Abstract
A series of new Smac mimetics have been designed, synthesized and evaluated. The most potent compound 10 binds to XIAP, cIAP-1 and cIAP-2 BIR3 proteins with Ki values of 3.9, 0.37 and 0.25 nM, respectively. Compound 10 antagonizes XIAP in a cell-free functional assay and induces rapid cIAP-1 degradation in cancer cells. Compound 10 inhibits cell growth in the MDA-MB-231 cancer cell line with an IC50 value of 8.9 nM.
Apoptosis is a critical cell process in normal development and homeostasis of multicellular organisms to eliminate unwanted or damaged cells. Evasion of apoptosis is now recognized as a hallmark of all cancers.1–2 Targeting key apoptosis regulators with a goal to promote apoptosis in cancer cells is a new strategy for anticancer drug design.3–4
Inhibitor of apoptosis proteins (IAPs)a are a class of key apoptosis regulators.5,6 Among them, cellular IAP-1 (cIAP-1) and cIAP-2 play a critical role in regulation of tumor necrosis factor (TNF) receptor-mediated apoptosis,7 and X-linked IAP (XIAP) is a central regulator of both death-receptor-mediated and mitochondria-mediated apoptosis pathways.8 XIAP inhibits apoptosis through direct binding to and inhibition of three cysteine proteases, an initiator caspase-9 and the two effectors caspase-3 and -7.5,6 XIAP contains three Baculoviral IAP Repeats (BIR) domains. While the third BIR domain (BIR3) of XIAP selectively targets caspase-9, the BIR2 domain, together with the immediate preceding linker, inhibits both caspase-3 and caspase-7.6,8 Since these caspases play a critical role in the execution of apoptosis, XIAP functions as an efficient inhibitor of apoptosis.6,8 Accordingly, these IAP proteins, especially XIAP, are considered as promising cancer therapeutic targets.8–9
Smac (Second Mitochondria-derived Activator of Caspases) is a potent pro-apoptotic protein and an endogenous antagonist of IAP proteins.10,11 Previous studies have firmly established that Smac interacts with XIAP and cIAP-1/2 proteins via its AVPI tetra-peptide motif.6,12–15 Smac, in its dimeric form, interacts with both BIR2 and BIR3 domains in XIAP and effectively removes the inhibition of XIAP to not only caspase-9 but also caspase-3/-7.
In the last few years, intense research efforts have been devoted to the design and development of small molecules to mimic the AVPI binding motif as antagonists of IAP proteins and as a new class of anticancer drugs.4,16–23 These small molecules are called Smac mimetics. To date, two different types of Smac mimetics have been reported, namely monovalent and bivalent Smac mimetics (Figure 1). 4,16–23 Monovalent Smac mimetics such as 1–4 are designed to mimic a single AVPI binding motif,16–19 whereas the bivalent compounds, such as 5 and 6, contain mimetics of two AVPI binding motifs tethered together through a linker.4,20–23 Bivalent Smac mimetics can achieve much higher affinities to XIAP and can be much more potent than the corresponding monovalent Smac mimetics in induction of apoptosis in tumor cells.20 However, because of their ideal low molecular weight (~500), monovalent Smac mimetics may possess major advantages for drug development as compared to bivalent Smac mimetics.
Figure 1.
Examples of monovalent (1–4) and bivalent (5–6) Smac mimetics.
Recent studies established that Smac mimetics induce rapid cIAP-1 degradation and cIAP-1 is a key cellular target for Smac mimetics.21–23 Furthermore, our recent study clearly showed that in order to most efficiently induce apoptosis in cancer cells by Smac mimetics, the apoptotic blockade by both cIAP-1 and XIAP needs to be concurrently removed.24 Thus, both cIAP-1 and XIAP are important cellular targets for Smac mimetics.
Our laboratory has previously reported the structure-based design, synthesis and initial evaluation of a series of conformationally constrained monovalent Smac mimetics.16–18,20 Among them, compound 7 (SM-122, Figure 2) binds to the XIAP BIR3 protein with a Ki value of 26 nM.20 Our subsequent binding studies showed that 7 also binds to cIAP-1, as well as to cIAP-2 proteins with high affinities (Table 1). Compound 7 potently inhibits cancer cell growth and effectively induces apoptosis in cancer cells.20 These data indicated that 7 is a promising lead compound for further optimization. We report herein the design, synthesis and evaluation of new analogues of 7. These efforts led to the discovery of highly potent monovalent Smac mimetics and yielded new insights into their structure-activity relationship.
Figure 2.
Chemical structures of designed Smac mimetics.
Table 1.
Binding affinities of Smac mimetics to XIAP, cIAP-1 and cIAP-2 BIR3 proteins. Ki and standard deviation (SD) values were calculated based upon 3–5 independent experiments.
| Compounds | (Ki ± SD, nM) | ||
|---|---|---|---|
| XIAP BIR3 | cIAP-1 BIR3 | cIAP-2 BIR3 | |
| 7 | 26 ± 5 | 0.80 ± 0.19 | 1.84 ± 0.62 |
| 8 | 14 ± 5 | 0.68 ± 0.15 | 1.00 ± 0.04 |
| 9 | 209 ± 73 | 29 ± 6 | 132 ± 17 |
| 10 | 3.9 ± 1.8 | 0.37 ± 0.25 | 0.25 ± 0.07 |
| 11 | 31 ± 15 | 1.27 ± 1.05 | 2.09 ± 0.78 |
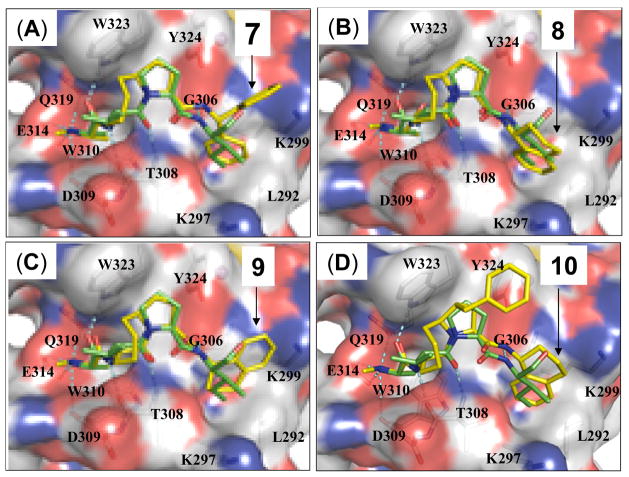
The predicted binding model of 7 in complex with XIAP BIR3 showed that while the pro-(R) phenyl group in 7 inserts into a hydrophobic pocket in XIAP, the pro(S) phenyl group does not have specific interactions with the protein (Figure 3). We have thus investigated if replacement of the diphenylmethyl group by a conformationally constrained 1-tetrahydronaphthalenyl group, which was previously used for the design of Smac peptidomimetics,17 could further improve the binding to XIAP. Modeling predicted that the compound with the (R)- configuration is preferred for binding to XIAP BIR3 (Figure 3). Compounds 8 and 9 with either the (R)- or the (S)- configuration were synthesized and evaluated. Compound 8 binds to XIAP, cIAP-1 and cIAP-2 BIR3 proteins with Ki values of 14 nM, 0.68 nM, and 1.0 nM, respectively, in competitive FP-based binding assays. In comparison, 9 binds to XIAP, cIAP-1 and cIAP-2 proteins with Ki values of 209, 29, and 132 nM, respectively, substantially less potent than 8.
Figure 3.
Predicted binding models of compounds 7, 8, 9 and 10 in complex with XIAP BIR3 protein. Predicted binding models are superimposed on the crystal structure of Smac in complex with XIAP BIR3. Protein is shown in surface with key binding residues shown and labeled. Compounds 7, 8, 9, 10 and AVPI peptide are shown in stick. Caron atoms in AVPI are shown in yellow and carbon atoms in 7, 8, 9, 10 are shown in green; oxygen and nitrogen atoms are shown in red and blue, respectively.
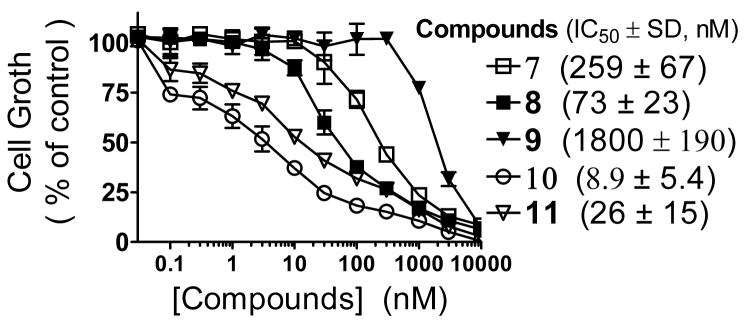
Consistent with their binding affinities, 8 is 4-times more potent than 7 in inhibition of cell growth in the MDA-MB-231 cancer cell line and has an IC50 value of 73 nM (Figure 4). In comparison, 9 is less potent than 7 and 8 and has an IC50 value of 1800 nM (Figure 4).
Figure 4.
Cell growth inhibition by Smac mimetics in the MDA-MB-231 cancer cell line. Cells were treated for 4 days and cell growth was determined by a WST-8 assay.
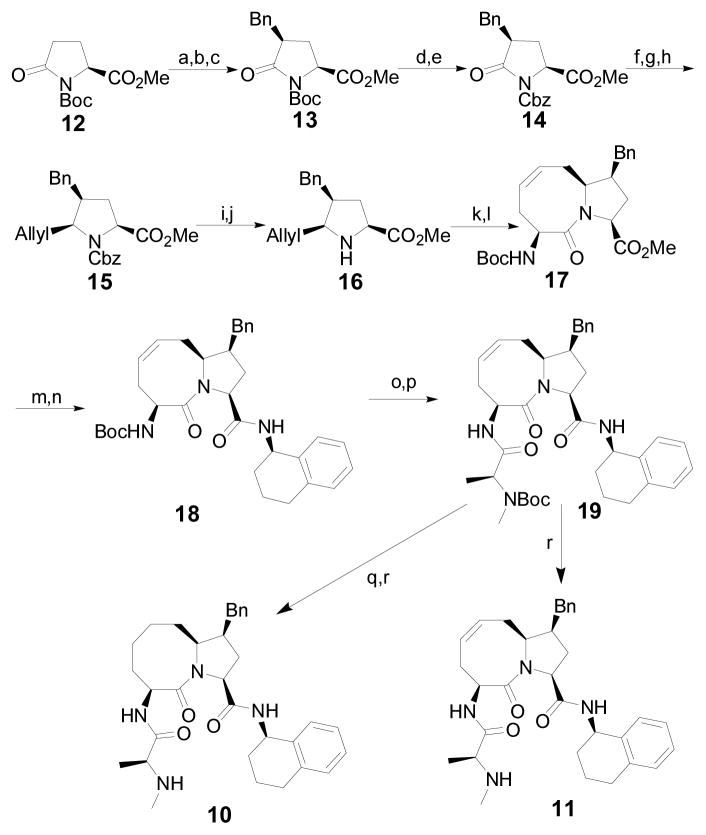
Our predicted binding model showed that there is a hydrophobic pocket on the surface of XIAP BIR3, between W323 and Y324 (Figure 3). We next designed and synthesized compound 10, in which a benzyl group is appended to the 5-membered ring, to test if targeting this hydrophobic pocket can further improve the binding affinities to IAP proteins and cellular activity. Modeling predicted that the pro-(S) configuration is preferred. Accordingly, we have developed a stereo-selective synthetic method for 10 (Scheme I). To test if the conformation of the 8-membered ring is critical for binding to IAP proteins, we have also synthesized compound 11, which has a double bond in its 8-membered ring.
Scheme I. Synthesis of compounds 10 and 11.
Reagents and conditions: (a) LiHMDS, Benzaldehyde, BF3·OEt2, THF, −78 °C; (b) MsCl, TEA, DCM, r.t.; (c) H2, Pd/C, MeOH, r.t., 75% (3 steps); (d) TFA, DCM, 0 °C; (e) LiHMDS, CbzCl, THF, −78 °C to r.t., 80% (2 steps); (f) Super-Hydrid, THF, −78 °C; (g) Ac2O, TEA, DMAP, DCM, r.t.; (h) Bu3SnAllyl, BF3·OEt2, toluene, −78 °C, 4, 5-syn:anti 5.6:1, 55% (3 steps); (i) BF3·OEt2, Me2S, DCM, 0 °C; (j) aq. sat. NaHCO3, 88% (2 steps); (k) (S)-N-Boc-Allylglycin, ClCOOiBu, NMM, THF, −20 °C; (l) Grubbs catalyst (1st generation), DCM, reflux, 67% (2 steps); (m) LiOH·H2O, THF/H2O/MeOH, 0 °C; (n) (R)−1,2,3,4-tetrahydronaphthalen−1-amine, EDCI, HOBt, DIPEA, DCM, r.t., 95% (2 steps); (o) HCl/MeOH, 0 °C; (p) Boc-N-Me-Ala-OH, EDCI, HOBt, DIPEA, DCM, r.t.; (q) H2, Pd/C, MeOH, r.t.; (r) HCl/MeOH, 0 °C, 85% (4 steps) for compound 10 and 91% (3 steps) for compound 11.
Compound 10 binds to XIAP, cIAP-1 and cIAP-2 with very high affinities and has Ki values of 3.9 nM, 0.37 nM and 0.25 nM to these three proteins, respectively (Table 1). In comparison, 11 has Ki values of 31 nM, 1.27 nM and 2.09 nM to XIAP, cIAP-1 and cIAP-2 proteins, respectively, significantly less potent than 10.
Consistent with its high affinities to IAP proteins, 10 potently inhibits cell growth in the MDA-MB-231 cancer cell line and has an IC50 value of 8.9 nM and is >25-times more potent than the initial lead 7 (Figure 4).
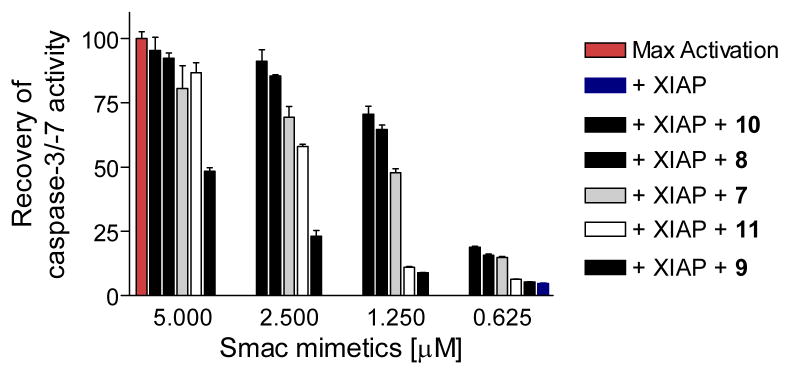
These Smac mimetics were evaluated for their ability to antagonize XIAP in a cell-free functional assay (Figure 5 and Supporting Information). While the XIAP BIR3 protein effectively inhibits the activity of caspase-3/-7, these Smac mimetics dose-dependently antagonize the inhibition of XIAP to caspase activity. Consistently with their binding affinities to XIAP BIR3, compounds 8 and 10 are the most potent antagonists of XIAP in this cell-free functional assay, while 9 is the least potent antagonist against XIAP BIR3.
Figure 5.
Functional antagonism of Smac mimetics against XIAP BIR3 in a cell-free functional assay. dATP and cyctochrome c were added into cell lystates to activate caspase-3/7 and recombinant XIAP BIR3 protein at 500 nM effectively inhibited casapse activity. Smac mimetics dose-dependently recovered the activity of casapse-3/-7. The caspase activity data at 60 min was used (Supporting Information).
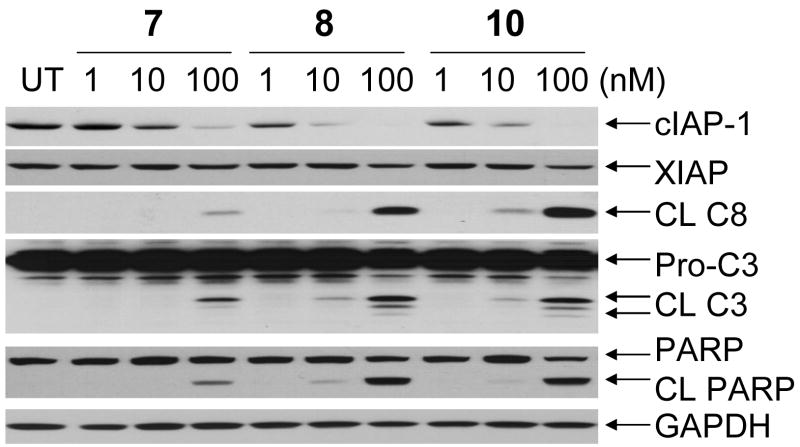
Recent studies showed that Smac mimetics induce cIAP-1 degradation in cells, which is crucial for apoptosis induction by Smac mimetics.21–23 Indeed, Western blotting analysis showed that compounds 7, 8 and 10 all effectively and dose-dependently induce the degradation of cIAP-1 (Figure 6). Compounds 8 and 10 are capable of inducing marked cIAP-1 degradation at concentrations as low as 10 nM, consistent with their high binding affinities to cIAP-1. These compounds have minimal effect on the levels of XIAP protein.
Figure 6.
Western blot analysis of the levels of cIAP-1, XIAP, cleaved caspase-8 (CL C8), pro- and cleaved caspase-3 (Pro-C3 and CL C3), cleaved PARP (CL PARP). MDA-MB-231 breast cancer cells were treated with Smac mimetics for 24 hours and proteins were probed with specific antibodies. GAPDH was used as the loading control.
We further examined the processing of caspase-8 and -3 and cleavage of poly(ADP-ribose) polymerase (PARP), several biochemical markers of apoptosis (Figure 6). Compounds 7, 8 and 10 effectively induce processing of caspase-8 and -3 and cleavage of PARP in a dose-dependent manner (Figure 6). Compounds 8 and 10 are capable of inducing processing of caspase-3 and -8 and cleavage of PARP at concentrations as low as 10 nM and are more effective than 7. These data indicated that these Smac mimetics not only inhibit cell growth but effectively and dose-dependently induce apoptosis in the MDA-MB-231 cancer cell line.
The synthesis of compounds 8 and 9 are similar to that for 720 and is provided in Supporting Information.
The synthesis of compounds 10 and 11 is shown in Scheme I. Briefly, compound 13 was prepared from the commercially available pyroglutamate ester 12.25 The Boc protective group in 13 was replaced by a Cbz to obtain 14 for achieving better stereo-selectivity in subsequent steps. The stereochemistry of the benzyl group in 13 and 14 was confirmed by two-dimensional (2D) Nuclear Overhauser Effect (NOE) data of 14 (Supporting Information) and was found to be same as that in the previous report.25 The carbonyl group in 14 was selectively reduced, followed by acetylation and introduction of a syn-allyl group at the C-5 position via the N-acyl iminium ion chemistry to afford compound 15.26 The Cbz protective group in 15 was selectively removed to generate 16, which was coupled with (S)-N-Boc-allylglycine, followed by cyclization using the olefin metathesis method to yield the key intermediate 17. The syn stereochemistry between the allyl and the benzyl groups of 16 between was confirmed by 2D NOE data of 16 and its stereoisomer 16i (Supporting Information). Compound 17 was hydrolyzed and then coupled with (R)-1,2,3,4-tetrahydronaphthalen-1-amine to give amide 18. Removal of the Boc protecting group in 18 yielded an ammonium salt, which was condensed with N-Boc-methyl-L-alanine to generate 19. Removal of the Boc protecting group in 19 produced the designed compound 11. Hydrogenation of the C=C double bond in 19 catalyzed by 10% Pd-C, followed by removal of the Boc protecting group, afforded compound 10.
In summary, we have designed and synthesized a number of novel Smac mimetics based upon lead compound 7 by modifications of two different regions. Our efforts led to the discovery of arguably the most potent, non-peptidic, monovalent Smac mimetic and insights into their structure-activity relationship in binding, functional and cellular levels. The most potent compound 10 (DM-28) binds to cIAP-1, cIAP-2 and XIAP with Ki values in subnanomolar and low nanomolar affinities, potently antagonizes XIAP in a cell-free functional assay and effectively induces cIAP-1 degradation, processing of caspase-8 and -3 and cleavage of PARP in the MDA-MB-231 cancer cell line at concentrations as low as 10 nM. Compound 10 potently inhibits cell growth in the MDA-MB-231 cell line with an IC50 value of 8.9 nM and is >25-times more potent than 7. Extensive in vitro and in vivo studies are under way to further elucidate mechanism of action and therapeutic potential of 10 as a new anticancer agent and the results will be reported in due course.
Supplementary Material
Acknowledgments
We are grateful for financial support from the Chinese Academy of Sciences and the National Natural Science Foundation of China (grants 20620120106, 20621062 & 90713047), the National Cancer Institute, National Institutes of Health, USA, (R01CA109025), the Breast Cancer Research Foundation, the Susan G. Komen Foundation, the Prostate Cancer Foundation, the University of Michigan Cancer Center Core grant (P30CA046592) and Ascenta Therapeutics Inc.
Footnotes
Abbreviations: IAP, inhibitor of apoptosis protein; XIAP, X-linked IAP; cIAP-1/-2, cellular IAP 1/2; Smac, second mitochondria-derived activator of caspases; BIR, baculoviral IAP repeats (BIR) domain; BIR2/BIR3, the second or third BIR domain; TNF, tumor necrosis factor; FP, fluorescence polarization; NOE, Nuclear Overhauser Effect.
Supporting Information Available: An experimental section including the information on the chemical data for compounds 8–10 and intermediates, structural assignments of 14, 16 and 16i by two-dimensional NOE data, FP competitive binding assays for XIAP and cIAP-1/-2 BIR3 proteins, cell-free functional assay of XIAP BIR3, cell growth assay, Western blotting analysis and molecular modeling is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 5.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;1:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 6.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 7.Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, Scheurich P, Schmid JA, Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 8.Holcik M, Gibson H, Korneluk RG. XIAP: Apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S. Inhibitor of apoptosis proteins as targets for anticancer therapy. Expert Rev Anticancer Ther. 2007;7:1255–1264. doi: 10.1586/14737140.7.9.1255. [DOI] [PubMed] [Google Scholar]
- 10.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 11.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 12.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Sun C, Olejniczak ET, Meadows R, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 15.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 16.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Liu M, Tomita Y, Pan H, Yoshioka Y, Krajewski K, Roller PP, Wang S. Structure-Based Design of Potent, Conformationally Constrained Smac Mimetics. J Am Chem Soc. 2004;126:16686–16687. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]
- 17.Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP, Olejniczak ET, Oleksijew A, Oltersdorf T, Rosenberg SH, Shoemaker AR, Tomaselli KJ, Zou H, Fesik SW. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47:4417–4426. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- 18.Sun H, Nikolovska-Coleska Z, Lu J, Qiu S, Yang CY, Gao W, Meagher J, Stuckey J, Wang S. Design, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimetic. J Med Chem. 2006;49:7916–7920. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]
- 19.Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol. 2006;1:525–533. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, Tomita Y, Ueda Y, Jiang S, Krajewski K, Roller PP, Stuckey JA, Wang S. Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc. 2007;129:15279–15294. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 23.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jianfeng Lu J, Longchuan Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, Meagher JL, Stuckey JA, Wang S. SM-164: A novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Research. 2008 doi: 10.1158/0008-5472.CAN-08-2655. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ezquerra J, Pedregal C, Yruretagoyena B, Rubio A, Carreno MC, Escribano A, Ruano JLG. Synthesis of enantiomerically pure 4-substituted glutamic acids and prolines: general Aldol reaction of pyroglutamate lactam lithium enolate mediated by Et2• BF3. J Org Chem. 1995;60:2925–2930. [Google Scholar]
- 26.Hanessian S, Margarita R, Hall A, Johnstone S, Tremblay M, Parlanti LJ. Total synthesis and structural confirmation of the marine natural product dysinosin A: A novel inhibitor of thrombin and factor VIIa. J Am Chem Soc. 2002;124:13342–13343. doi: 10.1021/ja0208153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.