Abstract
Expression patterns of six homeobox containing genes in a model chelicerate, the oribatid mite Archegozetes longisetosus, were examined to establish homology of chelicerate and insect head segments and to investigate claims that the chelicerate deutocerebral segment has been reduced or lost. engrailed (en) expression, which has been used to demonstrate the presence of segments in insects, fails to demonstrate a reduced deutocerebral segment. Expression patterns of the chelicerate homologs of the Drosophila genes Antennapedia (Antp), Sex combs reduced (Scr), Deformed (Dfd), proboscipedia (pb), and orthodenticle (otd) confirm direct correspondence of head segments. The chelicerate deutocerebral segment has not been reduced or lost. We make further inferences concerning the evolution of heads and Hox genes in arthropods.
Head segments and their appendages have long been considered of great importance in understanding the relationships of the main extant arthropod classes—insects, crustaceans, myriapods, and chelicerates (arachnids and horse-shoe crabs). The posterior regions of their bodies vary greatly, but several authors have found evidence for homologies between specific segments of the head in support of different phylogenetic schemes relating the arthropods (1–3). There is recently a general acceptance of the close relationship of insects and crustaceans and of direct homologies of their head segments and associated appendages (antennae, mandibles, etc.), some of the evidence for which comes from studies of gene expression (1, 4–6). Myriapod head segments also are widely presumed to be directly homologisable with those of insects and crustaceans, grouping the three classes into the mandibulates (3, 7). The chelicerates, on the other hand, are seen as fundamentally different in most schemes of arthropod evolution, and suggested homologies of their head segments with those of other arthropods seem, by their very variety, unconvincing (8–10).
One of the most widely held views concerning the chelicerate head is that they have secondarily lost or reduced the homolog of the front-most appendage-bearing segment, which in the other three classes carries the antennae (3, 7, 8, 11) (Fig. 1). This idea stems from two observations. Firstly, unlike all other groups (including the extinct trilobites and the onychophorans), the chelicerates have no antennae, the front-most appendages being the chelicerae. Secondly, the homolog of the mandibulate deutocerebrum, the brain region associated with the antennal segment found anterior to the mouth, is thought to have been lost or greatly reduced in chelicerates (8). Support for this view comes from studies claiming evidence of coelom remnants corresponding to the deutocerebral segment in a spider (10, 12, 13). This idea has direct consequences for attempts to assign homology to the segments and appendages posterior to the “missing” deutocerebral region and therefore for the understanding of morphological evolution in the arthropods.
Figure 1.
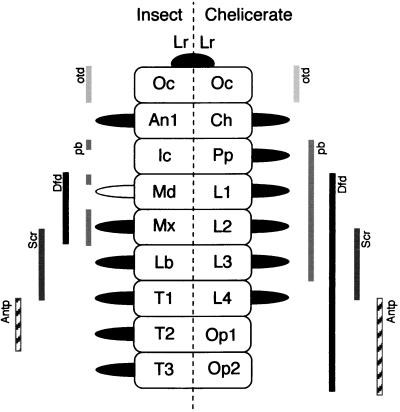
Two hypotheses of segmental homologies between chelicerate and crustacean/insect head regions. (A) Deutocerebral segment missing. The cross indicates the antennal segment thought to have been reduced or lost in the chelicerates relative to the insects and crustaceans. This theory supposes that the chelicerae (Ch) are homologous to the second antennal segment of crustaceans (An2) and the intercalary segment of insects (Ic). (B) Deutocerebral segment present. Diagonal lines between A and B emphasize the shift in segmental register. This theory supposes that the chelicerae are homologous to the first antennal segment of insects and crustaceans. Lr, labrum; Oc, ocular; Md, mandible; Pp, pedipalp; Mx1 and 2, maxilla 1 and 2; Lb, labium; L1–4, legs 1–4; T1–3, thorax 1–3; Op1–2, opisthosomal 1–2. More posterior segments are not shown.
In the insects, a secondarily reduced intercalary segment, corresponding to the crustacean second antennal (tritocerebral) segment, was demonstrated unequivocally by a stripe of expression of the gene engrailed (en) (14, 15). Region-specific expression of other genes has been used as a means of inferring homology of body regions (16), arthropod segments (17), and even parts of arthropod limbs (18). We have looked at the expression of several homeobox-containing genes in the embryos of a model chelicerate, the oribatid mite Archegozetes longisetosus, by using both in situ hybridization to see whether the segment corresponding to the insect/crustacean deutocerebrum has been reduced or lost secondarily in chelicerates and to deduce homologies between head segments of chelicerates and other arthropods. This gives us a powerful technique for predicting the ancestral morphology of the crustacean/insect clade by using the chelicerates as an outgroup.
MATERIALS AND METHODS
DNA Sources and Extraction and cDNA Preparation.
DNA extraction followed described methods (19). Reverse transcription–PCR used the primer CDNAI (GGATTTAGGTGACACTATAGCGGCCGCTTAAGA(T15)NN) attached to Dynabeads (Dynal, Great Neck, NY) to capture mRNA and to prime the first strand cDNA production by using Boehringer Mannheim products and protocols. Subsequent PCR amplification used a gene-specific 5′ primer (below) and CDNAB (TATAGCGGCCGCTTAAGA) at the 3′ end.
PCR Amplification, Genomic Clone Isolation, and in Situ Probe Template Cloning.
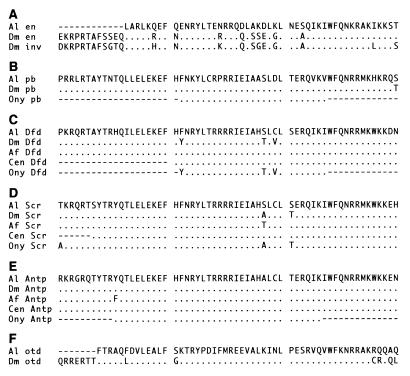
Archegozetes homologs of Antennapedia (Antp), Sex combs reduced (Scr), and probiscipedia (pb) (AlAntp, AlScr, and Alpb) were amplified by using a degenerate primer screening for all Hox genes. Primers were TLELEKEF [a 1:1 mixture of ACT TTG GAR TTR GAR AAR GAR TTY and ACT TTG GAR CTI GAR AAR GAR TTY (I = inosine)] at 5′ and WFQNRRXK (TTT NRY TCT TCT ATT YTG RAA CCT) at 3′. Clones isolated from this PCR screen were used to probe a genomic λ library for full length sequences. The Archegozetes homolog of orthodenticle (otd) (Alotd) was PCR amplified by using degenerate primers OTD5 (TTC ACA CGT GCN CAR YTN GAY GT) at 5′ and OTD3 (TGC AGY TGY TGN CKA CAY TTN GC) at 3′. The amplified and cloned product was used to screen the λ genomic library for full length sequences. λ clones were sequenced by using outward facing primers within the homeobox. Inward facing primers then were designed to amplify a coding region suitable in length for in situ hybridization. The Archegozetes homolog of deformed (Dfd) (AlDfd) was amplified by using DFD5B (STC GAY CCN AAR TTY CCN CC) at 5′ and WFQNRRXK at 3′ and was used directly for in situ probe production. The Archegozetes homolog of en (Alen) was amplified by using primers JM10 (GAI AAG CGI CGC ACI GCC TTC AC) at 5′ and WFQNRRXK at 3′. The 3′ terminus of the Alen gene was reverse transcription–PCR amplified by using a 5′ primer designed according to the amplified sequence (CCT GAA ATT AAA TGA ATC A). All sections of DNA for in situ hybridizations were cloned into pGEM T or pGEM TEASY (Promega) with the 3′ end closest to the T7 promoter. Alignment of these Archegozetes genes with homologs from other arthropods are shown in Fig. 2. DNA sequences used for in situ hybridizations are available from the GenBank database (accession nos. AF071402 to AF071407).
Figure 2.
Conceptual translations of homeodomains of Archegozetes (Al) genes compared with homologs from other arthropods and an onychophoran. (A) Engrailed (en) and invected (inv). (B) proboscipedia (pb). (C) Deformed (Dfd). (D) Sex combs reduced (Scr). (E) Antennapedia (Antp) (F) orthodenticle (otd). Insect sequences obtained from the GenBank database: Dm, Drosophila melanogaster; Af, Artemia franciscana; Sg, Schistocerca gregaria; Tc, Tribolium castaneum. Noninsect sequences from ref. 36: Cen, centipede (Ethmostigmus rubripes); Ony, onychophoran (Acanthokara kaputensis). Matches are indicated with a period and missing data by a dashed line.
Embryo Preparation.
Embryos were dechorionated in 5% bleach for 5 min, were fixed in n-heptane over 4% formaldehyde in PBS for 1 hr, and were devitellinized by placing the embryos in n-heptane prechilled on dry ice, adding room temperature methanol and shaking vigorously for 2–3 min. Embryos were digested partially in 7.5 μg/ml proteinase K for 8 min, were washed, were refixed in 4% formaldehyde in PBS with 0.2% Tween 20 (PTW) for 20 min, and were rewashed in PTW.
In Situ Hybridizations and Color Detection.
Digoxygenin labeled riboprobe production used Boehringer Mannheim T7 polymerase and buffers and Boehringer Mannheim digoxygenin-labeled ribonucleotides according to the manufacturer’s instructions. Prehybridization was in hybe (50% formamide/5× standard saline citrate/150 μg/ml yeast RNA/0.1% Tween 20/50 μg/ml heparin) at 65°C for 24 hr. Hybridizations were carried out by using 500 μl of 0.4–1 μg/ml of antisense riboprobe in hybe at 65°C overnight. Washes were 1 × 30 min in hybe at 65°C, 1 × 30 min in 1:1 hybe/PTW at 65°C, and 5 × 20 min in PTW at room temperature. Embryos were blocked in 1 mg/ml BSA, 5 mg/ml Boehringer Mannheim block, and 50 μl/ml normal goat serum in PTW for 1 hr followed by incubation overnight at 4°C with a preabsorbed Boehringer Mannheim alkaline phosphatase conjugated antidigoxygenin antibody diluted 1:2000 in PTW followed by washes in PTW and detection with nitroblue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt. Detailed protocols are available from the authors.
RESULTS
Expression of Alen.
Alen is expressed at the rear of segments in a fashion identical to that seen in other arthropod groups (6, 20, 21) (Fig. 3A). Five stripes appear initially, corresponding to the five visible limbs (the sixth limbs—fourth walking legs—are suppressed until after hatching in oribatid embryos), and more posterior stripes, including that corresponding to the sixth limb bearing segment, are added progressively (results not shown). If the chelicerate deutocerebral segment has been suppressed but not entirely lost, we would expect, by analogy with the insect intercalary segment, to see two regions of Alen expression anterior to the cheliceral stripe corresponding to the reduced deutocerebral segment and to the ocular segment. In fact, no Alen expression is seen anterior to the cheliceral stripe. We find no support from Alen expression patterns for a secondarily reduced deutocerebral segment in chelicerates. The lack of expression corresponding to the ocular expression of insects is puzzling but probably is explained by the tendency for this region of expression to be small (6, 20, 21). Furthermore, the size of the ocular en expression, at least in insects, seems to correspond with the size of the eyes (22); Archegozetes has no eyes.
Figure 3.
Expression patterns of Homeobox-containing genes in Archegozetes demonstrating homology of anterior segments. The embryo is ≈170 μm long in each case. Abbreviations as in Fig. 1. (A) Expression of the Archegozetes engrailed (Alen) homolog in an embryo after the formation of the labrum. Alen is expressed in the posterior portion of each segment with visible limbs. Despite strong staining in these segments, no engrailed expression is evident anterior to the chelicerae. (B) Alpb is expressed in all of the visible walking legs with an anterior boundary at the front of the pedipalp. (C) AlDfd is expressed throughout the posterior of the embryo, excepting the terminal segments, with an anterior boundary at the front of the first walking leg. (D) AlScr is expressed strongly in the third leg and weakly in the second. It appears to be expressed only in the more distal regions of the second leg though the staining is diffuse. (E) Close up of opisthosoma and fourth leg bud. AlAntp expression is strong in the opisthosoma and continues into the rear portion of the fourth leg bud. Because of the small size of the fourth limb bud and its position, tucked in next to the opisthosoma, it has proved very hard to photograph; this expression therefore is schematized in a camera lucida drawing in F. (F) Camera lucida drawing highlighting expression of AlAntp in the opisthosoma and rear of fourth leg bud. (G) Optical mid-sagittal expression showing AlAntp expressed strongly in the opisthosoma with no expression in the chelicerae, pedipalps, or legs 1–3. Leg 4, a small bud, is not in focus in this picture. (H) Alotd is expressed in the ocular lobes and in the ventral midline. The photograph is a sagittal optical section focusing on the expression in the ventral midline. No segmental expression is seen posterior to the ocular segment. (I) Alotd expression is seen in the ocular lobes but not in the chelicerae. View is anterior-ventral with the anterior to the left.
Expression of Alpb, AlDfd, AlScr, and AlAntp.
A lost or reduced segment has consequences for the assignment of homology of more posterior segments and appendages. If the deutocerebral segment has been reduced as generally supposed, then the first chelicerate appendages, the chelicerae, correspond to the second appendages of insects and crustaceans (crustacean second antennae, insect intercalary). The second chelicerate appendages (pedipalps) correspond to the third appendages of insects and crustaceans (mandible) (8), etc. (Fig. 1A). If, however, the deutocerebrum has not been lost or reduced, as indeed is suggested by lack of Alen expression, then the appendage bearing segments would align directly: chelicerae with first antennae, pedipalps with second antennae, and first legs with mandibles (9), etc. (Fig. 1B). By comparing the anterior boundaries of expression of segment-specifying homeobox genes, we sought to infer homology of segments directly (23). Anterior boundaries are stable evolutionarily and hence are good positional markers whereas posterior boundaries vary across different taxa under the influence of a variety of transrepressors. We make the assumption that the arthropods are monophyletic (24) and come from a segmented ancestor with appendages whose head segments were specified by Hox genes. All comparisons are made only with insects because expression data are not yet available outside the insects for the more anteriorly expressed arthropod Hox genes. Results from insects come from Rogers and Kaufman (22), who provide comparative expression data from distantly related insect orders. Results for Antp come from ref. 25.
The arthropod Hox cluster contains nine genes, and Archegozetes homologs of eight of these were discovered in a degenerate PCR screening (results not shown). For each separate gene unambiguously identified based on full homeobox amino acid sequence (Fig. 2), only a single nucleotide sequence was found. This suggests strongly that only a single Hox cluster exists in Archegozetes. We looked at expression patterns of four of these genes that specify anterior regions of metazoan embryos. The remaining genes we have identified are homologs of the Drosophila genes labial, Ultrabithorax, Abdominal-B (data not shown), and zen (26).
We first looked at expression of the Archegozetes proboscipedia homolog (Alpb). One of the most striking aspects of Hox gene expression is the co-linearity between the order of the genes on the chromosome and their anterior boundaries of expression. This co-linearity is maintained in both the vertebrates and insects. Drosophila proboscipedia (pb) is an exception to this rule because, according to its chromosomal position, it would be expected to have an anterior expression boundary in front of that of Dfd in the intercalary segment whereas it actually is expressed principally in the labial segment. There is, however, a limited amount of ectodermal expression in the maxillary, mandibular, and intercalary segments in crickets and milkweed bugs (22), placing its anterior boundary of ectodermal expression—as predicted from its chromosomal position and by comparison with its vertebrate homolog (Hox2)—anterior to that of Dfd in the second limb-bearing segment. In Archegozetes, Alpb is expressed equally in all three visible walking leg segments and in the pedipalpal segment (Fig. 3B) so its anterior expression boundary is in the second limb-bearing segment. From this comparison, we infer that the ancestral anterior boundary of pb expression was in the second limb-bearing segment as seen in a reduced fashion in crickets and milkweed bugs. We predict that full pb expression will be found in this segment in crustaceans and that the differences seen within the insects are secondary modifications.
The Archegozetes Deformed homolog (AlDfd) is expressed throughout the rear of the embryo with a discrete anterior boundary at the anterior of the third limb segment (first walking leg) (Fig. 3C). Insect Dfd is expressed in the fourth limb (maxillary) segment and has its anterior boundary at the front of the third limb (mandibular) segment.
In Archegozetes embryos, Sex combs reduced (AlScr) is expressed in two segments: strongly in the fifth limb (third leg) segment and less strongly in the fourth limb (second leg) segment (Fig. 3D). In the fourth limb segment, expression is in the limb rather than the ventral blastoderm. Insect Scr also is expressed strongly in the fifth limb (labial) segment and more weakly and restricted to the posterior portion of the fourth limb (maxillary) segment. Later expression of insect Scr in the fourth limb is in lateral epidermis (22).
The final Hox gene we studied, AlAntp, is expressed strongly in the more posterior segments of the opisthosoma. There is weaker expression in the first opisthosomal segment and the anterior boundary of expression is the rear of the fourth legs (sixth limb segment), which are small buds (Fig. 3 E–G). This corresponds closely with the early expression of Antp in Drosophila in which expression is in a broad band in parasegment 4 (anterior of the second thoracic and rear of the first thoracic) (25). In other words, initial expression in insects has its anterior boundary, like Archegozetes, in the rear of the sixth limb segment. Later, modulated expression of Antp in Drosophila also includes expression of a new transcript in a small subset of more anterior cells. AlAntp expression corroborates the inference drawn from Alpb, AlDfd, and AlScr expression that chelicerate and insect limb-bearing segments are directly alignable.
Expression of Alotd.
In Drosophila, otd is expressed in the ocular and first antennal segments as well as along the length of the ventral midline (27). In the beetle Tribolium, there are two otd genes expressed in similar but not identical patterns; both of these genes differ from the pattern seen in Drosophila in that, at the later stage, when expression includes the ventral midline, there is no expression in the antenna (28). We interpret the persistent expression in the Drosophila antennal segment as a Drosophila specific secondary adaptation. The expression seen in Archegozetes is strikingly similar to that seen in Tribolium (Fig. H and I), being restricted to expression in the ocular segment and the ventral midline.
DISCUSSION
Assignment of Homology Between Chelicerate and Insect Anterior Segments.
Each of the comparisons of genes expression patterns described support the conclusion proposed based on the lack of Alen expression anterior to the cheliceral segment, namely, that chelicerate and insect head segments line up directly: (Fig. 4) the antennal with the cheliceral, the second antennal (intercalary) with the pedipalpal (expressing pb), the mandibular with the chelicerate first leg (expressing Dfd), the first maxillary with chelicerate second leg (partially expressing Scr), the labial with the chelicerate third leg (fully expressing Scr), and the first thoracic with the chelicerate fourth leg (expressing Antp in the rear). We conclude that there has not been a loss or reduction of a segment in chelicerates relative to insects and crustaceans.
Figure 4.
Homologies between anterior segments of chelicerates and insects as determined by overlapping patterns of expression of homologous genes. Posterior boundary of otd and anterior boundaries of Dfd, Scr, and Antp are identical in insects and chelicerates. The anterior boundary of Archegozetes pb is as seen in some but not all insect groups. Appendages with Dll expression are colored black (results not shown). The inferred chelicerate homolog of the insect mandible (leg 1) does express Dll. Abbreviations as in Fig. 1.
Reappraisal of Evolution of Arthropod Anterior Segments.
In the light of our conclusion that the chelicerate deutocerebral segment is not missing and indeed corresponds to the cheliceral segment, we looked at the evidence that had given rise to this idea and the ramifications of its rejection. Ignoring the trivial observation that the anteriormost chelicerate appendages are not antennae, the most important idea suggesting that the deutocerebral segment is lost is the conviction that the deutocerebrum is missing from the chelicerate brain. This idea stems from the observation that insects have two segmental ganglia anterior to the mouth whereas, ancestrally at least, chelicerates only have one (8). As a result, the postoral cheliceral ganglion was homologized to the postoral tritocerebrum of the insects. In fact, having the deutocerebrum anterior to the mouth is probably a derived condition in crustaceans (and also, therefore, in insects) because certain crustacean orders have their first antennal ganglion lateral to the esophagus (29). The same tendency is seen in chelicerates in which the cheliceral ganglion is primitively on either side of the esophagus, but, in many chelicerate orders, the cheliceral ganglion is actually preoral and continuous with the protocerebrum (8, 30), though it only moves to this position during embryogenesis as the chelicerae move anterior. This presumably indicates a convergent tendency for ganglionic fusion and cephalization as well as suggesting that all of the appendages we see in extant arthropods were primitively postoral. The initial postoral position of the chelicerae also has been used to imply that they could not be homologs of the first antennae, which are thought of as preoral (31). In fact, studies of embryogenesis of insects, crustaceans, and myriapods show that, in all cases, the first antennae are initially postoral and the subsequent anteriorwards morphogenetic movements are strikingly similar in each case (32). This can be seen as further evidence supporting our view that cheliceral and first antennal segments are homologous.
Chelicerates as an Outgroup of Insects/Crustaceans.
Molecular phylogenetic studies show that the chelicerates are an outgroup to the insect/crustacean clade (33–36). This idea is confirmed by our demonstration that Dll is expressed in all chelicerate limbs (results not shown) whereas it is secondarily missing in crustacean and insect mandibles (4). As such, the chelicerates make an ideal outgroup to allow inference, through outgroup comparison, of the ancestral condition and, hence, the subsequent evolution of mandibulate anterior segments, their appendages, and the expression of the genes that specify them.
We already have inferred, for example, that pb was expressed as far forward as the second appendage in the common ancestor of insect/crustaceans and chelicerates. Furthermore, expression of AlDfd in the third limbs is contrary to the hypothesis (22) that the anterior boundary of Dfd was ancestrally in the fourth limb-bearing segment and that there was a migration forwards of this boundary in the insects and a concomitant loss of Dll expression and mandible tip. Further studies on chelicerates should be invaluable for the understanding of the evolution of the arthropods, and expression data from the anteriorly expressed genes described here from crustaceans and myriapods would be particularly welcome.
Acknowledgments
We are grateful to J. A. Mackenzie-Dodds, T. Snell, and D. Goode for technical assistance, R. A. Norton for providing our original Archegozetes stock, P. W. H. Holland for donating PCR primer JM10, and N. H. Patel and N. A. Williams for sharing protocols. This work was funded by the Biotechnology and Biological Sciences Research Council (Grant G01972).
ABBREVIATION
- PTW
PBS with 0.2% Tween 20
Note Added in Proof
Comparable results have been obtained from spiders, which are chelicerates phylogenetically distant from the mites (37).
References
- 1.Averof M, Akam M. Philos Trans Roy Soc Lond B. 1995;347:293–303. [Google Scholar]
- 2.Briggs D E G, Fortey R A. Science. 1989;246:241–243. doi: 10.1126/science.246.4927.241. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen C. Animal Evolution: Interrelationships of the Living Phyla. Oxford: Oxford Univ. Press; 1995. [Google Scholar]
- 4.Popadic A, Rusch D, Peterson M, Rogers B T, Kaufman T C. Nature (London) 1996;380:395. [Google Scholar]
- 5.Telford M J, Thomas R H. Nature (London) 1995;376:123–124. [Google Scholar]
- 6.Scholtz G. In: Arthropod Relationships. Fortey R A, Thomas R H, editors. London: Chapman & Hall; 1997. pp. 317–332. [Google Scholar]
- 7.Meglitsch P A, Schram F R. Invertebrate Zoology. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 8.Weygoldt P. In: Ontogeny of the Arachnid Central Nervous System. Barth F G, editor. Heidelberg: Springer; 1985. pp. 20–37. [Google Scholar]
- 9.Raff R A. The Shape of Life. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 10.Legendre R. Bull Soc Zool Fr. 1979;104:277–287. [Google Scholar]
- 11.Wegerhoff R, Breidbach O. In: Comparative Aspects of the Chelicerate Nervous Systems. Breidbach O, Kutsch W, editors. Basel: Birkhaüser; 1995. pp. 159–180. [Google Scholar]
- 12.Pross A. Z Morphol Ökol Tiere. 1966;58:38–108. [Google Scholar]
- 13.Pross A. Zoomorphologie. 1977;86:183–196. [Google Scholar]
- 14.Schmidt-Ott U, González-Gaitán M, Jäckle H, Technau G M. Proc Natl Acad Sci USA. 1994;91:8363–8367. doi: 10.1073/pnas.91.18.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt-Ott U, Technau G M. Development (Cambridge, UK) 1992;116:111–125. doi: 10.1242/dev.116.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Holland P W H, Holland L Z, Williams N A, Holland N D. Development (Cambridge, UK) 1992;116:653–661. doi: 10.1242/dev.116.3.653. [DOI] [PubMed] [Google Scholar]
- 17.Averof M, Akam M. Curr Biol. 1993;3:73–78. doi: 10.1016/0960-9822(93)90158-k. [DOI] [PubMed] [Google Scholar]
- 18.Averof M, Cohen S M. Nature (London) 1997;385:627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. NY: Cold Spring Harbor Lab. Press. Plainview; 1989. [Google Scholar]
- 20.Schmidt-Ott U, Sander K, Technau G M. Roux’s Arch Dev Biol. 1994;203:298–303. doi: 10.1007/BF00457800. [DOI] [PubMed] [Google Scholar]
- 21.Scholtz G. Zoology. 1995;98:104–114. [Google Scholar]
- 22.Rogers B T, Kaufman T C. Int Rev Cytol. 1997;174:1–84. doi: 10.1016/s0074-7696(08)62115-4. [DOI] [PubMed] [Google Scholar]
- 23.Abouheif E, Akam M, Dickinson W J, Holland P W H, Meyer A, Patel N H, Raff R A, Roth V L, Wray G A. Trends Genet. 1997;13:432–433. doi: 10.1016/s0168-9525(97)01271-7. [DOI] [PubMed] [Google Scholar]
- 24.Fortey R A, Thomas R H. Arthropod Relationships. London: Chapman & Hall; 1997. [Google Scholar]
- 25.Bermingham J R, Martinez-Arias A, Petitt M G, Scott M P. Development (Cambridge, UK) 1990;109:553–566. doi: 10.1242/dev.109.3.553. [DOI] [PubMed] [Google Scholar]
- 26.Telford, M. J. & Thomas, R. H. (1998) Dev. Genes Evol., in press. [DOI] [PubMed]
- 27.Finkelstein R, Smouse D, Capaci T M, Spradling A C, Perrimon N. Genes Dev. 1990;4:1516–1527. doi: 10.1101/gad.4.9.1516. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Brown S J, Hausdorf B, Tautz D, Denell R E, Finkelstein R. Dev Genes Evol. 1996;206:35–45. doi: 10.1007/s004270050028. [DOI] [PubMed] [Google Scholar]
- 29.Wallosek D, Müller K J. In: Arthropod Relationships. Fortey R A, Thomas R H, editors. London: Chapman & Hall; 1997. pp. 139–153. [Google Scholar]
- 30.Babu K S. In: Neurobiology of Arachnids. Barth F G, editor. Heidelberg: Springer; 1985. pp. 3–19. [Google Scholar]
- 31.Willmer P. Invertebrate Relationships: Patterns in Animal Evolution. Cambridge: Cambridge Univ. Press; 1990. [Google Scholar]
- 32.Anderson D T. Embryology and Phylogeny in Annelids and Arthropods. Oxford: Pergamon; 1973. [Google Scholar]
- 33.Regier J C, Shultz J W. Mol Biol Evol. 1997;14:902–913. doi: 10.1093/oxfordjournals.molbev.a025833. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler W C, Cartwright P, Hayashi C Y. Cladistics. 1993;9:1–39. doi: 10.1111/j.1096-0031.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 35.Turbeville J M, Pfeifer D M, Field K G, Raff R A. Mol Biol Evol. 1991;8:669–686. doi: 10.1093/oxfordjournals.molbev.a040677. [DOI] [PubMed] [Google Scholar]
- 36.Grenier J K, Garber T L, Warren R, Whitington P M, Carroll S. Curr Biol. 1997;7:547–553. doi: 10.1016/s0960-9822(06)00253-3. [DOI] [PubMed] [Google Scholar]
- 37.Damen W G M, Hausdorf M, Seyfarth E-A, Tautz D. Proc Natl Acad Sci USA. 1998;95:10665–10670. doi: 10.1073/pnas.95.18.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]