Abstract
Background and purpose:
Although it is well accepted that cannabinoids modulate intestinal motility by reducing cholinergic neurotransmission mediated by CB1 receptors, it is not known whether the endocannabinoids are involved in more complex circuits and if they interact with other systems. The aim of the present study was to examine possible interactions between cannabinoid CB1 receptors and purines in the control of spontaneous contractility of longitudinal muscle in mouse ileum.
Experimental approach:
The mechanical activity of longitudinally oriented ileal segments from mice was recorded as isometric contractions.
Key results:
The selective CB1 receptor agonist, N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA) reduced, concentration dependently, spontaneous contractions in mouse ileum. This effect was almost abolished by tetrodotoxin (TTX) or atropine. Inhibition by ACEA was not affected by theophylline (P1 receptor antagonist) or by P2Y receptor desensitization with adenosine 5′[β-thio]diphosphate trilithium salt, but was significantly reversed by pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (P2 receptor antagonist), by P2X receptor desensitization with α,β-methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP) or by 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino) bis(1,3,5-naphthalenetrisulphonic acid)] (P2X receptor antagonist). Contractile responses to α,β-MeATP (P2X receptor agonist) were virtually abolished by TTX or atropine, suggesting that they were mediated by acetylcholine released from neurones, and significantly reduced by ACEA.
Conclusion and implications:
In mouse ileum, activation of CB1 receptors, apart from reducing acetylcholine release from cholinergic nerves, was able to modulate negatively, endogenous purinergic effects, mediated by P2X receptors, on cholinergic neurons. Our study provides evidence for a role of cannabinoids in the modulation of interneuronal purinergic transmission.
Keywords: cannabinoids, CB1 receptors, purines, ATP, cholinergic transmission, P2X receptors, enteric nervous system
Introduction
Over the past decade, the identification of a system of endogenous cannabinoids has given the functional basis for the effects of the active constituents of Cannabis sativa. The endocannabinoid system contains specific receptors, specific ligands and specific enzymes for synthesis and degradation of the endocannabinoids (De Petrocellis et al., 2004). There are at least two established cannabinoid G-protein-coupled receptors, CB1 and CB2 receptors (nomenclature follows Alexander et al., 2008). CB1 receptors are highly expressed in the central and peripheral nervous systems, although non-neuronal expression sites have been also described (Cota et al., 2003). CB2 receptors are predominantly, but not exclusively, present in immune cells, suggesting that endocannabinoids may play a role as immunomodulators (Samson et al., 2003).
The involvement of the cannabinoid system in the regulation of different gastrointestinal functions has been well established (see Izzo and Coutts, 2005; Massa et al., 2005; Massa and Monory, 2006). In fact, several regulatory functions have been related to cannabinoids, including gut motility, ion transport, gastric and intestinal secretion, epithelial wound healing, feeding behaviour (Gomez et al., 2002; MacNaughton et al., 2004; Izzo and Coutts, 2005; Massa et al., 2005; Wright et al., 2005; Massa and Monory, 2006). CB1 receptors have been identified on enteric nerves and their activation results in inhibition of excitatory transmission in vitro (Coutts and Pertwee, 1997; Croci et al., 1998; Izzo et al., 1998; Storr et al., 2004; Mulèet al., 2007a,b;) and intestinal propulsion in vivo (Colombo et al., 1998; Izzo et al., 1999; 2001; Pinto et al., 2002; Casu et al., 2003; Carai et al., 2006).
There is substantial evidence that the endocannabinoid system is involved in the control of intestinal motility, in both the small and large intestines. However, besides the reduction of cholinergic excitatory neurotransmission through prejunctional CB1 receptors, little is known about the possible involvement of endocannabinoids in complex circuits and on crosstalk with other systems. Recent work has provided evidence for a cannabinoid role in the modulation of GABA function in the myenteric plexus-longitudinal muscle preparation of guinea pig ileum (Begg et al., 2002b), whereas Carai et al. (2006) reported that cannabinoid and opioid receptor systems did not interact in regulating gastrointestinal transit in mice. However, a crosstalk between κ-opioid and cannabinoid CB1 receptors may take place under inflammatory conditions (Capasso et al., 2008). Apart from a study by Begg et al. (2002a) showing that activation of pre-synaptic CB1 receptors reduces the electrically-evoked release of adenosine in guinea-pig ileum, to our knowledge, to date no study has addressed the issue of the functional relationship between cannabinoids and purines in the regulation of intestinal contractility.
Purine nucleotides and nucleosides play an important role in the modulation of motor functions in the gastrointestinal tract and both adenosine and ATP receptors participate in such regulation. Adenosine acts through P1 receptors that have been further subdivided in A1, A2a, A2B and A3 receptors (Ralevic and Burnstock, 1998). Selective receptors for ATP and ADP, designated as P2 receptors, have been divided into two families: P2X ionotropic receptors; and P2Y metabotropic G-protein coupled receptors (Abbracchio and Burnstock, 1994). Seven mammalian P2X receptor subtypes (P2X1 up to P2X7) and eight mammalian P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) have been cloned and functionally defined as P2 receptors (Burnstock, 2007). The motor effects (contractions and/or relaxation) induced by purines are species- and region-specific in the gastrointestinal tract. In particular, purines may act through postjunctional receptors to affect directly the muscular contractility or through neuronal receptors, to modulate neurotransmitter release, prejunctionally or pre-synaptically (Cunha and Ribeiro, 2000; Kadowaki et al., 2000; Galligan, 2002; Giaroni et al., 2002; Woods et al., 2003; Zizzo et al., 2006; Bornstein, 2008).
Recently, we have demonstrated that longitudinal smooth muscle of mouse ileum is under an excitatory tonic influence by neuronal acetylcholine, which can be negatively modulated by CB1 receptor activation (Baldassano et al., 2008). Therefore, the present study was undertaken in the attempt to examine a possible interaction between purinergic system and cannabinoids in this experimental model, and to clarify if modulation of the ongoing release of acetylcholine can be the final target for both systems. Specifically, we analysed the effects of blocking P1 and P2 (P2X and P2Y) receptors on the responses induced by N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA), a selective CB1 cannabinoid receptor agonist.
Methods
Animals
All animal procedures were in conformity with the Italian D.L. no. 116 of 27 January 1992 and associated guidelines in the European Communities Council Directive of 24 November 1986 (86/609/ECC). Adult male mice (C57BL/10SnJ; weighing 25.5 ± 0.5 g), obtained from Charles River Laboratories (Calco-Lecco, Italy) were used for the study. Animals were housed in standard conditions under a constant light–dark cycle, constant temperature (22 ± 1°C) and humidity (55 ± 5%), with free access to food and water.
General
Animals were killed by cervical dislocation. The abdomen was immediately opened and the ileum was removed and placed in a modified Krebs solution of the following composition (mmol·L−1): NaCl 119; KCl 4.5; MgSO4 2.5; NaHCO3 25; KH2PO4 1.2, CaCl2 2.5, glucose 11.1. Segments (20 mm in length) were suspended in a four-channel organ bath containing 8 mL of oxygenated (95% O2 and 5% CO2) Krebs solution maintained to 37°C. The distal end of each segment was tied to organ holders and the proximal end was secured with a silk thread to an isometric force transducer (FORT 25, Ugo Basile, Biological Research Apparatus, Comerio VA, Italy). Mechanical activity was digitized on an analog-to-digital converter, visualized, recorded and analysed on a personal computer using the PowerLab/400 system (Ugo Basile). Longitudinal preparations were subjected to an initial tension of 2 mN and were allowed to equilibrate for at least 30 min. Rhythmic spontaneous contractions developed in all preparations.
Experimental protocol
After equilibration responses of the preparations to cumulative concentrations of two CB1 cannabinoid receptor agonists, ACEA (0.01–10 µmol·L−1) or N-(cyclopropyl)-5Z,8Z,11Z,14Z-eicosatetraemamide (ACPA; 0.01–10 µmol·L−1), were evaluated. The concentration-response curves to ACEA were repeated after theophylline, a non-selective P1 purinoceptor antagonist (10 µmol·L−1 for 30 min), pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (PPADS), a non-selective P2 receptor antagonist (50 µmol·L−1 for 30 min), desensitization of P2Y receptors with adenosine 5′[β-thio]diphosphate trilithium salt (ADPβS; 10 µmol·L−1 for 30 min), desensitization of P2X receptors α,β-methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP) (50 µmol·L−1 for 30 min) or 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino) bis(1,3,5-naphthalenetrisulphonic acid)] (NF279), a P2X receptor antagonist (1 µmol·L−1 for 30 min). These agents were added to the organ bath before the agonists. ACEA was added to the bath at increasing concentrations in volumes of 80 µL. Each ACEA concentration was left in contact with the tissue for 10 min. Each preparation was tested with a single purinoreceptor inhibitor. The concentrations of the inhibitors used were determined from published data.
In order to evaluate the efficacy of purinergic antagonists or desensitizing procedures, the effects induced by adenosine – a P1 purinoceptor agonist (1–100 µmol·L−1), ATP – a P2 purinoceptor agonist (10 µmol·L−1–1 mmol·L−1), and α,β-MeATP – a P2X purinoceptor agonist (10–100 µmol·L−1), were examined before and after the respective antagonists or desensitizing agents. Agonists were added into the bath at increasing concentrations in volumes of 80 µL and were applied for 3 min at 20 min intervals. α,β-MeATP (10–100 µmol·L−1) was tested also in the presence of TTX (1 µmol·L−1), atropine (1 µmol·L−1) or ACEA (10 µmol·L−1). These agents were added to the organ bath 30 min before the agonists.
In a different set of experiments to further explore interactions between CB1 and P2X receptors in the modulation of cholinergic motor neurons, the effects of ACEA (10 µmol·L−1) on the contractions evoked by electrical field stimulation (EFS) were evaluated in the absence and presence of NF279 (1 µmol·L−1). EFS was applied by an S88 square-wave pulse generator (Grass Medical Instruments, Quincy, MA, USA) coupled via a stimulus isolation unit (Grass SIU5) to a pair of platinum electrodes placed in parallel on either side of the segments. EFS (0.5 ms duration, supramaximal voltage, in trains of 5 s, 8 Hz) was performed at 5 min intervals and stable and reproducible responses were observed for hours. The evoked contractions were abolished by the muscarinic receptor antagonist atropine (1 µmol·L−1) or by the neuronal blocker TTX (1 µmol·L−1) suggesting that they were mediated by cholinergic nerves.
Lastly, ACEA (0.01–10 µmol·L−1) was tested on ileum pre-contracted by carbachol (1 µmol·L−1).
Statistical analysis
Mean amplitude of spontaneous contractions was measured prior to and following drug administration after a new steady state was reached. Results are expressed as the changes in mean amplitude of the phasic contractions and reported as percentages of the values obtained in the control (e.g. −100% corresponds to the abolition of spontaneous activity). Relaxant and contractile effects induced by purinergic agonists were expressed respectively as a percentage of the maximal response. All data are expressed as mean values ± standard error of the mean. The letter n indicates the number of experimental animals. Statistical analysis was performed by means of two-way analysis of variance, followed by Bonferroni's post hoc test. A probability value of less than 0.05 was regarded as significant.
Drugs
The following drugs were used: ACEA, ACPA, adenosine, ATP, α,β-MeATP, ADPβS, atropine sulphate, carbachol, PPADS, theophylline, (Sigma Chemical Corp., St. Louis, MO, USA); NF279 (Tocris-Bioscience, Bristol, UK); TTX (Alomone Labs Ltd., Jerusalem, Israel). All drugs were dissolved in distilled water, except ACEA, adenosine and NF279, which were initially dissolved in dimethyl sulphoxide, and ACPA in ethanol. The working solutions were prepared freshly on the day of the experiments by diluting the stock solutions with Krebs solution. Control experiments using dimethyl sulphoxide alone, appropriately diluted, showed that it did not affect the contractility of the ileal segments.
Results
Isolated segments of mouse ileum displayed spontaneous activity, characterized by phasic contractions with amplitude of about 300 mg and frequency of about 30 cycles·min−1. As previously described, these contractions were induced by acetylcholine released from neurones because they were reduced by atropine (1 µmol·L−1) and increased by neostigmine (10 µmol·L−1) (Figure 1) (Baldassano et al., 2008).
Figure 1.
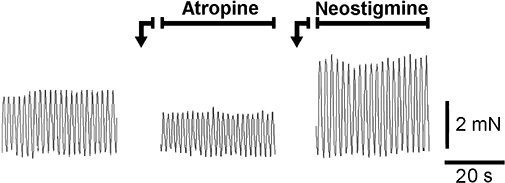
Typical tracings showing the effects of atropine (1 µmol·L−1) or neostigmine (10 µmol·L−1) on the spontaneous contractions of mouse ileum longitudinal muscle.
CB1 receptor activation and basal mechanical activity
The selective CB1 receptor agonists, ACEA (0.01–10 µmol·L−1) or ACPA (0.01–10 µmol·L−1), produced concentration-dependent inhibitory effects, consisting in a decrease of the mean amplitude of spontaneous contractions, without changes in the resting tone. As previously shown (Baldassano et al., 2008), the effects of ACEA were antagonized by SR141716A (0.1 µmol·L−1), a CB1 receptor antagonist, and it was almost abolished by the pretreatment with TTX (1 µmol·L−1) or atropine (1 µmol·L−1), suggesting that CB1 receptor activation reduces an ongoing neural release of acetylcholine (Figure 2).
Figure 2.

Typical tracings showing the inhibitory effects of the selective CB1 receptor agonist N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA; 10 µmol·L−1) on the spontaneous mechanical activity of mouse ileal longitudinal muscle in control conditions or after SR141716A (100 nmol·L−1), a CB1 receptor antagonist, tetrodotoxin (TTX; 1 µmol·L−1) or atropine (1 µmol·L−1).
The inhibitory effects induced by ACEA (0.01–10 µmol·L−1) were not affected by theophylline (10 µmol·L−1), which per se did not affect the spontaneous mechanical activity, but they were almost abolished by PPADS (50 µmol·L−1 for 30 min), indicating that P2, but not P1, receptors were involved in the effects evoked by the selective CB1 agonist (Figure 3).
Figure 3.

Concentration-response curves for the inhibitory effects induced by N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA) in the control, in the presence of theophylline (10 µmol·L−1), a P1 receptor antagonist or pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (PPADS; 50 µmol·L−1), a P2 receptor antagonist. The inhibitory response is expressed as percent change of the resting activity (−100% corresponds to the abolition of spontaneous activity). All values are means ± standard error of the mean (n = 5 for each treatment). *P < 0.05 compared with control value.
In order to clarify the subtype of P2 receptors involved in the cannabinoid modulation of spontaneous activity, we tested the effects of ACEA after desensitization of P2Y or P2X purinoceptors . Desensitization of P2Y receptors with ADPβS (10 µmol·L−1 for 30 min) did not affect the spontaneous mechanical activity. A transient relaxation occurred on addition of ADPβS to the bath, after which the muscle recovered its basal tone. Desensitization of P2X purinoceptors with α,β-MeATP (50 µmol·L−1 for 30 min) produced a significant reduction in the amplitude of the phasic spontaneous contractions (43 ± 6%, n = 5; P < 0.001) that was more or less maintained as long as the drug was present. A transient contractile response occurred on addition of α,β-MeATP to the bath, after which the muscle recovered its basal tone.
The inhibition of the spontaneous contractions induced by ACEA (0.01–10 µmol·L−1) were not affected by P2Y receptor desensitization with ADPβS, whereas they were almost abolished by P2X receptor desensitization with α,β-MeATP (Figure 4), indicating that P2X receptors are involved in these effects of ACEA. To corroborate this finding we tested the effects of NF279, a P2X receptor antagonist. NF279 (1 µmol·L−1) markedly and significantly decreased the ACEA-induced inhibitory responses (Figure 4). In addition NF279 per se caused a concentration-dependent decrease of the spontaneous contractions, which was prevented by TTX (1 µmol·L−1) or atropine (1 µmol·L−1) (Figure 5). Moreover, NF279 (10 µmol·L−1), abolished the increase of the spontaneous contraction amplitude induced by neostigmine (10 µmol·L−1) (Figure 5).
Figure 4.

Concentration-response curves for the inhibitory effects induced by N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA) in the control, after P2X receptor desensitization with α,β-methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP; 50 µmol·L−1 for 30 min), after P2Y receptor desensitization with adenosine 5′[β-thio]diphosphate trilithium salt (ADPβS; 10 µmol·L−1 for 30 min) or in the presence of NF279 (1 µmol·L−1), a P2X receptor antagonist. The inhibitory response is expressed as percent change of the resting activity (−100% corresponds to the abolition of spontaneous activity). All values are means ± standard error of the mean (n = 4 for each treatment). *P < 0.05 compared with control value.
Figure 5.

Inhibitory effects induced by NF279, on the amplitude of the spontaneous contractions in the control (n = 8), in the presence of tetrodotoxin (TTX; 1 µmol·L−1) (n = 4) or in the presence of atropine (1 µmol·L−1) (n = 4) and on the increase of the spontaneous contractions induced by neostigmine (10 µmol·L−1) (n = 4). The ordinate shows the amplitude of the contractions expressed as percent change of the resting activity. All values are means ± standard error of the mean. *P < 0.05 compared with control value. #P < 0.05 compared with neostigmine.
Purine-mediated effects
Adenosine (1–100 µmol·L−1) caused a concentration-dependent reduction of the amplitude of spontaneous contractions, which was significantly antagonized by theophylline (10 µmol·L−1) (Figure 6).
Figure 6.
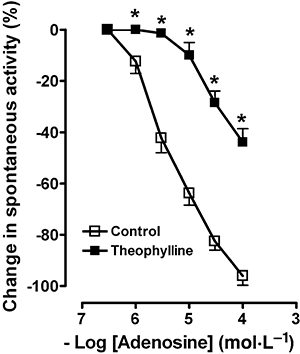
Concentration-response curves for the inhibitory effects induced by adenosine, a P1 receptor agonist, in the control or in the presence of theophylline (10 µmol·L−1), a P1 receptor antagonist. The inhibitory response is expressed as percent change of the resting activity (−100% corresponds to the abolition of spontaneous activity). All values are means ± standard error of the mean (n = 4). *P < 0.05 compared with control value.
ATP (10 µmol·L−1–1 mmol·L−1) evoked a rapid and transient concentration-dependent relaxation, which was followed at the highest concentrations (0.1–1 mmol·L−1) by a contraction. ATP responses were significantly reduced in the presence of PPADS (50 µmol·L−1). The relaxation to ATP was abolished by desensitization of P2Y receptors with ADPβS (10 µmol·L−1 for 30 min), whereas the contractile responses were blocked by desensitization of P2X receptors with α,β-MeATP (50 µmol·L−1 for 30 min) (Figure 7). α,β-MeATP (10–100 µmol·L−1), evoked a concentration-dependent contractile response, which was abolished by desensitization of P2X receptors with this agonist (50 µmol·L−1 for 30 min) and significantly antagonized by NF279 (10 µmol·L−1). Moreover, the contraction induced by α,β-MeATP (10–100 µmol·L−1) was significantly reduced by TTX (1 µmol·L−1) or atropine (1 µmol·L−1), suggesting the involvement of neuronal acetylcholine (Figure 8). Lastly, the contractile responses to α,β-MeATP were significantly reduced by ACEA (10 µmol·L−1) (Figure 9).
Figure 7.

Relaxant or contractile effects evoked by ATP (10 µmol·L−1–1 mmol·L−1), a P2 receptor agonist, in mouse ileal longitudinal muscle in control and after pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (PPADS; 50 µmol·L−1), a P2 receptor antagonist, desensitization of P2Y receptors with adenosine 5′[β-thio]diphosphate trilithium salt (ADPβS; 10 µmol·L−1 for 30 min) or desensitization of P2X receptors with α,β-methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP; 50 µmol·L−1 for 30 min). All values are means ± standard error of the mean. (n = 5 for each treatment) and are reported as a percentage of the maximal effect. *P < 0.05 compared with control value.
Figure 8.

Concentration-response curves for the contractile effects induced by α,β-methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP), a P2X receptor agonist in the control, after P2X receptor desensitization with α,β-MeATP (50 µmol·L−1 for 30 min), in the presence of NF279 (1 µmol·L−1), tetrodotoxin (TTX; 1 µmol·L−1) or atropine (1 µmol·L−1). All values are means ± standard error of the mean (n = 5 for each treatment) and are reported as a percentage of the maximal effect. *P < 0.05 compared with control value.
Figure 9.

Concentration-response curves for the contractile effects induced by α,β-methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP), P2X receptor agonist in the control or after N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA; 10 µmol·L−1). All values are means ± standard error of the mean (n = 4) and are reported as a percentage of the maximal effect. *P < 0.05 compared with control value.
CB1 receptor activation and cholinergic evoked responses
EFS (supramaximal voltage, 0.5 ms pulse duration, in trains of 5 s, 8 Hz) of the isolated mouse ileum caused a cholinergically mediated twitch contraction that was completely abolished by atropine (1 µmol·L−1) or by TTX (1 µmol·L−1). ACEA (10 µmol·L−1) markedly reduced the electrically evoked cholinergic contraction. This effect was significantly, but not completely, reduced in the presence of NF279 (1 µmol·L−1), which per se reduced the EFS-induced contraction (n = 5) (Figure 10).
Figure 10.
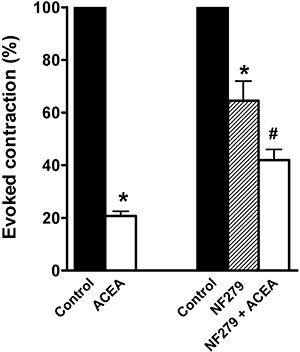
Inhibitory effects induced by N-(2-chloroethyl)5,8,11,14-eicosaetraenamide (ACEA; 10 µmol·L−1) on the contractions evoked by EFS (supramaximal voltage, 0.5 ms pulse duration, in trains of 5 s, 8 Hz) in the absence or in the presence of 8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino) bis(1,3,5-naphthalenetrisulphonic acid)] (NF279; 1 µmol·L−1). All values are means ± standard error of the mean (n = 5) and are expressed as a percentage of the respective control taken as 100%. *P < 0.05 compared with control value. #P < 0.05 compared with ACEA or NF279.
ACEA (0.01–10 µmol·L−1) had no significant influence on the smooth-muscle contraction level stimulated by carbachol- (% change of tension – 2.3 ± 3.1, n = 4).
Discussion and conclusions
The results of the present study suggest that, in mouse ileum, activation of CB1 receptors, apart from reducing acetylcholine release from cholinergic nerves, is able to modulate negatively an endogenous ongoing purinergic action on cholinergic neurons, mediated by P2X receptors.
In the gastrointestinal tract, acetylcholine is regarded as the major excitatory neurotransmitter and the prime regulator of gastrointestinal motility. The release of acetylcholine from enteric nerves is under a well-regulated pre-synaptic and/or pre-junctional control, involving specific neuronal receptors. These include cannabinoid CB1 (Coutts and Pertwee, 1997; Croci et al., 1998; Izzo et al., 1998; Storr et al., 2004; Mulèet al., 2007b) and purinergic P1 and P2 receptors, which, upon activation, enhance or inhibit the release of acetylcholine, depending on the purinoceptor subtype involved (Sawynok and Jhamandasn, 1976; Vizi and Knoll, 1976; Kadowaki et al., 2000; Lee et al., 2001; De Man et al., 2003a,b;).
In longitudinal muscle of mouse ileum, functional evidence for an ongoing release of neuronal acetylcholine, which increases the spontaneous mechanical activity has been reported (Vial and Evans, 2001; Baldassano et al., 2008). In this experimental model, activation of neuronal cannabinoid CB1 receptors decreases the spontaneous mechanical contractions by modulating negatively the cholinergic excitatory influence (Baldassano et al., 2008).
Our results suggest that the release of acetylcholine that sustains contraction through the activation of muscarinic receptors on the smooth muscle is facilitated by endogenous ATP, acting pre-synaptically on cholinergic neurons through P2X receptors. In fact, we performed experiments after prolonged exposure of the preparations to ADPβS or α,β-MeATP, which have been used as specific desensitizing agents to block P2Y or P2X receptor-mediated responses respectively (Ralevic and Burnstock, 1998; De Man et al., 2003a; Serio et al., 2003; Mulèet al., 2005). Desensitization of P2X purinergic receptors with α,β-MeATP, which antagonized the contraction to ATP or to α,β-MeATP in our preparations, reduced significantly the spontaneous contractions, whereas desensitization of P2Y receptors, which antagonized the relaxation evoked by ATP, failed to affect the amplitude of spontaneous contractions. These observations suggest a role for P2X receptors, but not for P2Y receptors, in control of the spontaneous motility in mouse ileum. On the other hand, theophylline, at a concentration effective in antagonizing the inhibitory effects on muscular contractions induced by adenosine, did not affect the spontaneous mechanical activity, ruling out a role for purinergic P1 receptors in the control of spontaneous contractility in the mouse ileum. In addition, NF279, a compound that preferentially blocks P2X1 receptors (Rettinger et al., 2000), but also inhibits P2X2 and P2X3 receptor-mediated responses (Damer et al., 1998; Klapperstuck et al., 2000), reduced in a concentration-dependent manner, the spontaneous contractions and this action was no longer observed after block of nerve action potentials with TTX or antagonism of muscarinic receptors with atropine. NF279, which antagonized the contraction to α,β-MeATP, also abolished the increase of spontaneous contractions caused by neostigmine, which prevents the metabolic deactivation of acetylcholine, supporting our hypothesis that endogenous activation of P2X receptors facilitates the ongoing release of neuronal acetylcholine. On the other hand, the hypothesis that in our preparation P2X receptors are functionally expressed on cholinergic nerves is supported by the observations that α,β-MeATP, which activates homomeric P2X receptors composed of P2X1 or P2X3 subunits (North, 2002), caused a contractile response virtually abolished by TTX or atropine and the block of P2X receptors by NF279 reduced significantly the cholinergic contractile response to electrical nerve activation. The relatively high concentration of α,β-MeATP required to evoke a response may reflect restricted access of the agonist to the P2X receptors on enteric neurons. Although immunohistochemistry failed to show P2X1 receptor reactivity in the myenteric plexus of the mouse ileum (Vial and Evans, 2001; Giaroni et al., 2002), there is functional evidence for the presence of pre-synaptic P2X1 receptors in the mouse enteric nervous system (Vial and Evans, 2001) and P2X3 receptors regulate intestinal peristalsis in the mouse (Bian et al., 2003). Indeed, Vial and Evans (2001) showed that in mouse ileum the contractions to α,β-MeATP were partially blocked by TTX, but not by atropine, and ATP evoked relaxation but no contraction. The discrepancy with our results could be explained by considering the pre-load of the muscular segments. In our experiments, longitudinal preparations were subjected to an initial tension of 200 mg rather than 1 g. It is well accepted that cholinergic nerve terminals are easily damaged by excessive stretching and 1 g in mouse ileum could represent a considerable tension, which facilitates the revealing of relaxation, but impairs cholinergic pathways.
To assess if modulation of the ongoing release of acetylcholine can be the final target for both the cannabinoid and purine systems, we tested the inhibitory effects induced by ACEA in the presence of purinergic antagonists or desensitizing agents. We found that the response to the CB1 agonist was virtually abolished by PPADS, desensitization of P2X receptors with α,β-MeATP or NF279 but not modified by theophylline or desensitization of P2Y receptors with ADPβS. These results suggest that there is a functional link between PPADS-sensitive P2X receptors and CB1 receptors. It is possible that the activation of CB1 receptors negatively modulates activity of purinergic neurones, which, in turn, via P2X receptors, triggers acetylcholine release from cholinergic neurones. Indeed, one might object that the reduction of the spontaneous contractions caused by the block of P2X with α,β-MeATP or NF279 could account for the inhibition of ACEA actions. However, this supposition can be ruled out because ACEA kept on inhibiting when the spontaneous mechanical activity was reduced by a sub-maximal concentration of adenosine (data not shown). To further support the hypothesis of a relationship between CB1 and P2X receptors we tested ACEA on electrically induced cholinergic contractions in the absence and presence of NF279. The observations that the CB1 agonist reduced the electrically evoked cholinergic responses and this effect was significantly, but not completely, reduced in the presence of NF279 would confirm that CB1 receptor activation is able to modulate not only the prejunctional release of acetylcholine but also the pre-synaptic purinergic pathway, which in turn activates P2X receptors. On the other hand, the cannabinoid agonist failed to affect the ileal tone in segments contracted by carbachol, which acts by activating muscarinic receptors on the smooth muscle, ruling out an involvement of post-junctional mechanisms in its action.
So far, an interaction between CB1 and P2X receptors has been documented only in nociceptive sensory neurons (Krishtal et al., 2006). To our knowledge, this is the first evidence for a possible role of cannabinoids in the modulation of enteric interneuronal purinergic transmission. Although recent data have ruled out an effect of anandamide on neuro-neuronal interaction in rodent small intestine (Yuece et al., 2007), our findings are in agreement with the studies by Lòpez-Redondo et al. (1997), who proposed, in guinea pig ileum, a neuro-neuronal action site of cannabinoids, because cannabinoid agonists were able to reduce neuronal excitatory postsynaptic potentials. Moreover, in the same preparation, Begg et al. (2002a) showed that, in addition to being able to reduce acetylcholine release, activation of pre-synaptic CB1 receptors also reduces the electrically evoked release of adenosine. Therefore, cannabinoids can have a more complex spectrum of actions than has previously been supposed. However, the observation that – in our experiments – contractile responses to the P2X receptor agonist, α,β-MeATP, which are mediated by acetylcholine release, were significantly reduced by ACEA, suggests that CB1 receptors are also located post-synaptically to inhibit cholinergic nerves, in line with previous observations suggesting a pre-junctional inhibitory role on the excitatory cholinergic neurotransnission (Coutts and Pertwee, 1997; Croci et al., 1998; Izzo et al., 1998; Storr et al., 2004; Mulèet al., 2007b).
In conclusion, in mouse ileum, cannabinoids, through CB1 receptors, negatively modulate acetylcholine release, acting not only prejunctionally on cholinergic neurons, but also interfering with the purinergic system. In fact, endogenous ATP, through P2X receptors, would sustain the ongoing release of acetylcholine and this action can be influenced by CB1 receptor activation. The proposed mechanism might participate in the suppression of gastrointestinal motor function following activation of the cannabinoid receptors in the bowel.
Acknowledgments
This work was supported by a grant from MIUR, Italy.
Glossary
Abbreviations:
- ACEA
N-(2-chloroethyl)5,8,11,14-eicosaetraenamide
- ACPA
N-(cyclopropyl)-5Z,8Z,11Z,14Z-eicosatetraemamide
- ADPβS
adenosine 5′[β-thio]diphosphate trilithium salt
- α,β-MeATP
α,β-methyleneadenosine 5′-triphosphate lithium salt
- EFS
electrical field stimulation
- NF279
8,8′-[carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino) bis(1,3,5-naphthalenetrisulphonic acid)]
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) tetrasodium salt
- SR 141716A
(N-piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- TTX
tetrodotoxin
Conflict of interest
None.
References
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano S, Serio R, Mulè F. Cannabinoid CB1 receptor activation modulates spontaneous contractile activity in mouse ileal longitudinal muscle. Eur J Pharmacol. 2008;582:132–138. doi: 10.1016/j.ejphar.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Begg M, Dale N, Llaudet E, Molleman A, Parson ME. Modulation of the release of endogenous adenosine by cannabinoids in the myenteric plexus-longitudinal muscle preparation of the guinea-pig ileum. Br J Pharmacol. 2002a;137:1298–1304. doi: 10.1038/sj.bjp.0704985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Molleman A, Parson M. Modulation of the release of endogenous γ-aminobutyric acid by cannabinoids in the guinea-pig ileum. Eur J Pharmacol. 2002b;434:87–94. doi: 10.1016/s0014-2999(01)01530-8. [DOI] [PubMed] [Google Scholar]
- Bian X, Ren J, Devries M, Schnegelsberg B, Cockayne DA, Ford APDW, et al. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol. 2003;551:309–322. doi: 10.1113/jphysiol.2003.044172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC. Purinergic mechanisms in the control of gastrointestinal motility. Purinergic Signal. 2008;4:497–212. doi: 10.1007/s11302-007-9081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, et al. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between k-opioid and cannabinoid CB1 receptors. Br J Pharmacol. 2008;155:681–689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carai MAM, Colombo G, Gessa GL, Yalamanchili R, Basavarajppa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motilità in mice. Br J Pharmacol. 2006;148:1043–1050. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu MA, Porcella A, Ruiu S, Saba P, Marchese G, Carai MA, et al. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol. 2003;459:97–105. doi: 10.1016/s0014-2999(02)02830-3. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Gessa GL. Cannabinoid modulation of intestinal propulsion in mice. Eur J Pharmacol. 1998;344:67–69. doi: 10.1016/s0014-2999(97)01555-0. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci T, Manara L, Aureggi G, Guagnini F, Rinaldi-Carmona M, Maffrand JP, et al. In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br J Pharmacol. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha AR, Ribeiro JA. ATP as a presynaptic modulator. Life Sci. 2000;68:119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- Damer S, Niebel B, Czeche S, Nickel P, Ardanuy U, Schmalzing G, et al. NF279: a novel potent and selective antagonist of P2X receptor-mediated responses. Eur J Pharmacol. 1998;350:R5–R6. doi: 10.1016/s0014-2999(98)00316-1. [DOI] [PubMed] [Google Scholar]
- De Man JG, De Winter BY, Seerdeen TC, De Schepper U, Herman AG, Pelckamans PA. Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br J Pharmacol. 2003a;140:1108–1116. doi: 10.1038/sj.bjp.0705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man JG, Seerdeen TC, De Winter BY, Van Marck EA, Herman AG, Pelckamans PA. Alteration of the purinergic modulation of the enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br J Pharmacol. 2003b;139:172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, et al. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005;168:573–598. doi: 10.1007/3-540-26573-2_19. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Borrelli F, Capasso F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid 1 receptors. Br J Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Borrelli F, Capasso F. Defaecation, intestinal fluid accumulation and motility in rodents: implications of cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:65–70. doi: 10.1007/pl00005325. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice n a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Takeda M, Tokita K, Hanaoka K, Tomoi M. Molecular identification and pharmacological characterization of adenosine receptors in the guinea-pig colon. Br J Pharmacol. 2000;129:871–876. doi: 10.1038/sj.bjp.0703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapperstuck M, Buttner C, Nickel P, Schmalzing G, Lambrecht G, Markwardt F. Antagonism by the suramin analogue NF279 on human P2X(1) and P2X(7) receptors. Eur J Pharmacol. 2000;387:245–252. doi: 10.1016/s0014-2999(99)00826-2. [DOI] [PubMed] [Google Scholar]
- Krishtal O, Lozovaya N, Fedorenko A, Savelyew I, Chizhmakov I. The agonists for nociceptors are ubiquitous, but the modulators are specific: P2X receptors in the sensory neurons are modulated by cannabinoids. Pflugers Arch. 2006;453:353–360. doi: 10.1007/s00424-006-0094-1. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Talubmook C, Parsons ME. Activation of presynaptic A1-receptors by endogenous adenosine inhibits acetylcholine release in the guinea-pig ileum. J Auton Pharmacol. 2001;21:29–38. doi: 10.1046/j.1365-2680.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- Lòpez-Redondo F, Less GM, Pertwee RG. Effects of cannabinoid receptor ligands on electrophysiological properties of myenteric neurones of the guinea-pig ileum. Br J Pharmacol. 1997;122:330–334. doi: 10.1038/sj.bjp.0701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol. 2004;286:G863–G871. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- Massa F, Monory K. Endocannabinoids and the gastrointestinal tract. J Endocrinol Invest. 2006;29:47–57. [PubMed] [Google Scholar]
- Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- Mulè F, Naccari D, Serio R. Evidence for the presence of P2y and P2x receptors with different functions in mouse stomach. Eur J Pharmacol. 2005;513:135–140. doi: 10.1016/j.ejphar.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Mulè F, Amato A, Baldassano S, Serio R. Evidence for a modulatory role of cannabinoids on the excitatory NANC neurotransmission in mouse colon. Pharmacol Res. 2007a;56:132–139. doi: 10.1016/j.phrs.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Mulè F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res. 2007b;56:185–192. doi: 10.1016/j.phrs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G, Damer S, Muller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X(1) receptor. Neuropharmacology. 2000;39:2044–2053. doi: 10.1016/s0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Samson MT, Small-Howard A, Shimoda LM, Koblan-Huberson M, Stokes AJ, Turner H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J Immunol. 2003;170:4953–4962. doi: 10.4049/jimmunol.170.10.4953. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Jhamandasn KH. Inhibition of acetylcholine release from cholinergic nerves by adenosine, adenine nucleotides and morphine: antagonism by theophylline. J Pharmacol Exp Ther. 1976;197:379–390. [PubMed] [Google Scholar]
- Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mulè F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol. 2003;460:183–190. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- Storr M, Sibaev A, Marsicano G, Lutz B, Schusdziarra V, Timmermans J-P, et al. Cannabinoid receptor type 1 modulates excitatory and inhibitory neurotransmission in mouse colon. Am J Physiol. 2004;286:G110–G117. doi: 10.1152/ajpgi.00148.2003. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. Smooth muscle does not have a common P2X receptor phenotype: expression, ontogeny and function of P2X1 receptors in mouse ileum, bladder and reproductive systems. Auton Neurosci. 2001;92:56–64. doi: 10.1016/S1566-0702(01)00319-8. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Knoll J. The inhibitory effect of adenosine and related nucleotides on the release of acethylcholine. Neuroscience. 1976;1:391–398. doi: 10.1016/0306-4522(76)90132-9. [DOI] [PubMed] [Google Scholar]
- Woods CM, Toouli J, Saccone GT. A2A and A3 receptors mediate the adenosine-induced relaxation in spontaneously active possum duodenum in vitro. Br J Pharmacol. 2003;138:1333–1339. doi: 10.1038/sj.bjp.0705165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Yuece B, Sibaev A, Broedl UC, Morsicano G, Göke B, Lutz B, et al. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil. 2007;19:744–753. doi: 10.1111/j.1365-2982.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mulè F, Serio R. Inhibitory responses to exogenous adenosine in murine proximal and distal colon. Br J Pharmacol. 2006;148:956–963. doi: 10.1038/sj.bjp.0706808. [DOI] [PMC free article] [PubMed] [Google Scholar]


