bjp.0705050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MK, Hiroi J. Inhibition of IgE-dependent histamine release from human dispersed lung mast cells by anti-allergic drugs and salbutamol. Br J Pharmacol. 1987;90:421–429. doi: 10.1111/j.1476-5381.1987.tb08972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Knoll BJ, Barber R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharmacol Sci. 1999;20:279–286. doi: 10.1016/s0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet. 1993;342:833–837. doi: 10.1016/0140-6736(93)92695-p. [DOI] [PubMed] [Google Scholar]
- Ennis M. Current techniques of histamine determination: automated fluorometric assays. Handbook Exp Pharmacol. 1991;97:31–38. [Google Scholar]
- Gilbert HS, Ornstein L. Basophil counting with a new staining method using alcian blue. Blood. 1975;46:279–286. [PubMed] [Google Scholar]
- Holgate ST, Hardy C, Robinson C, Agius RM, Howarth PH. The mast cell as a primary effector cell in the pathogenesis of asthma. J Allergy Clin Immunol. 1986;77:274–282. doi: 10.1016/s0091-6749(86)80104-x. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol. 2003;111:S18–S36. doi: 10.1067/mai.2003.25. [DOI] [PubMed] [Google Scholar]
- Howarth PH, Durham SH, Lee TM, Kay AB, Church MK, Holgate ST. Influence of albuterol, cromolyn sodium and ipratropium bromide on the airway and circulating mediator responses to allergen bronchial provocation in asthma. Am Rev Respir Dis. 1985;132:986–992. doi: 10.1164/arrd.1985.132.5.986. [DOI] [PubMed] [Google Scholar]
- January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, et al. β2-Adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem. 1997;272:23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. The classification of drugs and drug receptors in isolated tissues. Pharmacol Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- Ketchell RI, Jensen MW, Spina D, O'Connor BJ. Dose-related effects of formoterol on airway responsiveness to adenosine 5' monophosphate and histamine. Eur Respir J. 2002;19:611–616. doi: 10.1183/09031936.02.00332001. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- Jokic R, Swystun VA, Davis BE, Cockcroft DW. Regular inhaled salbutamol. Effect on airway responsiveness to methacholine and adenosine 5'-monophosphate and tolerance to bronchoprotection. Chest. 2001;119:370–375. doi: 10.1378/chest.119.2.370. [DOI] [PubMed] [Google Scholar]
- Lau HYA, Wong PLE, Lai CKW, Ho JKS. Effects of long-acting β2-adrenoceptor agonists on mast cells of rat, guinea pig, and human. Int Arch Allergy Immunol. 1994;105:177–180. doi: 10.1159/000236821. [DOI] [PubMed] [Google Scholar]
- Lowman MA, Rees PH, Benyon RC, Church MK. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung, adenoids, tonsils and colon in response to IgE-dependent and non-immunologic stimuli. J Allergy Clin Immunol. 1988;81:590–597. [PubMed] [Google Scholar]
- Nials AT, Ball DI, Butchers PR, Coleman RA, Humbles AA, Johnson M, et al. Formoterol on airway smooth muscle and human lung mast cells – a comparison with salbutamol and salmeterol. Eur J Pharmacol. 1994;251:127–135. doi: 10.1016/0014-2999(94)90392-1. [DOI] [PubMed] [Google Scholar]
- Nightingale JA, Rogers DF, Barnes PJ. Differential effect of formoterol on adenosine monophosphate and histamine reactivity in asthma. Am J Respir Crit Care Med. 1999;159:1786–1790. doi: 10.1164/ajrccm.159.6.9809090. [DOI] [PubMed] [Google Scholar]
- O'Connor BJ, Aikman SL, Barnes PJ. Tolerance to the nonbronchodilator effects of inhaled β2-agonists in asthma. N Engl J Med. 1992;327:1204–1208. doi: 10.1056/NEJM199210223271704. [DOI] [PubMed] [Google Scholar]
- O'Connor BJ, Fuller RW, Barnes PJ. Non-bronchodilator effects of inhaled β2-agonists. Greater protection against adenosine monophosphate- than methacholine-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1994;150:381–387. doi: 10.1164/ajrccm.150.2.8049819. [DOI] [PubMed] [Google Scholar]
- O'Donnell SR, Wanstall JC. Evidence that ICI 118,551 is a potent, highly beta2-selective adrenoceptor antagonist and can be used to characterize beta-adrenoceptor populations in tissues. Life Sci. 1980;27:671–677. doi: 10.1016/0024-3205(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Orange RP, Austen WG, Austen KF. Immunological release of histamine and slow reacting substance of anaphylaxis from human lung. I. Modulation by agents influencing cellular levels of cyclic 3'5'-adenosine monophosphate. J Exp Med. 1971;134:136–148. [PMC free article] [PubMed] [Google Scholar]
- Pittman RN, Reynolds EE, Molinoff PB. Relationship between intrinsic activities of agonists in normal and desensitized tissue and agonist-induced loss of beta-adrenergic receptors. J Pharmacol Exp Ther. 1984;230:614–618. [PubMed] [Google Scholar]
- Proud D, Reynolds CJ, Lichtenstein LM, Kagey-Sobotka A, Togias A. Intranasal salmeterol inhibits allergen-induced vascular permeability but not mast cell activation or cellular infiltration. Clin Exp Allergy. 1998;28:868–875. doi: 10.1046/j.1365-2222.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Russo C, Zeng D, Prosperini G, Spicuzza L, Guarino F, Polosa R. Effect of salbutamol on nasal symptoms and mast cell degranulation induced by adenosine 5' monophosphate nasal challenge. Clin Exp Allergy. 2005;35:1192–1196. doi: 10.1111/j.1365-2222.2005.02318.x. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: respiratory tolerance to regular β2-agonist use in patients with asthma. Ann Intern Med. 2004;140:802–813. doi: 10.7326/0003-4819-140-10-200405180-00010. [DOI] [PubMed] [Google Scholar]
- Schild H. Histamine release in anaphylactic shock of isolated lungs of guinea pigs. Q J Exp Physiol. 1937;26:165–179. [Google Scholar]
- Schleimer RP, Schulman ES, MacGlashan DW, Peters SP, Hayes EC, Adams GK, et al. Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest. 1983;71:1830–1835. doi: 10.1172/JCI110938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola A-M, Chong LK, Chess-Williams R, Peachell PT. Influence of agonist intrinsic activity on the desensitisation of β2-adrenoceptor-mediated responses in mast cells. Br J Pharmacol. 2004a;143:71–80. doi: 10.1038/sj.bjp.0705905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola A-M, Chong LK, Suvarna SK, Chess-Williams R, Peachell PT. Desensitization of mast cell β2-adrenoceptor-mediated responses by salmeterol and formoterol. Br J Pharmacol. 2004b;141:163–171. doi: 10.1038/sj.bjp.0705599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MGH, Swan C, Jobson TM, Rees S, Hall IP. Effects of a range of β2-adrenoceptor agonists on changes in intracellular cyclic AMP and on cyclic AMP driven gene expression in cultured human airway smooth muscle cells. Br J Pharmacol. 1999;128:721–729. doi: 10.1038/sj.bjp.0702829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Sommerhoff CP. Mast cell tryptases and airway remodeling. Am J Respir Crit Care Med. 2001;164:S52–S58. doi: 10.1164/ajrccm.164.supplement_2.2106058. [DOI] [PubMed] [Google Scholar]
- Su Y-F, Harden TK, Perkins JP. Catecholamine-specific desensitization of adenylate cyclase; evidence for a multi-step process. J Biol Chem. 1980;255:7410–7419. [PubMed] [Google Scholar]
- Swystun VA, Gordon JR, Davis B, Zhang X, Cockcroft DW. Mast cell tryptase release and asthmatic responses to allergen increase with regular use of salbutamol. J Allergy Clin Immunol. 2000;106:57–64. doi: 10.1067/mai.2000.107396. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Jensen MW, Aikman SL, Harris JG, Barnes PJ, O'Connor BJ. Comparison of salmeterol and albuterol-induced bronchoprotection against adenosine monophosphate and histamine in mild asthma. Am J Respir Crit Care Med. 1997;156:1731–1737. doi: 10.1164/ajrccm.156.6.9703047. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Peachell PT, Lichtenstein LM. Isoproterenol-induced inhibition of immunoglobulin E-mediated release of histamine and arachidonic acid metabolites from the human lung mast cell. J Pharmacol Exp Ther. 1988;247:209–217. [PubMed] [Google Scholar]
- Van der Heijden PJCM, Van Amsterdam JGC, Zaagsma J. Desensitization of smooth muscle and mast cell β-adrenoceptors in the airways of the guinea pig. Eur J Resp Dis. 1984;65(Suppl.)(135):128–134. [PubMed] [Google Scholar]
- Waldeck B. β-adrenoceptor agonists and asthma – 100 years of development. Eur J Pharmacol. 2002;445:1–12. doi: 10.1016/s0014-2999(02)01728-4. [DOI] [PubMed] [Google Scholar]
- Williams CMM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]


