Abstract
Background
Deletion of phenylalanine-508 (ΔF508) from the first nucleotide-binding domain (NBD1) in the wild-type cystic fibrosis (CF) transmembrane-conductance regulator (wtCFTR) causes CF. However, the mechanistic relationship between ΔF508-CFTR and the diversity of CF disease is unexplained. The surface location of F508 on NBD1 creates the potential for protein-protein interactions and nearby, lies a consensus sequence (SYDE) reported to control the pleiotropic protein kinase CK2.
Methods
Electrophysiology, immunofluorescence and biochemistry applied to CFTR-expressing cells, Xenopus oocytes, pancreatic ducts and patient biopsies.
Results
Irrespective of PKA activation, CK2 inhibition (ducts, oocytes, cells) attenuates CFTR-dependent Cl− transport, closing wtCFTR in cell-attached membrane patches. CK2 and wtCFTR coprecipitate and CK2 co-localized with wtCFTR (but not ΔF508-CFTR) in apical membranes of human airway biopsies. Comparing wild-type and ΔF508CFTR expressing oocytes, only ΔF508-CFTR Cl− currents were insensitive to two CK2 inhibitors. Furthermore, wtCFTR was inhibited by injecting a peptide mimicking the F508 region, whereas the ΔF508-equivalent peptide had no effect.
Conclusions
CK2 controls wtCFTR, but not ΔF508-CFTR. Others find that peptides from the F508 region of NBD1 allosterically control CK2, acting through F508. Hence, disruption of CK2-CFTR interaction by ΔF508-CFTR might disrupt multiple, membrane-associated, CK2-dependent pathways, creating a new molecular disease paradigm for deleted F508 in CFTR.
Keywords: ATP-binding cassette transporter, CFTR, Chloride ion channel, Channel regulation, Cystic fibrosis, Protein kinase CK2
Introduction
Cystic fibrosis (CF) is a common autosomal recessive multi-system disease resulting from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) [1, 2]. CFTR is a Cl− channel with intricate regulation that plays an essential role in fluid and electrolyte transport across epithelia [2, 3]. However, the molecular mechanisms that control CFTR function are complex and incompletely understood [4]. CFTR belongs to the ATP-binding cassette (ABC) family of membrane transporters that utilise the energy of ATP hydrolysis to transport a wide spectrum of substrates across cell membranes [5]. Unique among ABC proteins, CFTR is an ion channel, gated by ATP-driven nucleotide-binding domain (NBD) dimerisation [6].
Deletion of F508 (ΔF508) from NBD1 of CFTR is by far the commonest pathogenic mutation (accounting for 80-90% of alleles) and induces a multi-system disease [2]. The resultant clinical features are difficult to reconcile with defects in a single type of ion channel [7-10] because ΔF508-CFTR perturbs inflammation, cell metabolism and multiple ion channels and transporters in epithelial cells. ΔF508-CFTR attenuates CFTR biosynthesis [11], cell surface expression [12] and channel gating [13]. However, it is unknown how ΔF508 CFTR alters the function of unrelated proteins, including other epithelial ion channels [14].
CFTR is part of a macromolecular complex in the apical membrane of epithelia [4] comprising (amongst others) a number of protein kinases, syntaxins, ezrin-binding phosphoprotein 50 [15] and CAP70 [16]. Provided ATP is available for NBD binding, CFTR channel function is activated by protein kinases such as protein kinase A (PKA) and protein kinase C (PKC) [17], but inhibited by the AMP-activated kinase (AMPK) [18, 19]. We noted that F508 is located on the surface of the crystal structure of NBD1 of CFTR, suggesting that it might be accessible for protein-protein interactions [20-22]. However, in CF model systems no such inter-molecular interactions have yet been assigned to the F508 residue or its adjacent region in wild-type CFTR. This report investigates whether a regulatory protein interacts with this region in an F508-dependent manner.
We hypothesised that an explanation for the multi-system nature of CF might reside in differences between proteins bound to wild-type and ΔF508 CFTR. The amino-acid sequence of CFTR adjacent to F508 contains a consensus sequence (KENIIF508GVS511 YDEYR; consensus motif underlined) with potential for phosphorylation by protein kinase CK2 (formerly known as casein kinase II) [23], with a potential target serine located at serine 511 (S511). This protein kinase is essential during development and has many hundreds of targets [24-26]. Given the diversity of unexplained defects in CF, this plurality of action makes CK2 an attractive candidate for study in CF cells. CK2 can exist as a heterotetramer containing two 47 kDa α (catalytic) and two 26 kDa β (regulatory) subunits that target over 300 proteins, linking its promiscuous activity to essential cellular functions [26]. Unusually, CK2 can also use either ATP or GTP as a phosphate donor for kinase activity towards multiple substrates thus adding to the complexity [26]. However, the physiological mechanisms regulating CK2 are not understood despite four decades of effort. Principally, this difficulty arises because unlike most protein kinases, CK2 is often described as constitutively active; but CK2 function is regulated, first by restricting its subcellular localisation and, second, by modulating regulatory interactions between the α and β subunits (following the poorly-understood ‘auto’phosphorylation of the β subunit) [27]. Specific inhibitors of CK2 are either directed towards the nucleotide-binding α subunit (exploiting its rather different structure compared to most kinases) or to the site of polyamine binding on the acidic groove in the regulatory β subunit. In this study, we investigated whether CK2 interacts functionally with the F508 region of CFTR and whether deletion of F508 disrupts binding of this signalling molecule using CFTR peptides from the F508 region of NBD1, pharmacological inhibitors of CK2, site-directed mutagenesis of S511-CFTR and biochemical, cell biological and functional assays.
Materials and Methods
Ethical approval
Informed consent for nasal biopsy was obtained in writing from normal volunteers and CF patients. Ethical approval was granted by the Tayside Committee on Medical Research Ethics, Dundee, UK. Guinea pigs were killed by cervical dislocation and the pancreas removed. The experimental protocol was approved by the local Ethical Board of the University of Szeged, Hungary (1-74-130/2007). The harvesting of oocytes from Xenopus for CFTR expression studies complied in full with the German laws for animal experimentation (§6 Abs. 1 Satz 2, Nr. 4 of the German animal protection laws).
Cell culture
For this study, we used human epithelial cells endogenously expressing CFTR and mammalian cell lines expressing recombinant CFTR. The human airway epithelial cell lines 16HBE14o- and CFBE41o- [28-30] were cultured in M199 media containing 10% foetal calf serum, 2 mM L-glutamine, 1% antibiotics (penicillin-streptomycin, 10,000 U/ml; Invitrogen Ltd., Paisley, UK) and 1% fungizone at 37 °C in a humidified atmosphere of 5% CO2. Mouse mammary epithelial (C127) cells stably expressing wild-type human CFTR and baby hamster kidney (BHK) cells stably expressing wild-type and mutant CFTRs were cultured as previously described [31, 32]. C127 and BHK cells expressing wild-type and mutant CFTRs were generous gifts of Dr. C.R. O’Riordan (Genzyme Corp., Framingham, MA, USA) and Prof. M.D. Amaral (University of Lisboa, Portugal), respectively. Unless otherwise specified, reagents were of analytical grade and purchased from Sigma-Aldrich.
Immunofluorescence
Ciliated nasal epithelial cells harvested from the inferior turbinate of patients undergoing unrelated surgery (approved by local ethical committee) were maintained in cell culture medium M199 prior to fixation in 4% paraformaldehyde. Cells were permeabilised using 1% Triton X-100, washed 3 times in PBS, then blocked in 1 mM glycine for 15 min, followed by 5% donkey serum for 15 min. Pelleted cells were resuspended in PBS containing primary antibodies (goat anti-CK2α (Santa Cruz) and mouse anti-CFTR NBD1 (Neomarkers, originally developed by J.R. Riordan) at a 1:100 dilution) and incubated at room temperature, with shaking, overnight. After 3 washes in PBS, pelleted cells were resuspended in PBS containing fluorescein isothiocyanate (FITC)-labelled anti-goat and rhodamine-labelled anti-mouse IgG secondary antibodies (Jackson, 1:100). After a 2 h incubation, with shaking, the cells were washed five times in PBS and resuspended in 15 μl anti-fade mountant (6% n-propyl gallate in 70% glycerol, 100 mM Tris/HCl, pH 7.4) for mounting on glass slides. Coverslips were sealed with nail varnish for image capture using a Zeiss 510 laser scanning confocal microscope.
Co-immunoprecipitation of CK2 with CFTR
16HBE14o- and CFBE41o- cells were cultured to confluence, harvested and membranes isolated as previously described [33]. Membranes were solubilised in RIPA buffer (10 mM Tris; 1% Triton X-100; 0.5% Na deoxycholate; 0.5% Nonidet P-40; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; pH 7.4 with HCl) containing protease and phosphatase inhibitors before immunoprecipitations were performed. Immunoprecipitates were washed stringently 3 × in RIPA buffer and once in TBST (Tris buffered saline containing 0.05% Tween 20) with 0.5 M NaCl. Samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Immunoblotting
Immunoblotting was performed as previously described [34]. In brief, blotted membranes were blocked for 30 minutes in TBST with 5% milk powder followed by 4 × 15 minute washes, antibody was applied for 1.5 hours followed by 4 × 15 minute washes, then horseradish peroxidase- (HRP) labelled anti-mouse secondary antibody was applied at a 1/5000 dilution for 45 minutes followed by 4 × 15 minute washes. Bound HRP was visualised using a chemiluminescence system and exposure to X-ray film.
Single-channel patch-clamp studies
CFTR Cl− channels were recorded in either cell-attached or excised inside-out membrane patches using an Axopatch 200B patch-clamp amplifier (Molecular Devices Corp., Union City, CA, USA) and pCLAMP data acquisition and analysis software (versions 6.03 and 9.2, Molecular Devices Corp.) as previously described [31, 35]. The established sign convention was used throughout; currents produced by positive charge moving from intra- to extracellular solutions (anions moving in the opposite direction) are shown as positive currents.
For cell-attached recordings, the pipette solution contained (mM): 140 N-methyl-D-glucamine (NMDG), 3 MgCl2, 10 N-tris[Hydroxymethyl]methyl-2-aminoethanesulfonic acid (Tes) and 1 CsEGTA, pH 7.3 with HCl ([Cl−], 147 mM). The bath solution contained (mM): 137 NaCl, 4 KCl, 3 MgCl2 and 10 Tes, pH 7.3 with NaOH ([Cl−], 147 mM) and was maintained at 37 °C; pipette potential was +50 mV. CFTR Cl− channels were activated by pre-treating cells with forskolin (20 μM; Sigma-Aldrich) prior to seal formation. To inhibit CK2, we added 4,5,6,7-tetrabromobenzotriazole (TBB; 10 μM; Sigma-Aldrich), a selective inhibitor of CK2 to the bath solution in the continuous presence of forskolin (20 μM).
For excised inside-out membrane patch recordings, the pipette (extracellular) solution contained (mM): 140 NMDG, 140 aspartic acid, 5 CaCl2, 2 MgSO4 and 10 Tes, pH 7.3 with Tris ([Cl−], 10 mM). The bath (intracellular) solution contained (mM): 140 NMDG, 3 MgCl2, 1 CsEGTA, and 10 Tes, pH 7.3 with HCl, ([Cl−], 147 mM; [Ca2+]free, <10−8 M) and was maintained at 37 °C; voltage was −50 mV. After excision of inside-out membrane patches, CFTR Cl− channels were activated by the addition of ATP (1 mM) and the catalytic subunit of PKA (75 nM; purified from bovine heart; Promega UK) to the intracellular solution within 5 min of patch excision. To test the effects of TBB (10 μM), the inhibitor was added to the intracellular solution in the continuous presence of ATP (1 mM) and PKA (75 nM).
We recorded, filtered and digitised data as described previously [31], with the exception that cell-attached data were digitally filtered at 100 Hz after digitisation. For the purposes of illustration, (i) data were filtered at 500 Hz and digitised at 1 kHz and (ii) baseline correction was applied to cell-attached recordings using pCLAMP (version 9.2). To measure single-channel current amplitude (i), Gaussian curves were fit to current amplitude histograms. To measure open probability (Po), we created lists of open- and closed-times and calculated Po as described previously [35]. The number of active channels in a membrane patch was determined from the maximum number of simultaneous channel openings observed during an experiment using the strategies described in [35] to minimise errors when counting the number of active channels.
Xenopus oocyte studies
Oocytes were isolated and microinjected as described previously [36]. In brief, cDNAs encoding (i) wild-type or mutant CFTRs and (ii) CK2 were linearised in pBluescript or pTLN with Notl and in vitro transcribed using T7, T3 or SP6 promotor and polymerase (Promega, USA). After isolation from adult Xenopus laevis (African clawed frog) (Kähler, Germany), oocytes were dispersed and defolliculated with collagenase (45 min, type A, Boehringer, Germany). Subsequently, oocytes were rinsed and stored at 18 °C in ND96-buffer (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes and 2.5 Na-pyruvate, pH 7.55, supplemented with theophylline (0.5 mM) and gentamicin (5 mg/l). Oocytes were injected with cRNA (1-10 ng) in 47 nl double-distilled water (Nanoliter Injector WPI, Germany). Water-injected oocytes served as controls. 2-4 days after injection, oocytes were impaled with two microelectrodes (Harvard Apparatus), which had resistances of <1 MΩ when filled with 2.7 mol/l KCl. Using two bath electrodes and a virtual-ground headstage, the voltage drop across Rserial was effectively zero. Membrane currents were measured by voltage-clamping the oocytes (Warner oocyte clamp amplifier OC725C) in intervals from −90 to +30 mV, in steps of 10 mV, each 1 s. Conductances were calculated using Ohm’s law. Oocytes were perfused continuously with physiological solutions at a rate of 5-10 ml/min. All experiments were conducted at room temperature (22 °C). For studies of poly E:Y peptide, 43 nl of the peptide (Sigma) were injected into CFTR-expressing oocytes to a final concentration of 10-20 μM and CFTR Cl− currents assessed 3-24 h after injection. For studies using the KENIIF and KENII peptides, peptides were injected into CFTR-expressing oocytes to a final concentration of 100 nM and CFTR Cl− currents assessed 20 h after injection. To investigate the intracellular pH-dependence of CFTR Cl− currents, CFTR-expressing Xenopus oocytes were exposed to extracellular solutions titrated to pH 7.0, 7.5 and 8.0 in the presence of the protonophore carbonyl cyanide m-chloro phenylhydrazone (CCCP; 10 μM). For experiments using excised oocyte macropatches, at the high levels of CFTR expression employed, run down of preactivated (with forskolin/IBMX) CFTR Cl− currents was sufficiently slow to permit a solution change allowing addition of TBB. The macropatch methods are described elsewhere [37] and were made in the presence of ATP (3 mM) in the intracellular solution.
Pancreatic duct studies
Small intra/interlobular ducts were isolated from guinea pigs as described previously [38]. The ducts were cultured overnight in a 37 °C incubator gassed with 5% CO2/95% air. The duct lumen was microperfused using a modification of the method of Ishiguro et al. [39] with a standard HCO3−-buffered solution containing (mM) 115 NaCl, 25 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2 and 10 D-glucose, gassed with 95 % O2/5 % CO2 to set pH to 7.4 at 37 °C. Two concentric pipettes were used for microperfusion. One end of a sealed duct was cut off and the other end was aspirated into the outer, holding pipette. Then, while applying a negative pressure to the holding pipette with a syringe, the inner perfusion pipette was gently advanced into the duct lumen. The duct was then perfused at a rate of 10-30 μl/min with the luminal perfusate flowing out at the open end. The high rate of bath perfusion (5-6 ml/min), which was in the same direction as the flow of luminal perfusate, ensured that the escaping luminal perfusate did not gain access to the basolateral surface of duct epithelial cells. Replacement of the luminal perfusate took up to 2 min.
Intracellular pH (pHi) was estimated using the pH-sensitive fluorescent dye BCECF-AM. Briefly, ducts were bathed in standard Hepes solution at 37 °C and loaded with the membrane permeable acetoxymethyl derivative of BCECF (2 μmol/l) for 20-30 min. After loading, the ducts were continuously perfused with solutions at a rate of 5-6 ml/min. pHi was measured using a microspectrofluorimeter system (Cairn Research Ltd., Faversham, UK). A small area of 5-10 cells was excited with light at wavelengths of 490 nm and 440 nm and the 490/440 fluorescence emission ratio was measured at 535 nm. Four pHi measurements were obtained per second. In situ calibration of the fluorescence signal was performed using the high K+-nigericin technique [40, 41]. During the calibration, ducts were bathed in high K+ Hepes solution and extracellular pH stepped between 5.95 and 8.46.
We utilized the inhibitory stop method to estimate the rate of HCO3− efflux across the luminal membrane. Briefly, basolateral Na+/HCO3− cotransporters (NBC) and Na+/H+ exchangers (NHE) were blocked using H2DIDS (0.5 mM) and amiloride (0.2 mM), respectively, administered from the basolateral side for 3 minutes. The inhibition of these transporters caused a marked decrease in pHi. The rate of pHi acidification after exposure to H2DIDS and amiloride reflects the intracellular buffering capacity and the rate at which HCO3− effluxes (i.e. is secreted) across the luminal membrane through Cl−/HCO3− exchangers and/or CFTR Cl− channels [42, 43]. The initial rate of intracellular acidification (dpH/dt) from the administration of inhibitors over the first 60 s was calculated by linear regression analysis using 60 data points (one pHi measurement per second).
The total buffering capacity (βtotal) of duct cells was estimated according to the NH4+ pre-pulse technique [42-45]. Pancreatic duct cells were exposed to various concentrations of NH4Cl in a Na+ and HCO3− free solution. βi (which refers to the ability of intrinsic cellular components to buffer changes of pHi) was estimated by the Henderson-Hasselbach equation. βtotal was calculated from: βtotal = βi + βHCO3- = βi + 2.3 × [HCO3−]i, where βHCO3- is the buffering capacity of the HCO3−/CO2 system. The rates of pHi change measured in the inhibitor stop experiments were converted to transmembrane base flux J(B−) using the equation: J(B−) = dpH/dt × βtotal. We denoted base efflux (secretion) as −J(B−).
Statistics
Results are expressed as means ± SEM of n observations. To test for differences between groups of data, an analysis of variance (ANOVA) was used. To compare only two sets of data, we used Student’s t-test. Differences were considered statistically significant when P < 0.05. Tests were performed using SigmaStat™ (version 2.03, Jandel Scientific GmbH, Erkrath, Germany) or Excel (Microsoft).
Results
Localisation of CK2 and CFTR in ciliated nasal epithelial cells from normal subjects and CF patients
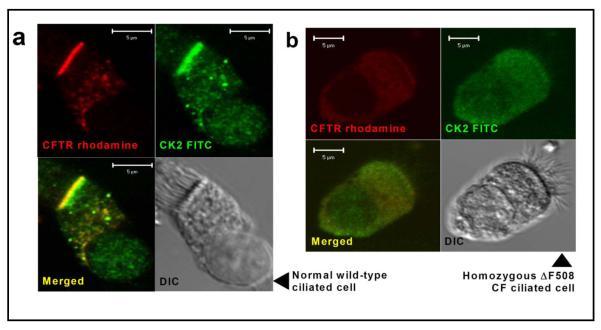
Using immunofluorescence and confocal microscopy, we investigated whether the subcellular localisation of the pleiotropic protein kinase CK2 coincides with the apical membrane distribution of CFTR in ciliated human nasal epithelial cells harvested from normal volunteers (age range 16-35 years). Moreover, we were interested to learn whether the defective processing and trafficking of the common CF mutant ΔF508-CFTR altered the distribution of CK2 in ciliated nasal epithelial cells obtained from CF patients of a similar age homozygous for the ΔF508-CFTR mutation. Figure 1a demonstrates that, like wild-type CFTR, the α (catalytic) subunit of CK2 (CK2α) is localised at the apical membrane of ciliated nasal epithelial cells from normal subjects. Consistent with previous data acquired using recombinant and native cells [11, 46], ciliated nasal epithelial cells from CF patients homozygous for the ΔF508-CFTR mutation show very little staining for CFTR protein at the apical membrane (Fig. 1b). Figure 1b also demonstrates that the localization of CK2α to the apical membrane is abrogated in nasal epithelial cells from CF patients bearing the ΔF508-CFTR mutation. We interpret these results to suggest that the localisation of CK2 to the apical membrane of nasal epithelial cells is dependent on CFTR.
Fig. 1.
CK2 colocalisation with wild-type, but not ΔF508 CFTR 4% paraformaldehyde-fixed human ciliated nasal airway epithelial cells from wild type (a) and Pseudomonas-free homozygous ΔF508 CF (b) subjects stained with anti-CFTR rhodamine and anti-CK2α FITC. Images were acquired with a Zeiss LSM510 microscope using identical laser output and gain and are typical of samples from at least three separate individuals from each group. Scale bar is 5 μm. Controls omitting primary antibodies were blank at the same settings.
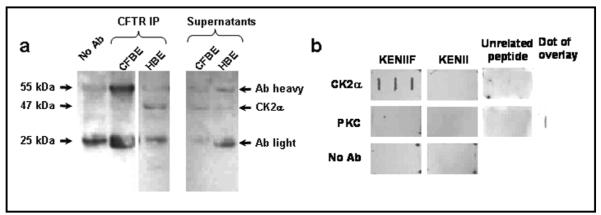
To explore further the association of CK2 with CFTR, we isolated cell membranes from human bronchial epithelial cells endogenously expressing wild-type or ΔF508-CFTR (HBE, 16HBE14o- and CFBE, CFBE41o-[28-30, 47], respectively) and tested whether CK2α co-immunoprecipitates with CFTR. Figure 2a demonstrates that CK2α was detected in CFTR immunoprecipitates from HBE membranes, whereas it was undetectable in CFTR immunoprecipitates from CFBE membranes. CK2 was detected in the input membranes from both cell lines (data not shown). We confirmed that CK2α can interact with the F508 region of CFTR using a peptide overlay approach. We made detergent extracts of cell membranes from HBE cells and overlaid them onto peptide dot blots followed by detection with an anti-CK2α antibody. Figure 2b demonstrates that CK2α only binds the peptide sequence corresponding to wild-type CFTR (KENIIF; Fig. 2b). This was confirmed using a second anti-CK2α antibody (data not shown). Figure 2b also shows that no interaction was detected with cell membranes from HBE cells using either the ΔF508-CFTR peptide (KENII) or a control peptide from another region of CFTR (unrelated peptide from NBD2). As a further control, we also used PKC, a protein kinase that regulates CFTR activity [48-50] and demonstrated that no peptide bound to PKC (Fig. 2b).
Fig. 2.
CFTR, but not ΔF508-CFTR associates with CK2 (a) Co-immunoprecipitation (IP) of CK2 with CFTR using identical input protein content of membranes from cystic fibrosis bronchial epithelia (CFBE) and human bronchial epithelia (HBE). (b) 1% octyl-gluco-side extracted HBE membranes overlaid onto dot blots of wild-type/ΔF508 peptide (GTIKENIIFGVSYDEYRYR/GTIKENIIGVSYDEYRYR; labelled as KENIIF/KENII, respectively), and probed for CK2α or PKC (both Santa Cruz). Bottom panels omit primary antibody and fourth PKC panel is positive control; unrelated peptide is from NBD2 (QRVGLLGRTGSGKSTLL); results shown are representative of n = 3 experiments.
Taken together, these biochemical and cell biological data suggest that CFTR associates with CK2α and that the ΔF508 mutation disrupts this interaction. This interpretation is consistent with recently published evidence demonstrating that NBD1 of CFTR interacts with CK2α in vitro and that the loss of F508 alters this interaction [27].
CK2 modulates cAMP-dependent anion transport
CFTR plays a central role in cAMP-dependent fluid and electrolyte transport across epithelia by providing a pathway for anion movement across the apical membrane and regulating the function of other ion channels and transporters [3, 51]. To investigate CK2-dependent regulation of CFTR-mediated anion transport, we employed the CK2-selective inhibitor, TBB [52]. TBB targets the unusual ATP binding site on CK2α, but is without effect on 30 other protein kinases including recognised modulators of CFTR such as PKA, PKC and AMPK [52].
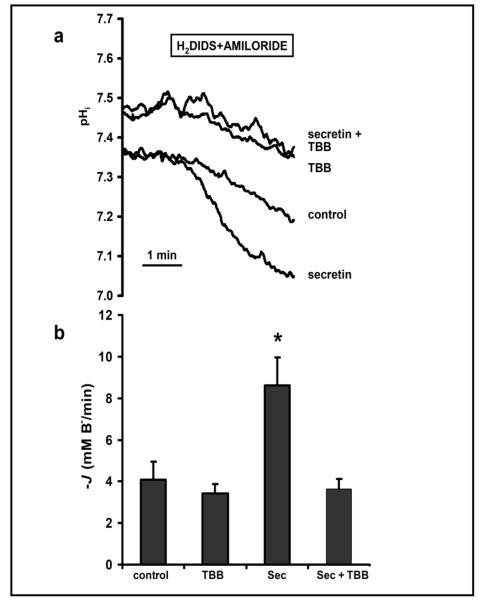
To test the effects of TBB on CFTR-dependent anion transport at the whole tissue level we employed isolated pancreatic ducts from the guinea pig [43, 53]. Intact pancreatic ducts from the guinea pig and other species are a robust model of CFTR-dependent HCO3− secretion [38, 39, 42, 54]. Using the inhibitor-stop technique, we measured rates of luminal HCO3− efflux (see Methods); amiloride and DIDS were applied to inhibit basolateral Na+/H+ exchangers (NHEs) and Na+/HCO3− cotransporters (NBCs), respectively. Figure 3a demonstrates that exposure of a single microperfused pancreatic duct to secretin (10 nM) increased HCO3− secretion (measured as a fall in pHi) ~2 fold above basal levels (data summarised in Figure 3b). Secretin is the most important physiological regulator of pancreatic HCO3− secretion and activates CFTR by a cAMP-dependent mechanism [55, 56]. Addition of TBB (40 μM) alone to the luminal membrane caused a small alkalinisation of intracellular pH (Fig. 3a), but this CK2 inhibitor had no significant effect on basal rates of HCO3− efflux (Fig. 3b). By contrast, TBB (40 μM) completely abolished secretin-stimulated HCO3− efflux (Fig. 3b), consistent with an important role of CK2 in regulating CFTR-dependent HCO3− secretion. Note that the increase in pHi caused by TBB is unlikely to account for the observed inhibition of secretin-stimulated HCO3− secretion because CFTR exhibits little pHi-dependence between pHi 7.3 and 7.5 [56]. Moreover, when we exposed CFTR-expressing Xenopus oocytes to extracellular solutions titrated to pH 7.0, 7.5 and 8.0 in the presence of the protonophore CCCP (10 μM) there was little or no change in CFTR conductance (pHi 7.0, 24.8 ± 4.3 μS; pHi 7.5, 25.1 ± 3.3 μS; pHi 8.0, 24.9 ± 4.1 μS; n = 5).
Fig. 3.
Luminal TBB inhibits secretin-stimulated bicarbonate secretion in pancreatic ducts (a) Representative traces from an inhibitor stop experiment using a guinea pig pancreatic duct exposed to a basolateral solution containing amiloride (0.2 mM) and H2DIDS (0.5 mM) for 3 min. (b) Summary data showing the initial rates of transmembrane HCO3− efflux (−JB−) measured over 60 s. Measurements were made, in the absence and presence of a 6-10 minute pre-treatment of pancreatic ducts with TBB (40 μM), from control ducts (albumin; 1% w/v; the vehicle for secretin) or ducts treated with secretin (Sec; 10 nM) in the basolateral solution. Data are means + SEM (control, n = 4; TBB, n = 10; secretin, n = 5; secretin + TBB, n = 3). The asterisk indicates a value that is significantly different from the control value (P < 0.05).
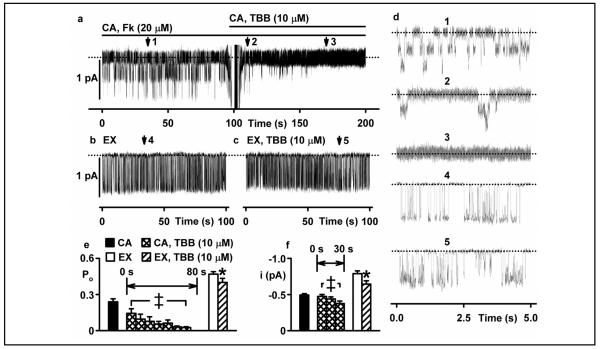
To understand better how CK2 inhibition affects CFTR function, we used the patch-clamp technique to investigate the single-channel activity of CFTR expressed in C127 cells. Figure 4a demonstrates that addition of TBB (10 μM) to the solution bathing an intact cell caused a prompt inhibition of channel activity in a cell-attached membrane patch (compare expanded trace 1 with expanded traces 2 and 3 in Figure 4a and d). TBB mediated its effects in two ways: first, it caused a time-dependent prolongation of the closed-time interval between bursts of channel openings and hence, decreased open probability (Po) to zero (Fig. 4e histogram, left). Second, it caused a small decrease in current flow through individual channels (Fig. 4f histogram, right). Interestingly, Figure 4b - f demonstrates that when TBB (10 μM) was added directly to the solution bathing the intracellular side of the membrane using an excised inside-out membrane patch, the drug had little effect on channel gating and hence, Po, but still reduced current flow through individual channels. Two conclusions can be drawn from these data. First, the small effect of TBB on CFTR-mediated current flow most likely represents the direct interaction of TBB with CFTR, itself. Second, the effects of TBB on CFTR channel gating that are observed in intact cells, but lost on excision of membrane patches suggest that TBB exerts its effect via a cytosolic CFTR-interacting protein that may be lost during excision of the membrane patch from C127 cells (which does not seem to be the case in excised oocyte membranes; c.f. below). Given that TBB is a selective inhibitor of CK2 at the low micromolar concentrations used here, the simplest interpretation of our data is that CK2 regulates CFTR channel gating in intact cells.
Fig. 4.
TBB inhibits CFTR channel gating in intact cells, but not excised membrane patches (a) Top, representative recording of two CFTR Cl− channels in a cell-attached (CA) membrane patch from a C127 cell expressing wild-type human CFTR. During the periods indicated by the bars, forskolin (20 μM) and TBB (10 μM) were present in the bath solution. (b, c) Middle, representative recording of a single CFTR Cl− channel in an excised (EX) inside-out membrane patch in the absence (b) and presence (c) of TBB (10 μM). ATP (1 mM) and PKA (75 nM) were continuously present in the intracellular solution. Dotted lines indicate where channels are closed and downward deflections correspond to channel openings. (d) 5 s portions indicated by numbered arrows in (a), (b) and (c) are shown on an expanded time scale (right). (e, f) Bottom Values of open probability (Po, (e)) and current amplitude (i, (f)) of CFTR Cl− channels recorded in cell-attached and excised inside-out membrane patches made in the absence and presence of TBB (10 μM). Columns and error bars are means + SEM (CA, n = 4-12; EX, n = 6). Data from cell-attached membrane patches in the presence of TBB (10 μM) are shown in consecutive 10 s intervals for 80 s (Po) and 30 s (i). The asterisks and double crosses indicate values that are significantly different from the control values (P < 0.05).
TBB fails to inhibit ΔF508-CFTR Cl−currents
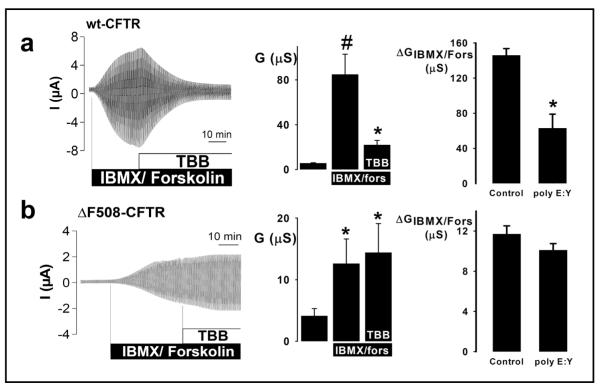
To investigate further how CK2 regulates the CFTR Cl− channel, we used the Xenopus oocyte expression system. Because the amino acid sequence of Xenopus CK2 is almost identical to that of human CK2 (α subunit, 94% identity; β subunit, 98% identity), we began by expressing only CFTR constructs in Xenopus oocytes. To inhibit CK2 activity pharmacologically, we added TBB (1 μM) to the solution bathing oocytes or injected oocytes with poly E:Y peptide 4:1 (10 μM), a peptide inhibitor of CK2, structurally-unrelated to TBB, which targets the β subunit of CK2. Figure 5a demonstrates that cAMP-stimulated CFTR Cl− currents were inhibited markedly by both TBB (1 μM) and poly E:Y peptide (10 μM); similar results were obtained when CFTR-expressing oocytes were pre-treated with TBB (1 μM) prior to the addition of cAMP agonists (Fig. 5, legend). These data suggest that the effects on CFTR Cl− currents of CK2 inhibition are dominant over those of PKA activation. Moreover, when membranes were excised as macropatches from IBMX/forskolin stimulated CFTR-expressing oocytes, the conductance of the macropatches (56 ± 4.1 nS) was significantly inhibited by 1 μM TBB (31 ± 3.8 nS; n = 5), suggesting that CK2 directly controls CFTR. Consistent with our hypothesis that CK2 would not regulate CFTR when the F508 residue is absent, we found that neither agent inhibited ΔF508-CFTR Cl− currents (Fig. 5b). Because inhibition of CK2 attenuates markedly wild-type CFTR Cl− current, but is without effect on ΔF508-CFTR Cl− current, we suggest that the CF mutation ΔF508 abrogates CFTR regulation by protein kinase CK2.
Fig. 5.
CK2 inhibition blocks wild-type, but not ΔF508-CFTR Cl− currents in Xenopus oocytes (a) Data for oocytes expressing wild-type human CFTR. (b) Equivalent data for oocytes expressing ΔF508 CFTR. Left, time-courses of cAMP-stimulated Cl− currents. During the periods indicated by the bars, IBMX (1 mM) + forskolin (Fors; 2 μM) and TBB (1 μM) were added to the bath solution. Oocytes were voltage-clamped from −90 to +30 mV, in steps of 10 mV, each 1 s. Middle, effects of TBB on cAMP-stimulated Cl− currents ((a) # and * indicate significant differences from control and IBMX/fors, respectively, P < 0.05; (b) * indicates significant difference from control, P < 0.05; n = 7 for (a) and (b)). Right, effects of poly E:Y peptide 4:1 (10 μM) on cAMP-stimulated Cl− currents. Data are means + SEM ((a) control, n = 8; poly E:Y, n = 13; (b) control, n = 7; poly E:Y, n = 12); * indicates significant difference in magnitude of IBMX/fors-activated conductance, P < 0.05. When oocytes expressing wild-type CFTR were pre-treated with TBB (1 μM) prior to activating CFTR Cl− currents with cAMP agonists (IBMX (1 mM) and forskolin (2 μM)) current magnitude was blunted severely. The magnitude of activated CFTR Cl− conductance in these oocytes (22 ± 4 μS, n = 8) is equivalent to the residual CFTR Cl− conductance following the inhibition of activated CFTR Cl− currents by TBB (1 μM) (Fig. 5a, middle panel).
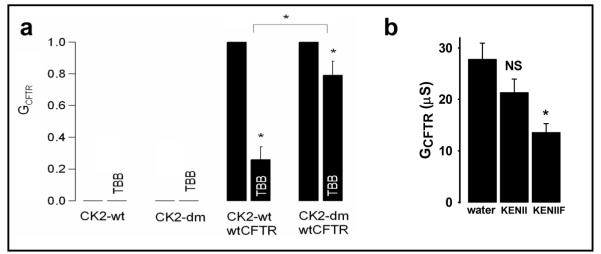
Despite the reported specificity of TBB for CK2 around the concentrations used in the above experiments [52], the application of any pharmacological inhibitor always raises the question of specificity. CK2 is both essential and constitutively active targeting hundreds of proteins. Therefore, to demonstrate that TBB exerts its effects on CFTR by acting specifically on CK2, we used CK2-dm, a TBB-insensitive CK2 α subunit variant (V66A and I174A, gift of L.A. Pinna, Padua, Italy) [57]. CK2-dm retains full kinase activity, but is not inhibited by TBB and is thus a valuable tool to compete away endogenous, TBB-inhibitable, CK2. Figure 6a (left) demonstrates that expression of either wild-type CK2α (CK2-wt) or CK2-dm alone did not generate any endogenous Cl− conductance and that, correspondingly, TBB (1 μM) was without effect. Figure 6a (right) demonstrates that co-expression of wild-type CFTR with CK2α-wt generated a TBB-inhibitable CFTR Cl− conductance, whereas co-expression of wild-type CFTR with CK2-dm attenuated greatly the inhibition of CFTR Cl− conductance by TBB (1 μM). The residual inhibition of CFTR in oocytes co-expressing CFTR and CK2-dm most likely represents the inhibition of CFTR by endogenous wild-type Xenopus CK2α, which is almost identical to human CK2α. Taken together, our functional data argue that the effects of TBB on the CFTR Cl− channel are mediated by protein kinase CK2. This finding is consistent with our biochemical data which used surface plasmon resonance to demonstrate a high affinity interaction between CK2 and the NBD1 region of CFTR [27].
Fig. 6.
Demonstration of the interaction of CK2 and CFTR using a TBB-insensitive CK2 construct and CFTR peptides (a) Oocytes were injected with cRNA encoding either CK2 alone (left two panels) or CK2 with wild-type CFTR (right two panels), stimulated with IBMX/forskolin and subsequently exposed to TBB (1 μM). CK2-dm indicates the double mutant form of CK2α that is unaffected by the CK2 inhibitor, TBB. The Y-axis shows CFTR conductance (GCFTR) normalised to each individual pre-TBB value, and is expressed as a fraction of the IBMX (1 mM) and forskolin (2 μM) stimulated level. Data are means + SEM (CK2 alone, n = 4; CK2-dm, n = 4; CK2 + CFTR, n = 8; CK2-dm + CFTR, n = 20). The co-expression of CK2 with CFTR generated cAMP-stimulated CFTR Cl− conductances in the expected range (30 - 50 μS). (b) Wild-type (KENIIF), but not ΔF508 (KENII) peptide inhibits CFTR Cl− current. Xenopus oocytes expressing wild-type human CFTR were injected with 100 nM of GTIKENIIFGVSYDEYRYR (wild-type, WT, KENIIF) or GTIKENIIGVSYDEYRYR (ΔF508, KENII) peptides or water control, 20 h prior to stimulation with IBMX (1 mM) and forskolin (2 μM). Data are means + SEM, n = 23. NS indicates not significantly different from water control, * indicates significantly different from control, P < 0.01.
CK2 associates with wild-type, but not ΔF508 CFTR
Despite our consistent results with two unrelated pharmacological CK2 inhibitors, it remained to be determined whether CK2, by itself, directly affected CFTR Cl− channel function. One reason for caution is that CK2 has so many targets, including the epithelial Na+ channel (ENaC) [58], which is defectively regulated in CF [14]. To overcome this problem, we used the peptide employed in Figure 2b, corresponding to the wild-type sequence of CFTR around the F508 site to sequester CK2 away from CFTR. Such CFTR derived peptides of approximately 10-20 amino acids in length across this region of NBD1 were recently found to interfere with the ‘auto’phosphorylation of CK2β in vitro [27]. We applied the KENIIF (wild-type) and KENII (ΔF508) peptides, which both contain the putative CK2 consensus sequence around serine 511, to determine their effects on CFTR function. Figure 6b demonstrates that injection of Xenopus oocytes expressing wild-type CFTR with KENIIF inhibited cAMP-stimulated CFTR Cl− current by ~50%, whereas injection of oocytes expressing wild-type CFTR with KENII did not reduce significantly the magnitude of cAMP-stimulated CFTR Cl− current.
Mutation of S511 abrogates TBB inhibition, without altering single channel behaviour
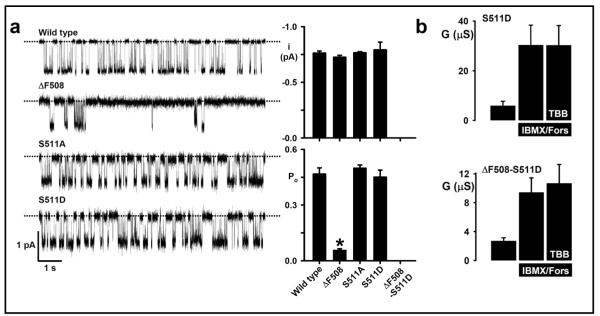
To investigate the contribution of S511 to CFTR function, we used site-directed mutagenesis to replace this uncharged polar side chain with (i) a nonpolar side chain (alanine, A) and (ii) an acidic side chain (aspartic acid, D) and recorded CFTR Cl− currents in BHK-cells expressing these constructs. Figure 7a demonstrates that mutation of S511 in wild-type CFTR is without effect on the single-channel activity of CFTR; neither the i nor the Po of S511A- and S511D-CFTR differed from those of wild-type CFTR when measured in excised inside-out membrane patches from BHK cells stably expressing CFTR constructs. By contrast, the Po of ΔF508-CFTR was attenuated sharply compared with that of wild-type CFTR and no channel activity was detected in BHK cells expressing the construct ΔF508-S511D-CFTR, when BHK cells were cultured at 37 °C (Fig. 7a).
Fig. 7.
Mutation of S511-CFTR abrogates CFTR inhibition by TBB, but does not disrupt CFTR channel gating (a) Single-channel analysis of CFTR constructs expressed in BHK cells. Left, representative single-channel recordings show the gating behaviour of the indicated CFTR constructs in the presence of ATP (1 mM) and PKA (75 nM) at −50 mV in the presence of a large Cl− concentration gradient ([Cl−]int = 147 mM; [Cl−]ext = 10 mM). The dotted lines indicate where channels are closed and downward deflections correspond to channel openings. Right, quantification of single-channel current amplitude (i) and Po. Values are means + SEM (n = 6, except S511A where n = 4). The asterisk indicates a value that is significantly different from wild-type CFTR, P < 0.05). No channel activity was detected in 8 membrane patches excised from BHK cells expressing ΔF508-S511D grown at 37 °C. (b) TBB insensitivity of S511D-CFTR. S551D and S511D-ΔF508-CFTR constructs were expressed in Xenopus oocytes and tested for TBB (1 μM) sensitivity in the presence of cAMP agonists. CFTR Cl− conductance was determined as described in Figure 5 and the Methods. Data are means + SEM (n = 7). It was not possible to study the construct S511A-CFTR in Xenopus oocytes because for unknown reasons oocytes expressing this construct all died immediately after microelectrode impalement.
Nevertheless, a critical role for S511 in CK2-dependent regulation of CFTR was revealed using the Xenopus oocyte expression system. Figure 7b demonstrates that TBB (1 μM) was without significant effect on cAMP-stimulated CFTR Cl− currents in oocytes expressing S511D-CFTR. This result contrasts sharply with the marked inhibition of wild-type CFTR Cl− currents by TBB (1 μM) (Fig. 5a). Processing of ΔF508-S511D-CFTR is more efficient in oocytes and thus ΔF508-S511D-CFTR generated a cAMP-stimulated CFTR Cl−current that was also unaffected by TBB (1 μM). Taken together, these data suggest that S511 plays an important role in mediating CFTR inhibition by the CK2-selective inhibitor TBB, but that mutation of this residue is without significant effect on the single-channel activity of CFTR.
Discussion
Our studies have explored the role of protein kinase CK2 in CFTR regulation. We demonstrate that inhibition of CK2 closes the CFTR Cl− channel. The consistency of our results using mammalian cells and Xenopus oocytes expressing recombinant CFTR and epithelial cells expressing endogenous CFTR (including an intact, perfused pancreatic duct) argues that the CK2-CFTR interaction is a general feature of CFTR-expressing cells. In addition, we established that deletion of F508 disrupts the interaction of this kinase with CFTR.
Because CK2 is an essential early gene in development with over three hundred in vivo targets [25, 26], dominant negative, inhibitory or knockdown studies inevitably impact on multiple cellular pathways making interpretation of results problematic given that CK2 may control 10% of all phosphorylated proteins. The constitutive ‘always-on’ activity of CK2 makes it necessary to apply site-directed mutagenesis to both the kinase itself (creating an inhibitor-insensitive mutant) and the putative target as discussed below. We complemented this approach with experiments using specific, competitive peptides to disrupt the local association of CK2 and CFTR. We find that the KENIIF (wild-type) peptide inhibits wild-type CFTR Cl− channel function in oocytes. We undertook these studies because TBB is itself an anion; at high concentrations TBB is an open-channel blocker of the CFTR Cl− channel (Z Xu, J-H Chen and DN Sheppard, unpublished observations). One possible interpretation of the peptide data is that KENIIF can sequester CK2 away from wild-type CFTR, and that CK2 is essential for normal CFTR function. Our hypothesis that ΔF508 abrogates CK2 binding is strengthened by the finding that the KENII (ΔF508) peptide cannot inhibit CFTR function, suggesting that it cannot sequester CK2 (but see alternative allosteric regulatory loop arguments below). The contrast in CFTR sensitivity to TBB using the cell-attached and the excised patch configurations shown in Figure 4 is striking. These data suggest a protein-protein interaction between CK2 and CFTR in the intact cell.
CK2 is a very complex kinase whose physiological regulators are unknown. In the present work, we show that when CFTR fails to traffic to the apical membrane of CF nasal epithelial cells, CK2 is absent from the apical membrane. This result is reminiscent of our recent studies of the interaction between CK2 and ENaC [58]. In that study, we found that the transition between cytosolic and membrane-bound CK2 is dependent on the phosphorylation status of ENaC. Of note, when both recognised CK2 targets in ENaC were mutated to non-phosphorylatable alanines (αβS631AγT599A-ENaC), no CK2 migrated to the membrane despite abundant amounts in the cell interior [58]. Moreover, other studies have described complex interactions between CK2 and the cytoskeleton (for review, see [59]). Thus, trafficking of CK2 is not a simple matter of diffusion from the cytosol to a molar excess of target protein in the cell membrane. In another study [60], we identified a related phenomenon for membrane local effects when investigating CFTR regulation by AMPK. In results reminiscent of those observed with ENaC [58], adding millimolar quantities of the AMPK activator AMP to the cytosol failed to induce CFTR channel closure [60]. By contrast, when we manipulated local AMP concentrations using pharmacological tools to prevent AMP formation at the cell membrane, CFTR Cl− channels opened promptly [60]. For these and other reasons (see below), we believe that adding complex, multi-subunit kinases (e.g. AMPK and CK2) to the solution bathing the intracellular face of excised inside-out membrane patches from cells expressing CFTR is unlikely to yield meaningful results. Our studies began with the notion that S511 in the F508 region of NBD1 is a potential target serine for phosphorylation by CK2 because the CFTR sequence S511YDE has the appropriate acid residues C-terminal of the S511. However, when Pagano et al. [27] investigated interactions between CK2 and NBD1 of murine CFTR in vitro, no phosphorylation of S511 was identified despite it being an excellent potential CK2 target. Instead, CK2 phosphorylated S422, S423 and S670; S422 and S670 are conserved in the human sequence [27]. We have also been unable to demonstrate phosphorylation of S511 either using radiolabelled ATP in the presence of recombinant CK2 and purified human NBD1 or by applying a site specific antibody for phosphoserine-511 to recombinant CFTR (KJ Treharne and A Mehta, unpublished observations). Nevertheless, the present results using intact cells and a variety of assays suggest strongly that some form of functional interaction occurs between CK2 and the F508 region of CFTR. However, further work is required to resolve why S511 appears to be important in conferring CK2 sensitivity on CFTR when expressed in the Xenopus oocyte system, but has no major impact on the single-channel behaviour of CFTR in excised inside-out membrane patches (Fig. 7). Pagano et al. [27] suggest that this region of CFTR confers the first ever described allosteric regulation towards the CK2 holoenzyme which alters when F508 is missing and when S511 is mutated. They also suggest that this region might inhibit the catalytic site of CK2 should CK2α be present without its β subunit. Thus, the simplest interpretation of our data is that when a patch of cell membrane is excised a local feedback loop involving CK2 is disrupted. Given that CK2 controls so many processes at the cell membrane, including PKA [61] and the cytoskeleton [59], this idea seems to be a reasonable working hypothesis.
Using biochemical assays, Pagano et al. [27] also analysed the interplay between the KENIIF and KENII peptides, NBD1, CK2α and CK2β. In their in vitro phosphorylation experiments, Pagano et al. [27] applied peptides corresponding to the F508 region of CFTR and measured the activation status of CK2. They found that these peptides inhibited the phosphorylation of the β subunit of CK2, which is known to be essential for the activation of the holoenzyme, yet the same peptides enhanced the phosphorylation of NBD1 and could even modulate CK2 targeting towards physiologically important substrates such as calmodulin, inhibitor-2 of protein phosphatase 1 and heat shock protein 90 [27]. The authors state that “the sequence around F508 represents, with respect to CK2, at the same time a docking site and an allosteric effector, whose dual function is increased by F508 deletion.” The complexity of their discoveries using purified proteins and in vitro biochemical approaches makes direct comparisons with our physiological data difficult. In particular, the data shown in Figure 7 demonstrate that S511D-CFTR is insensitive to TBB inhibition even though S511 is not a CK2 target. Clearly, there may be other regions outside the NBDs that might be CK2 targets such as T1471 near the C-terminus of CFTR [62], but their functional significance remains to be determined. Nevertheless, our data when combined with those of Pagano et al. [27] suggest that the S511 region has a regulatory function in the reciprocal interaction of CK2 with CFTR such that CK2 controls CFTR and, in turn, CFTR controls CK2.
What might be the pathophysiological consequences of ΔF508-induced CK2 mislocalisation away from the apical membrane of epithelial cells? Some of the cellular processes reported to be defective in CF are dependent upon CK2. For example, polyamines, such as spermidine, which are stimulators of CK2 activity, are increased in CF [63-65], suggesting a compensatory reaction to the loss of CK2 function. CFTR maturation is dependent on its association with calnexin in the ER [66]; this chaperone is phosphorylated by CK2 [67]. The residence time for ΔF508-CFTR at the apical membrane is reduced due to abnormal endocytic cycling [68, 69] and coating and uncoating of such vesicles is a CK2-dependent process [70]. As discussed above, CK2 is targeted to the cell membrane by ENaC [57], whose function is disrupted in CF by unknown mechanisms. Interestingly, the CF antigen, which is over-expressed in both CF patients and model systems, is a regulator of CK2 kinase activity [71, 72]. Thus, some of the reported targets of CK2 [26, 73] include proteins controlling unexplained aspects of CF disease [74, 75]. Overall, our proposed role for CK2 is consistent with published data on the cell biological consequences of the commonest CFTR mutation.
One approach to CF therapy aims to rescue defective processing and trafficking of ΔF508-CFTR [76]. Our data suggest that CK2-dependent regulation of ΔF508-CFTR might still be defective under conditions where ΔF508-CFTR has been induced to traffic to the apical membrane, consistent with published data showing that restoration of trafficking fails to fully restore CFTR-mediated Cl−current [13, 76, 77]. Outside the CF field, understanding the CK2-CFTR interaction might generate new drugs for the treatment of secretory diarrhoeas because inhibitors of CFTR, selected for their impact on transepithelial ion transport, have been proposed as treatments for cholera [78].
In conclusion, the data in this paper suggest not only that CK2 is a novel regulator of CFTR function, but also that CK2-dependent regulation of ΔF508-CFTR is dysfunctional. Although the ΔF508 mutation is responsible for most cases of CF, further work will be needed to determine whether other pathogenic mutations in CFTR act by a CK2-related mechanism. Our data suggest a radically different explanation for the commonest CF defect that links the deletion of F508 with defective function of a pleiotropic kinase that controls diverse pathways currently thought to lie outside CF pathophysiology. Our work complements the data of Pagano et al. [27], who suggest that the F508 region of CFTR, in isolation, can regulate CK2 activity towards specific targets, including NBD1, itself. Here, we present the corollary, regulation of the CFTR Cl− channel by CK2, a kinase with hundreds of potential targets that acts dominantly over PKA regulation.
Acknowledgements
We thank L.A. Pinna, D. Litchfield, A.C. Boyd, W. Skach, J.R. Riordan, C.R. O’Riordan and M.D. Amaral for gifts of reagents, vectors, antibodies and cells. We thank our laboratory colleagues, especially Z. Cai for advice and assistance and R.E. Olver and D. Meek for critical review. Supported by DFG SFB A6 to DFG SFB699 A6/A7, the Wellcome Trust (069150/z/02/z), Cystic Fibrosis Trust and Russell Trust. ZX was supported by the University of Bristol and an ORS award. PH was supported by OTKA (K060242).
References
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui L-C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 2.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill Inc.; New York: 2001. pp. 5121–5188. [Google Scholar]
- 3.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 4.Riordan JR. Assembly of functional CFTR chloride channels. Annu Rev Physiol. 2005;67:701–718. doi: 10.1146/annurev.physiol.67.032003.154107. [DOI] [PubMed] [Google Scholar]
- 5.Holland IB, Cole SPC, Kuchler K, Higgins CF. ABC Proteins: From Bacteria to Man. Academic Press; London: 2003. [Google Scholar]
- 6.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabary O, Escotte S, Couetil JP, Hubert D, Dusser D, Puchelle E, Jacquot J. High susceptibility for cystic fibrosis human airway gland cells to produce IL-8 through the IkB kinase a pathway in response to extracellular NaCl content. J Immunol. 2000;164:3377–3384. doi: 10.4049/jimmunol.164.6.3377. [DOI] [PubMed] [Google Scholar]
- 8.Tirouvanziam R, de Bentzmann S, Hubeau C, Hinnrasky J, Jacquot J, Péault B, Puchelle E. Inflammation and infection in naïve human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol. 2000;23:121–127. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 9.Tirouvanziam R, Khazaal I, Péault B. Primary inflammation in human cystic fibrosis small airways. Am J Physiol. 2002;283:L445–451. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 10.Mehta A. CFTR: more than just a chloride channel. Pediatr Pulmonol. 2005;39:292–298. doi: 10.1002/ppul.20147. [DOI] [PubMed] [Google Scholar]
- 11.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 12.Lukacs GL, Chang X-B, Bear C, Kartner N, Mohamed A, Riordan JR, Grinstein S. The DF508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane: determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–21598. [PubMed] [Google Scholar]
- 13.Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, Lazdunski M. Altered chloride ion channel kinetics associated with the DF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 14.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 15.Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–108. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhu T, Hinkson DA, Dahan D, Evagelidis A, Hanrahan JW. CFTR regulation by phosphorylation. Methods Mol Med. 2002;70:99–109. doi: 10.1385/1-59259-187-6:99. [DOI] [PubMed] [Google Scholar]
- 18.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem. 2003;278:998–1004. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- 20.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis HA, Zhao X, Wang C, Sauder JM, Rooney I, Noland BW, Lorimer D, Kearins MC, Conners K, Condon B, Maloney PC, Guggino WB, Hunt JF, Emtage S. Impact of the DF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–1353. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- 22.Thibodeau PH, Brautigam CA, Machius M, Thomas PJ. Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol. 2005;12:10–16. doi: 10.1038/nsmb881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meggio F, Marin O, Pinna LA. Substrate specificity of protein kinase CK2. Cell Mol Biol Res. 1994;40:401–409. [PubMed] [Google Scholar]
- 24.Wang H, Davis A, Yu S, Ahmed K. Response of cancer cells to molecular interruption of the CK2 signal. Mol Cell Biochem. 2001;227:167–174. [PubMed] [Google Scholar]
- 25.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 27.Pagano MA, Arrigoni G, Marin O, Sarno S, Meggio F, Treharne KJ, Mehta A, Pinna LA. Modulation of protein kinase CK2 activity by fragments of CFTR en-compassing F508 may reflect functional links with cystic fibrosis pathogenesis. Biochemistry. 2008;47:7925–7936. doi: 10.1021/bi800316z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 29.Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC. Isolation of CF cell lines corrected at DF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9:683–685. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- 30.Illek B, Maurisse R, Wahler L, Kunzelmann K, Fischer H, Gruenert DC. Cl transport in complemented CF bronchial epithelial cells correlates with CFTR mRNA expression levels. Cell Physiol Biochem. 2008;22:57–68. doi: 10.1159/000149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheppard DN, Robinson KA. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. J Physiol. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt A, Hughes LK, Cai Z, Mendes F, Li H, Sheppard DN, Amaral MD. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–1323. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treharne KJ, Marshall LJ, Mehta A. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am J Physiol. 1994;267:L592–601. doi: 10.1152/ajplung.1994.267.5.L592. [DOI] [PubMed] [Google Scholar]
- 34.Muimo R, Hornickova Z, Riemen CE, Gerke V, Matthews H, Mehta A. Histidine phosphorylation of annexin I in airway epithelia. J Biol Chem. 2000;275:36632–36636. doi: 10.1074/jbc.M000829200. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Taddei A, Sheppard DN. Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel. J Biol Chem. 2006;281:1970–1977. doi: 10.1074/jbc.M510576200. [DOI] [PubMed] [Google Scholar]
- 36.Mall M, Hipper A, Greger R, Kunzelmann K. Wild type but not DF508 CFTR inhibits Na+ conductance when coexpressed in Xenopus oocytes. FEBS Lett. 1996;381:47–52. doi: 10.1016/0014-5793(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 37.Bachhuber T, König J, Voelcker T, Mürle B, Schreiber R, Kunzelmann K. Chloride interference with the epithelial Na+ channel ENaC. J Biol Chem. 2005;280:31587–31594. doi: 10.1074/jbc.M504347200. [DOI] [PubMed] [Google Scholar]
- 38.Argent BE, Arkle S, Cullen MJ, Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986;71:633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- 39.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 41.Hegyi P, Rakonczay Z, Jr, Gray MA, Argent BE. Measurement of intracellular pH in pancreatic duct cells: a new method for calibrating the fluorescence data. Pancreas. 2004;28:427–434. doi: 10.1097/00006676-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Szalmay G, Varga G, Kajiyama F, Yang XS, Lang TF, Case RM, Steward MC. Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J Physiol. 2001;535:795–807. doi: 10.1111/j.1469-7793.2001.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegyi P, Gray MA, Argent BE. Substance P inhibits bicarbonate secretion from guinea pig pancreatic ducts by modulating an anion exchanger. Am J Physiol. 2003;285:C268–276. doi: 10.1152/ajpcell.00574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machen TE, Townsley MC, Paradiso AM, Wenzl E, Negulescu PA. H and HCO3 transport across the basolateral membrane of the parietal cell. Ann N Y Acad Sci. 1989;574:447–462. doi: 10.1111/j.1749-6632.1989.tb25183.x. [DOI] [PubMed] [Google Scholar]
- 45.Weintraub WH, Machen TE. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989;257:G317–327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- 46.Penque D, Mendes F, Beck S, Farinha C, Pacheco P, Nogueira P, Lavinha J, Malhó R, Amaral MD. Cystic fibrosis F508del patients have apically localized CFTR in a reduced number of airway cells. Lab Invest. 2000;80:857–868. doi: 10.1038/labinvest.3780090. [DOI] [PubMed] [Google Scholar]
- 47.Gruenert DC, Willems M, Cassiman JJ, Frizzell RA. Established cell lines used in cystic fibrosis research. J Cyst Fibros. 2004;3(Suppl 2):191–196. doi: 10.1016/j.jcf.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 48.Jia Y, Mathews CJ, Hanrahan JW. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- 49.Dahan D, Evagelidis A, Hanrahan JW, Hinkson DA, Jia Y, Luo J, Zhu T. Regulation of the CFTR channel by phosphorylation. Pflügers Arch. 2001;443(Suppl 1):S92–96. doi: 10.1007/s004240100652. [DOI] [PubMed] [Google Scholar]
- 50.Chappe V, Hinkson DA, Zhu T, Chang X-B, Riordan JR, Hanrahan JW. Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol. 2003;548:39–52. doi: 10.1113/jphysiol.2002.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 52.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2′) FEBS Lett. 2001;496:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 53.Hegyi P, Rakonczay Z, Jr, Tiszlavicz L, Varró A, Tóth A, Rácz G, Varga G, Gray MA, Argent BE. Protein kinase C mediates the inhibitory effect of substance P on HCO3− secretion from guinea pig pancreatic ducts. Am J Physiol. 2005;288:C1030–1041. doi: 10.1152/ajpcell.00430.2003. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25:5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988;105:131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- 56.O’Reilly CM, Winpenny JP, Argent BE, Gray MA. Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: inhibition by bicarbonate ions. Gastroenterology. 2000;118:1187–1196. doi: 10.1016/s0016-5085(00)70372-6. [DOI] [PubMed] [Google Scholar]
- 57.Meggio F, Pagano MA, Moro S, Zagotto G, Ruzzene M, Sarno S, Cozza G, Bain J, Elliott M, Deana AD, Brunati AM, Pinna LA. Inhibition of protein kinase CK2 by condensed polyphenolic derivatives: an in vitro and in vivo study. Biochemistry. 2004;43:12931–12936. doi: 10.1021/bi048999g. [DOI] [PubMed] [Google Scholar]
- 58.Bachhuber T, Almaça J, Aldehni F, Mehta A, Amaral MD, Schreiber R, Kunzelmann K. Regulation of the epithelial Na+ channel by the protein kinase CK2. J Biol Chem. 2008;283:13225–13232. doi: 10.1074/jbc.M704532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canton DA, Litchfield DW. The shape of things to come: an emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18:267–275. doi: 10.1016/j.cellsig.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem. 2009;284:5645–5653. doi: 10.1074/jbc.M806780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosuge S, Sawano Y, Ohtsuki K. A novel CK2-mediated activation of type II cAMP-dependent protein kinase through specific phosphorylation of its regulatory subunit (RIIα) in vitro. Biochem Biophys Res Commun. 2003;310:163–168. doi: 10.1016/j.bbrc.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Ostedgaard LS, Randak C, Vermeer D, Karp P, Rokhlina T, Welsh M. Role of the conserved acidic cluster in CFTR channel activity and protein trafficking. Pediatr Pulmonol Suppl. 2005;28:206. [Google Scholar]
- 63.Rennert OM, Frias J, Shukla JB. Polyamine metabolism in cystic fibrosis. Tex Rep Biol Med. 1976;34:187–197. [PubMed] [Google Scholar]
- 64.Russell DH, Rosenblum MG, Beckerman RC, Durie BG, Taussig LM, Barnett DR. Altered polyamine metabolism in cystic fibrosis. Pediatr Res. 1979;13:1137–1140. doi: 10.1203/00006450-197910000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Russell DH. Clinical relevance of polyamines. Crit Rev Clin Lab Sci. 1983;18:261–311. doi: 10.3109/10408368209085073. [DOI] [PubMed] [Google Scholar]
- 66.Pind S, Riordan JR, Williams DB. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994;269:12784–12788. [PubMed] [Google Scholar]
- 67.Ou WJ, Thomas DY, Bell AW, Bergeron JJ. Casein kinase II phosphorylation of signal sequence receptor a and the associated membrane chaperone calnexin. J Biol Chem. 1992;267:23789–23796. [PubMed] [Google Scholar]
- 68.Gentzsch M, Chang X-B, Cui L, Wu Y, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild type and DF508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:2684–2696. doi: 10.1091/mbc.E04-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okiyoneda T, Lukacs GL. Cell surface dynamics of CFTR: the ins and outs. Biochim Biophys Acta. 2007;1773:476–479. doi: 10.1016/j.bbamcr.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 70.Korolchuk VI, Banting G. CK2 and GAK/auxilin2 are major protein kinases in clathrin-coated vesicles. Traffic. 2002;3:428–439. doi: 10.1034/j.1600-0854.2002.30606.x. [DOI] [PubMed] [Google Scholar]
- 71.Murao S, Collart FR, Huberman E. A protein containing the cystic fibrosis antigen is an inhibitor of protein kinases. J Biol Chem. 1989;264:8356–8360. [PubMed] [Google Scholar]
- 72.Tugizov S, Berline J, Herrera R, Penaranda ME, Nakagawa M, Palefsky J. Inhibition of human papillomavirus type 16 E7 phosphorylation by the S100 MRP-8/14 protein complex. J Virol. 2005;79:1099–1112. doi: 10.1128/JVI.79.2.1099-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishihara K, Yamagishi N, Hatayama T. Protein kinase CK2 phosphorylates Hsp105a at Ser 509 and modulates its function. Biochem J. 2003;371:917–925. doi: 10.1042/BJ20021331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Treharne KJ, Mehta A, Muimo R. NDPK, a protein kinase defective in CF, binds to and is regulated by AMPK, a CFTR-associated protein. Pediatr Pulmonol Suppl. 2001;22:195. [Google Scholar]
- 75.Amaral MD. CFTR and chaperones: processing and degradation. J Mol Neurosci. 2004;23:41–48. doi: 10.1385/JMN:23:1-2:041. [DOI] [PubMed] [Google Scholar]
- 76.Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in DF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med. 1998;157:484–490. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- 77.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 78.Thiagarajah JR, Verkman AS. CFTR pharmacology and its role in intestinal fluid secretion. Curr Opin Pharmacol. 2003;3:594–599. doi: 10.1016/j.coph.2003.06.012. [DOI] [PubMed] [Google Scholar]