Abstract
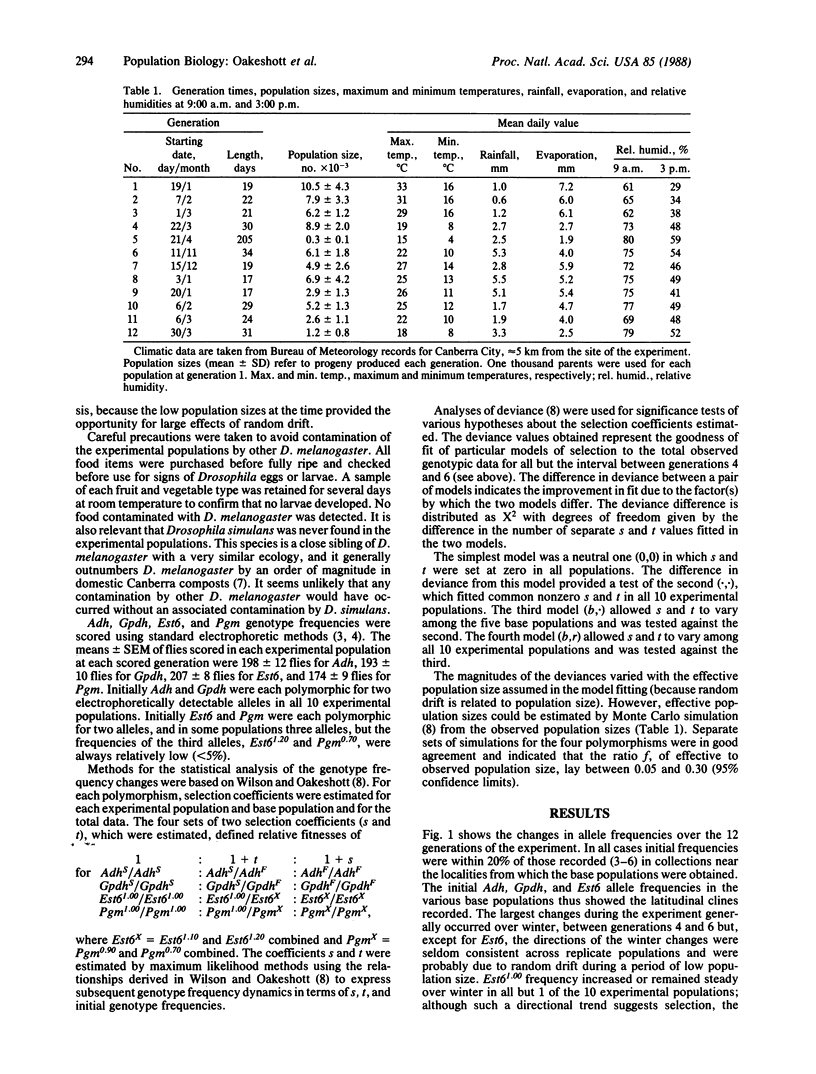
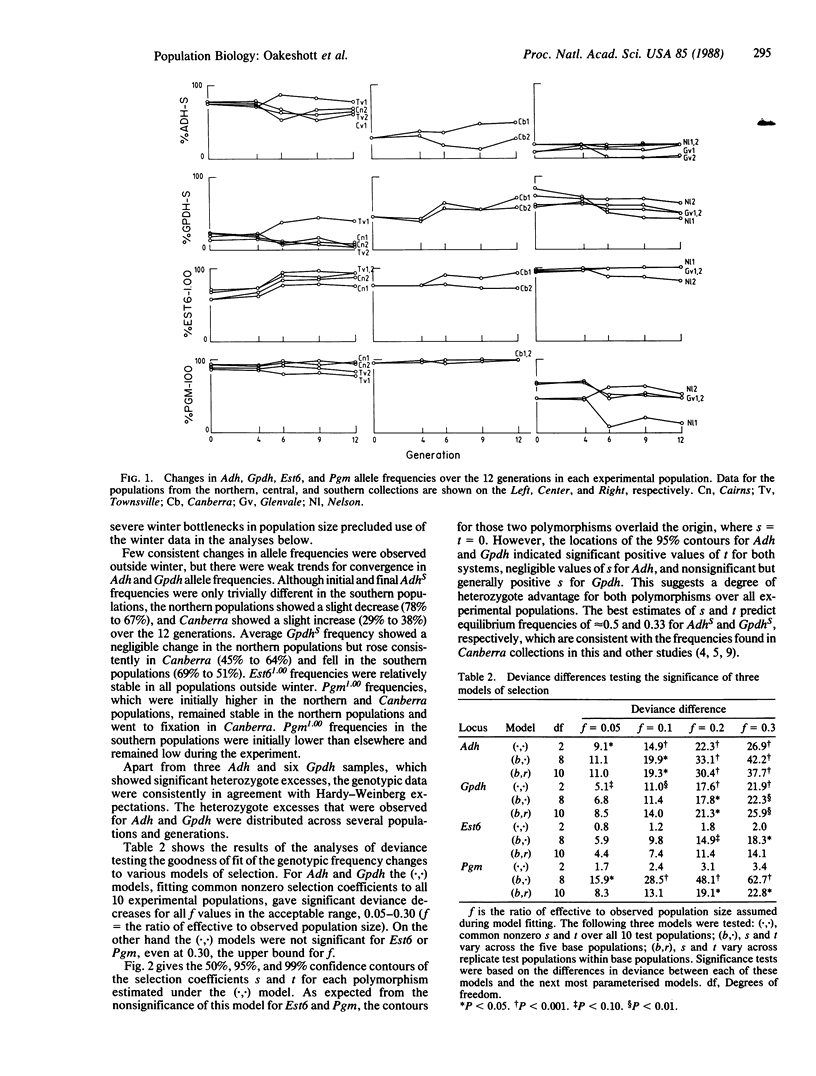
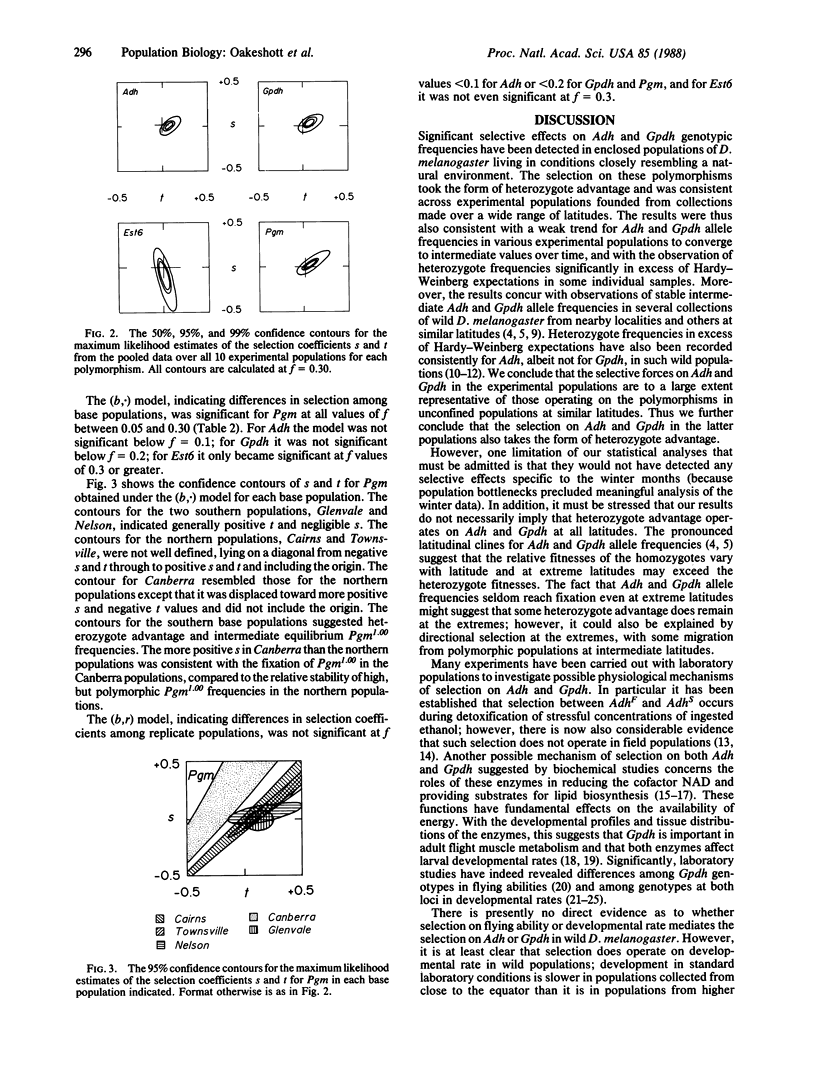
Allele frequencies for the Adh, Gpdh, and Est6 enzyme polymorphisms of Drosophila melanogaster show large-scale latitudinal clines, whereas those for Pgm do not vary systematically with latitude. To elucidate possible mechanisms of selection underlying these distributions, large collections of the species were made from five Australasian localities spanning 24 degrees of latitude. Two replicate experimental populations were established from each collection, and each replicate was then released into an enclosure surrounding a natural habitat at a central-latitude locality. Genotype frequencies at the four loci were monitored for 15 months, covering 12 discrete generations, and selection coefficients on each polymorphism were then estimated by maximum likelihood procedures. For Est6 no coefficients were found to be significantly different from zero. For Pgm some nonzero coefficients were estimated, but these were heterogeneous across experimental populations of different geographic origins. For both Adh and Gpdh, nonzero selection coefficients were estimated that were homogeneous across populations and indicated heterozygote advantage. Predicted Adh and Gpdh equilibrium allele frequencies were consistent with those found in adjacent free-living populations. It is concluded that, at such intermediate latitudes at least, selection operates on the Adh and Gpdh polymorphisms to the advantage of heterozygotes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. T., Laurie-Ahlberg C. C. Genetic variability of flight metabolism in Drosophila melanogaster. III. Effects of Gpdh allozymes and environmental temperature on power output. Genetics. 1986 Feb;112(2):267–294. doi: 10.1093/genetics/112.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R., Clegg M. T. Dynamics of correlated genetic systems. IV. Multilocus effects of ethanol stress environments. Genetics. 1978 Nov;90(3):629–644. doi: 10.1093/genetics/90.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. The Response of Enzyme Polymorphisms to Developmental Rate Selection in DROSOPHILA MELANOGASTER. Genetics. 1983 Sep;105(1):105–113. doi: 10.1093/genetics/105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluster P. D., Marinković D., Allard R. W., Ayala F. J. Correlations between development rates, enzyme activities, ribosomal DNA spacer-length phenotypes, and adaptation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Jan;84(2):610–614. doi: 10.1073/pnas.84.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer B. W., Langevin M. L., McKechnie S. W. Dietary ethanol and lipid synthesis in Drosophila melanogaster. Biochem Genet. 1985 Aug;23(7-8):607–622. doi: 10.1007/BF00504295. [DOI] [PubMed] [Google Scholar]
- Geer B. W., McKechnie S. W., Langevin M. L. Regulation of sn-glycerol-3-phosphate dehydrogenase in Drosophila melanogaster larvae by dietary ethanol and sucrose. J Nutr. 1983 Aug;113(8):1632–1642. doi: 10.1093/jn/113.8.1632. [DOI] [PubMed] [Google Scholar]
- Geer B. W., McKechnie S. W., Langevin M. L. The effect of dietary ethanol on the composition of lipids of Drosophila melanogaster larvae. Biochem Genet. 1986 Feb;24(1-2):51–69. doi: 10.1007/BF00502978. [DOI] [PubMed] [Google Scholar]
- Lewontin R. C. Population genetics. Annu Rev Genet. 1985;19:81–102. doi: 10.1146/annurev.ge.19.120185.000501. [DOI] [PubMed] [Google Scholar]
- Oakeshott J. G., Chambers G. K., Gibson J. B., Willcocks D. A. Latitudinal relationships of esterase-6 and phosphoglucomutase gene frequencies in Drosophila melanogaster. Heredity (Edinb) 1981 Dec;47(Pt 3):385–396. doi: 10.1038/hdy.1981.99. [DOI] [PubMed] [Google Scholar]
- Oakeshott J. G., Gibson J. B., Wilson S. R. Selective effects of the genetic background and ethanol on the alcohol dehydrogenase polymorphism in Drosophila melanogaster. Heredity (Edinb) 1984 Aug;53(Pt 1):51–67. doi: 10.1038/hdy.1984.62. [DOI] [PubMed] [Google Scholar]
- Oakeshott J. G. Selection at the alcohol dehydrogenase locus in Drosophila melanogaster imposed by environmental ethanol. Genet Res. 1975 Dec;26(3):265–274. doi: 10.1017/s0016672300016062. [DOI] [PubMed] [Google Scholar]
- Singh R. S., Hickey D. A., David J. Genetic Differentiation between Geographically Distant Populations of DROSOPHILA MELANOGASTER. Genetics. 1982 Jun;101(2):235–256. doi: 10.1093/genetics/101.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Delden W., Kamping A. The alcohol dehydrogenase polymorphism in populations of Drosophila melanogaster. 3. Differences in developmental times. Genet Res. 1979 Feb;33(1):15–27. doi: 10.1017/s0016672300018139. [DOI] [PubMed] [Google Scholar]


