Abstract
Purpose
Clinical studies have suggested that seizures in newborns are more damaging that seizures occurring in older children. However, these studies are difficult to interpret for a variety of factors including differing etiologies of seizures across ages. Animal studies can provide insights into the question of whether age of seizure onset in children is a factor in cognitive outcome.
Methods
To evaluate effect of age on seizure-induced cognitive impairment we subjected rats to 50 seizures from postnatal day (P) 0 to P10 or P15–P25. As adults the rats were studied in the Morris water maze, radial-arm water maze, open field, and active avoidance. To assess synaptic strength and network excitatory and inhibitory function animals were evaluated with long-term potentiation (LTP) and paired-pulse facilitation/inhibition.
Results
Compared to controls, both groups of rats with recurrent seizures were impaired in spatial memory in both water maze tests, had altered activity in the open field, and did not differ from controls in active avoidance. Rats with recurrent seizures had impaired LTP but showed no deficits in paired-pulse facilitation or inhibition. While rats with later onset showed a trend to worse performance than rats with earlier seizures, the differences were not substantial.
Conclusions
Recurrent seizures during development are associated with long-term behavioral deficits in learning, memory and activity level as well as impaired synaptic efficiency. Age of seizure onset was not a strong predictor of outcome.
Keywords: Development, seizures, radial-arm water maze, open field, active avoidance, paired pulse, long term potentiation
1.0 Introduction
Age plays a major role in virtually all aspects of epilepsy (Hauser, 1992). Children are at substantially higher risk for epilepsy than young and middle aged adults (Hauser, 1994;1995; Forsgren et al., 2005). In addition to the higher incidence of epilepsy in children than adults, precipitating factors such as fever are far more likely to induce a seizure in a young child than adult (Hauser, 1992;Fetveit, 2008). Age is also a determinant for prognosis. Intellectual impairment (Huttenlocher and Hapke, 1990; Glosser et al., 1997; Bulteau et al., 2000; Bjornaes et al., 2001; Hermann et al., 2002; Cormack et al., 2007), learning disabilities (Sillanpaa, 2004; Soria et al., 2007; Fastenau et al., 2008),social outcome (Lindsay et al., 1979; Sillanpaa, 1983) and medical refractoriness (Berg et al., 1996; Casetta et al., 1999; Camfield and Camfield, 2007) all appear to be influenced by age of onset. In a number of studies an early age of onset of seizures has been associated with more cognitive impairment than a later onset of seizures (Huttenlocher and Hapke, 1990; Glosser et al., 1997; Bulteau et al., 2000; Bjornaes et al., 2001; Hermann et al., 2002; Cormack et al., 2007). However, not all investigators have found a relationship between early onset of seizures and cognitive outcome (Sturniolo and Galletti, 1994; Bailet and Turk, 2000; Jokeit and Ebner, 2002).
Determining if age of onset of childhood epilepsy is a factor in outcome is important since it could alter how aggressively seizures are treated at various ages. However, interpreting clinical studies is difficult due to different seizure variables across different age groups. Etiology of the seizures, seizure type, frequency and duration of seizures, genetics, and antiepileptic drugs are but a few of these variables. The use of animal models allows the investigator to stringently control many of these variables and provides insight into the behavioral consequences of early-life seizures (Huang et al., 1999; de Rogalski Landrot et al., 2001; Cha et al., 2002; Hoffmann et al., 2004; Zhao et al., 2005).
In this study we compared the effects of recurrent brief seizures at two developmental stages on subsequent learning and memory. We used the rat model of recurrent flurothyl seizures and then studied the rats during adulthood in a variety of tasks designed to assess hippocampal, prefrontal cortex, and amygdala function. We report here that rats subjected to seizures during early development have long-standing deficits in learning, memory, and activity level and have deficits in synaptic efficiency, as measured by long-term potentiation (LTP). The age of seizure onset was not a strong predictor of subsequent outcome.
2.0 Methods
2.1 Overview of Experiments
Male Sprague-Dawley rats (n = 56) from Charles River Laboratories were used throughout the study and were treated in accordance to the guidelines set by the National Institute of Health and the Animal Care and Use Committee of Dartmouth College for the humane treatment of animals. Animals had access to food and water ad libitum and were group housed in plastic cages under diurnal lighting conditions, with lights on from 8.00 to 20.00.
The animals underwent recurrent flurothyl-induced seizures or sham-seizures between P0–P10 or P15–P25. The rats then underwent sequential testing in the Morris water maze, the radial-arm water maze, the open field test, and the active avoidance test. In an effort to restrict animal number we used the same rats for all of the studies. These tests were designed to evaluate a range of behavioral tasks mediated by hippocampus, prefrontal cortex, and amygdale (Table 1). We elected to test the two experimental groups at the same time rather than at a uniform interval following the last seizure. The interval between the last seizure and the Morris water maze was 15 days in the E2 group, a time sufficient to make any postictal effects unlikely (Boukhezra et al., 2003). We separated the water maze tasks to try to reduce carry-over learning from one maze to the other. Active avoidance was done last since this results in considerable stress to the animal which could alter the other behavioral studies. The behavioral studies were followed by two electrophysiological tests: i) Long-term potentiation, a test of synaptic efficiency; and ii) Paired pulse facilitation/inhibition, a measure of the balance between excitation and inhibition in neuronal ensembles. Rats were then sacrificed and the brains examined for histological lesions. Table 2 summarizes the groups, animal numbers, and tests.
Table 1.
Behavioral tasks and primary brain area responsible for function.
| Test | Behavioral Measure | Anatomical Structures | References |
|---|---|---|---|
| Water Maze | Working and reference memory | Hippocampus, prefrontal cortex | (Morris, 2007) |
| Radial arm water maze | Working and reference memory | Hippocampus, prefrontal cortex | (Bolhuis et al., 1985;Buresova et al., 1986) |
| Open field | Locomotor activity, hyperactivity, and exploratory behaviors. | Prefrontal, motor cortex | (Walsh and Cummins, 1976;Gewiss et al., 1989;Kalsbeek et al., 1989) |
| Active Avoidance | Emotional memory | Amygdala | (Grossman et al., 1975) |
| LTP | Synaptic plasticity | Multiple | (Bliss et al., 2003;Cooke and Bliss, 2006) |
| Paired pulse inhibition/facilitation | Network excitability/inhibition | Multiple | (Austin et al., 1989;Huang et al., 1999) |
Table 2.
Experimental Design
| Groups (Animal #) |
Ages with Seizures |
Morris Water maze |
Open field | Radial-arm water maze |
Active Avoidance |
LTP | Paired-Pulse |
|---|---|---|---|---|---|---|---|
| 1 (E1)/16 | P0-P11 | P42 | P60 | P80 | P100 | P120 | P120 |
| 2 (E2)/14 | P15-P25 P0-P11/ |
P42 | P60 | P80 | P100 | P120 | P120 |
| Cont. 1/11 | No flurothyl P15-P25/ |
P42 | P60 | P80 | P100 | P120 | P120 |
| Cont. 2/11 | No flurothyl | P42 | P60 | P80 | P100 | P120 | P120 |
2.2 Flurothyl-induced seizures
Male rat pups derived from 10 litters were divided into four groups and subjected to fluorthyl or sham seizures. The day of delivery was designated as P0. The rat pups were divided into four groups: Group 1 (E1)(n = 18) animals received 50 flurothyl-induced seizures from P0–P10; Group 2 (E2)( n = 16) animals received 50 flurothyl-induced seizures from P15–P25. Controls (Cont.)(n = 22) were handled in the same manner as E1 and E2 but were not exposed to flurothyl. The control and experimental groups came from the same litter. All of the control and experimental rats were handled daily for the first 25 days of life.
Serial seizures were induced by fluorthyl (bis-2,2,2-trifluoroethyl ether, Aldrich Chemical Co) inhalation. Animals were subjected to five seizures per day, spaced two hours apart. For each trial 4–5 rats were placed into a plastic chamber (length = 28 cm, width = 18 cm, height = 26 cm) Liquid flurothyl was delivered through a plastic syringe and dripped slowly (3 cc/hr) onto filter paper in the center of the container where it evaporated. Rats were exposed to flurothyl until all of the rats had tonic extension of both the forelimbs and hindlimbs. Animals were then removed from the chamber and allowed to recover before being returned to their cages. The chamber was flushed with room air and cleaned between fluorothyl exposures. Controls were placed in the chamber but not exposed to flurothyl. Controls and experimental rats were separated from the dams for the same amounts of time until weaned at P25.
2.3 Water maze
To assess spatial memory function, we used the Morris water maze (Morris et al., 1982a; 1986; Morris, 1989). The Morris water maze is a test of hippocampal-dependent spatial memory (Morris et al., 1982b; Morris, 1984), the closest parallel to episodic memory in humans (Jeltsch et al., 2001; Spiers et al., 2001a; 2001b). Rats underwent water maze testing on P42 using techniques previously described in our laboratory (Rutten et al., 2002; Liu et al., 2003). This test measures both working and reference memory. Working memory is measured by the ability of the rat to find the escape platform during a single testing session whereas reference memory is a measure of how well the rat does on subsequent testing days. Working memory is primarily served by frontal cortex (Ragozzino et al., 1998; Jones, 2002) and reference memory by the hippocampus (Morris, 2006; 2007).
Apparatus
A stainless-steel circular swimming pool (2 m in diameter, 50 cm high) was filled to a depth of 25 cm with water. Non-toxic white paint was added to make the water opaque and prevent the rats from seeing the platform. Room cues visible from the water surface were constant from day to day. Four points on the perimeter of the pool were designated north (N), east (E), south (S), and west (W), thus dividing the pool into four quadrants (NW, NE, SE, SW). A clear plexiglass escape platform 8 cm in diameter was positioned in the center of one of the quadrants, 2 cm below the water surface.
Behavioral procedures
On the first day each rat was placed in the pool for 60 seconds without the platform present; this free swim enabled the rat to become habituated to the training environment. Starting three hours after habituation the rats began the hidden escape platform portion of the test. The rats underwent 6 timed, hidden platform trials with the platform in the same quadrant across days, for four days (Days 1–4). The point of immersion into the pool varied between N, E, S, and W in a random order for each trial, so that the rat was not able to predict the platform location from the point at which it was placed in the pool. The latency from immersion into the pool to escape onto the platform was recorded for each trial, and the observer also recorded the route taken by the rat to reach the platform. On mounting the platform, rats were given a 30-second rest period, after which the next trial was started. If the rat did not find the platform in 120 seconds, it was manually placed on the platform for a 30-second rest. At the start of each trial, the rat was held facing the perimeter and dropped into the pool to ensure immersion.
On day 5 the platform was removed and animals underwent the probe test for 60 seconds when the time spent in the quadrant where the platform had previously been located was recorded. The test began with the rat in the quadrant opposite to the trained platform location. The path and time spent in the quadrant where the platform had previously been placed was recorded. In this part of the water maze, termed the probe test, normal animals typically spend more time in the quadrant where the platform had been previously located than in the other quadrants. The testing procedure used during the four days of locating the hidden platform provides a measure of spatial reference memory, while the probe trial is a measure of the strength of spatial learning (Jeltsch et al., 2001).
2.4 Open Field Test
The open field chamber consisted of a square piece of plywood (61 × 61 cm), divided into 64 squares, with wooden side boards (30 cm high). At age P60, animals were placed in the open field chamber for 120 seconds on two consecutive days. The number of squares crossed, number of rears, and number of stools were recorded. The chamber was cleaned after each trial and ambient lighting and noise remained constant. The open field is a measure of the animal’s reaction to a novel environment and indicates the animal’s excitability.
2.5 Radial-arm water maze
An 8-arm radial-arm water maze was used to measure spatial learning and memory (Sayin et al., 2004; Mortazavi et al., 2005; Cornejo et al., 2007). The test allows assessment of working and reference memory performance simultaneously (French et al., 2006) and is considered to require a greater memory load than the Morris water maze (Hyde et al., 1998). Its utilization also omits the necessity of a food reward and is hippocampus dependent (Mesches et al., 2004).
Apparatus
The radial-arm maze consisted of eight stainless steel arms (length – 50 cm, width – 15 cm) extending radially from a central area (diameter 40 cm) placed in a stainless-steel circular swimming pool filled with water. One arm was chosen as the target arm, in which a clear plexiglass escape platform was placed that allowed the rat to climb atop it, thus exiting the water, and rest. The escape platform remained in a constant position for the duration of the experiment. White paint was added to make the water opaque and prevent the rats from seeing the platform. Cue cards were distributed around the maze and remained constant throughout the experiment.
Behavioral procedures
Testing in the radial-arm water maze began at P80. Rats received trials with 30-minute intertribal intervals. Each rat was placed in the center of the maze and the trial continued until the escaped platform was found or until 2 minutes had elapsed. A visit to an arm was scored if all four limbs of the rat were within an arm. On mounting the platform the rats were given a 30-second rest period, and were taken to the home cage for a 30-minute intertrial interval. If the rat did not find the platform in 120 seconds, it was manually placed on the platform for a 30-second rest before being taken to the cage. Rats were placed in the maze at randomly selected different start arms with the same goal arm position. Time to completion was measured as the time taken to reach the escape platform and complete the trial. It was recorded for each trial and the observer also manually recorded the arms visited by the rat before it found the platform. The rats were trained until they reached the criteria of no more than one error in a single trial and no more than two errors in total for three consecutive trials. If the rat entered into an incorrect arm the error was coded as a reference error. Re-entrance into an arm during a trial was coded as a working memory error.
2.6 Active Avoidance
Active avoidance was measured in a stainless steel cage measuring 9”×10.875”×19” divided into two compartments of equal size separated by an inner divider (PACS-30, Columbus Instruments, Columbus, Ohio). Each floor had an electrical grid that provided a shock to the animal. An infrared-type beam assembly was used to detect subject transfers.
On the first day of testing rats were placed randomly in one of the two compartments and allowed five minutes to acclimate to the chamber. The next day the rat was placed in one chamber with the light off. After a 30 second delay an overhead light was turned on and simultaneously a 5,000 Hz tone was emitted. The rat then had five seconds to escape from the chamber before an electric shock occurred consisted of a five second shock administered at 0.5 mA. Rats were given thirty trials separated by one minute on two consecutive days. Trials were coded as avoidance if the rat left the chamber prior to the shock. If the rat left the chamber during the shock the trial was coded as an escape.
2.7 Long-term Potentiation (LTP)
A randomly selected subset of the rats (n = 23; E1 = 8; E2 = 6; Cont. = 9) were used for the LTP study. Under urethane anesthesia (1.2g/kg, ip), a rat was placed in a stereotaxic frame and holes were drilled in appropriate regions of the skull. Both recording and stimulating electrodes are bipolar 125-µm twisted wires (Plastics One Inc. Roanoke, VA). Stimulating electrode was placed in the ventral hippocampal commissure (AP, 1.4; L, 0.05; D, 3.8) and for the recording electrode, which is placed in the CA1 subfield of right hippocampus, the coordinates are (AP, 3.8; L, 2.5; D, 2.4) initially, and the final position was adjusted based on the field excitatory postsynaptic potential (fEPSP). A screw placed over the cerebellum was used as the ground.
Input/output curve was drawn and the stimulus intensity was set to 70% of the maximum response for LTP study. Baseline response was recorded for 30 minutes (stimulation frequency-0.05Hz; stimulation pulse duration-0.2ms). LTP was evoked by high frequency stimulation (HFS) consisted of 3 trains with 10 seconds intertrain intervals. Each train consisted of 1 second of 200 Hz stimulations which intensity was 90% of maximum response and 0.3ms in duration. Right after HFS, the stimulation setting was changed back to baseline parameters. Responses were stimulated and recorded for another three hours. Every 10 minutes, 30 normalized responses were averaged to represent one point on figure. The rat was kept on a warming pad throughout the recording period. The f-EPSPs were recorded using Digidata 1322A acquisition system (Axon instruments, CA) and the off line analyzed with Mini analysis program (Synaptosoft Inc. NJ).
2.8 Paired-Pulse Facilitation/Inhibition
After the LTP recording, paired stimulations was delivered through the ventral hippocampal commissure electrode and paired pulse inhibition was tested. Stimulation intensity was the same as LTP test. Pulses were 0.2 ms in duration. Interpulse intervals were set to be 30ms, 40ms, 50ms, 75ms and 100ms. The amplitude of population spikes were averaged for 10 pairs at each interpulse interval using Mini Analysis program. Three frequencies were tested, 0.1Hz, 0.5 Hz and 1 Hz. The paired-pulse index (PPI) was used as a measure of the net short-term facilitation or interneuronally-mediated inhibition effective at the time of the paired-pulse test and was computed by dividing the amplitude of the second population spike (p2) by the amplitude of the first population spike (p1).
2.9 Histology
Animals were sacrificed on P120. They were deeply anesthetized with sodium pentobarbital (65 mg/kg). Then they were transcardially perfused with 200 ml of normal saline followed by 200 ml of 4% paraformaldehyde (PFA). Brains were removed and postfixed overnight in 4% PFA and were subsequently placed in 30% sucrose solution. Coronal sections through the entire hippocampus were cut on a freezing microtome and stored in phosphate buffered saline. Coronal sections through the entire extent of the hippocampus were cut at 30 µm on a freezing microtome, and sections were stored in phosphate buffered saline (pH 7.3). Every forth section was stained with thionin for cell loss. The loss of neurons in the prefrontal cortex, amygdala, CA1, CA3 of hippocampus and hilum of dentate gyrus was evaluated by the following scoring scale: i) normal = 0 (10 cell loss); ii) mild =1 (10–25% cell loss); iii) moderate =2 (25–75% cell loss), and iv) severe = 3 (>75% cell loss (Mikati et al., 1994; Schmid et al., 1999). This scale provides a rough measure of cell loss but has not been validated with cell counting techniques.
2.10 Statistical analysis
The Kolmogorov-Smirnov goodness-of-fit test was used to assess normality (Gaussian-shaped distribution) for all continuous variables. Mean escape latency to water maze platform was compared using the repeated measures ANOVA. Trials to criteria, time to complete trials, and reference and working memory errors were compared using the ANOVA. The probe test was compared using the one-way ANOVA with post hoc testing using Tukey’s Multiple Comparison Test. Total blocks crossed, rears, and stools in the open field across days were compared using the repeated measures ANOVA. Group scores in the active avoidance task were compared using the ANOVA. For all ANOVA testing post-hoc analysis was performed using the Tukey's Multiple Comparison Test. Histological scores were compared using the Mann-Whitney test. Results are presented as means±standard errors and a p <0.05 was used to defined statistical significance.
3.0 Results
3.1 Flurothyl-Induced Seizures
As we previously described (Gatt et al., 1993), age-related differences in the behavioral features were noted. In both E1 and E2 flurothyl inhalation initially resulted in agitation with increased exploring of the testing chamber. Swimming movements were prominent in pups from P0–P5, followed by abrupt onset of tonus. From P10 onward prominent clonic and myoclonic activity occurred before the tonic activity. From P20 onward clonus and myoclonus was followed by wild running and then the tonic phase. All of the rats developed tonic seizures at which point they were removed from the chamber. Four rats (two each from E1 and E2) died during the course of the study.
No spontaneous seizures were seen in any of the rats during any of the testing. However, EEG monitoring was not performed.
3.2 Water maze
The results of the water maze are presented in Figure 1. All three groups learned to find the escape platform over the 24 trials conducted over four days. Latency to the escape platform decreased with trials (F23 = 25.32; p<0.0001). Rats improved during the course of six trials each day. After a night of rest the first trial (7th, 13th, and 19th trials) had longer latencies that the last trial of the preceding day (6th, 12th, and 18th trials), indicating a component of both reference memory and working memory during testing days 2–4. Significant differences were noted in the three groups (F2 = 4.761; p = 0.012) with a time/group interaction (F69 = 2.493; p<0.0001). The controls differed from both E1 (F1 = 5.285, p = 0.027) and E2 (F1 = 11.62, p = 0.002). No differences were seen between E1 and E2 (F1 = 0.1383, p = 0.712).
Figure 1.
Morris water maze. Compared to controls, rats with recurrent seizures (E1 and E2) were slower in reaching the escape platform than the controls. The insert shows the probe test. The E2 group spent less time in the target quadrant than the control group.
There was a significant difference between groups in the probe test (F2 = 4.076, p = 0.0243) with the control rats spending more time swimming in the target quadrant than the E2 (P <0.05)(Fig. 1, Insert). No differences were noted between the controls and E1.
3.3 Radial-arm water maze
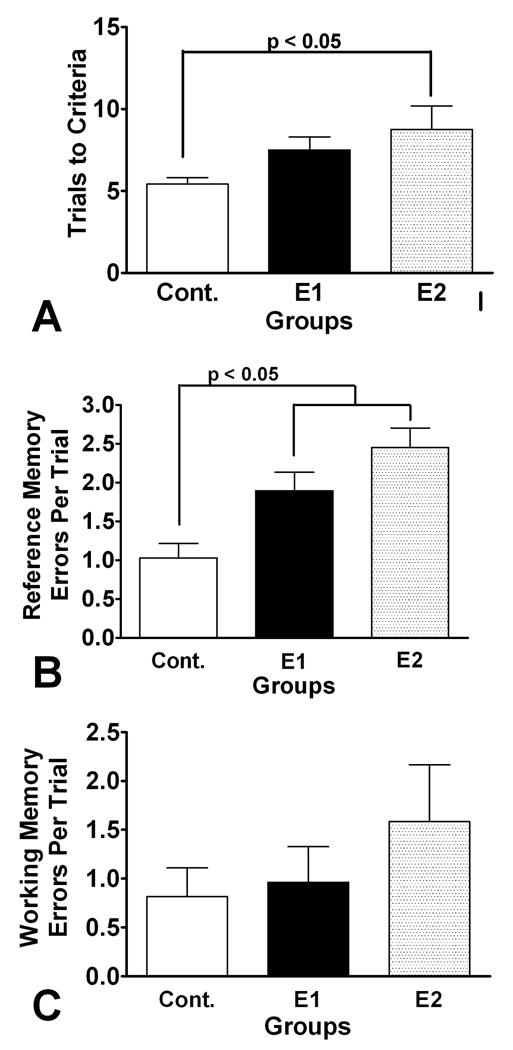
A significant difference in trials to criteria between the three groups was seen (F2 = 3.511, p = 0.042) with the controls reaching criteria faster than the E2 group (p < 0.05) (Fig. 2). There was a significant difference in groups in number of total incorrect arms entered across trials (F3 = 3.51, p = 0.046) with E2 group having more incorrect arms than the controls (F1 = 6.82, p = 0.019). No differences were seen between E1 and E2 (p > 0.05) or between the controls and E1 (p > 0.05). Mean number of reference members per trial was significantly different in the three groups (F2 = 10.07, p <0.0001) with higher references errors in both E1 (1.90±0.24 errors/trial) and E2 (2.45±0.25 errors/trial) than the controls (1.03±0.19 errors/trials). No differences between E1 and E2 sere noted (p > 0.05). Working memory errors did not differ between the three groups: E1 (0.96±0.37 errors), E2 (1.58±0.58 errors), controls (0.95±0.24 errors)(F2 = 0.5234, p = 0.600).
Figure 2.
Radial-arm water maze results. A. Trials to criteria. Control rats required fewer trials to reach criteria than the E2 rats. B. Reference memory errors per trial. Both the E1 and E2 rats had more reference memory errors than the controls. C. Working memory errors per trial. No significant differences between groups were found.
3.4 Open Field
There was a significant difference between groups (F2 = 6.607, p <0.004; repeated measures ANOVA) with a group/time interaction (F2 = 12.00, p <0.0001). As seen in Figure 3, the controls had reduced activity in the open field from day one to two (p < 0.05) whereas the E1 and E2 groups did not. There were no differences between the E1 and E2 groups (F1 = 0.823; p = 0.373). No differences were found between groups in the number of stools or rearing during the two sessions (P > 0.05).
Figure 3.
Comparison of activity level in controls and rats with recurrent seizures. Note that control rats had a reduction in activity level from day one to day two whereas neither of the two experimental groups (E1 and E2) had a reduction in activity level. There were no differences between the E1 and E2 groups.
3.5 Active Avoidance
No significant differences were noted between the three groups in either escape (F2 = 1.113, p = 0.347; repeated measure ANOVA) or avoidance (F2 = 2.685, p = 0.0915).
3.6 LTP
Figure 4 shows the results of the LTP. As can be seen, there were significant differences between the controls and rats with recurrent seizures (F2 = 6.877, p = 0.006). The results also varied as a factor of time (F2 = 26.60, p <0.0001). There were significant differences between the controls and E1 (F1 = 11.43, p = 0.004) and E2 (F1 = 5.926, p = 0.032) rats with recurrent seizures. No differences were noted between the E1 and E2 rats (P > 0.05).
Figure 4.
Long-term potentiation in controls and two recurrent seizure groups (E1 and E2). Graph shows post-tetanic change of slope from normalized slope prior to tetanic stimulation. The slopes from both the E1 and T2 groups were significantly lower than the controls (p = 0.006).
3.7 Paired-Pulse Facilitation/Inhibition
In both the controls and experimental rats there was a change in the ratio of the amplitudes of the EPSPs as a function of interpulse interval. At short IPI (30–40 msec) there was inhibition of the second EPSP whereas at longer IPIs facilitation of the second EPSP was seen. At 0.1 Hz (F4 = 6.757, p = 0.0005), 0.5 Hz (F4 = 8.763, p = 0.0003) and 1.0 Hz (F4 = 3.010, p = 0.043) there were significant changes between P2/P1 amplitudes as a function of IPI in the controls. The rats with recurrent seizures did not have significant differences in P2/P1 amplitudes as a function of IPI. However, no significant differences in facilitation or inhibition was seen when the three groups were compared at each IPI for 0.1, 0.5, or 1.0 Hz.
3.8 Histology
No discernible cell loss was seen in the hippocampus (CA3, CA1, hilus), prefrontal cortex or amygdale. Cell loss scores did not differ between groups for any of the regions (data not shown).
4.0 Discussion
In this study we found that recurrent seizures during early development were associated with long-standing changes in learning and memory. In tests of hippocampal function both seizure groups were found to be impaired: i) the Morris water maze did not show any differences between age group in either the hidden platform task or the probe test; ii) both age groups were impaired in the radial-arm water maze. Paralleling these behavioral measures both seizure groups were similarly impaired in LTP. In the open field test, a measure of prefrontal and motor cortex function, we found that both seizure age groups differed from the controls but did not differ significantly from each other. Active-avoidance, a test that is dependent on an intact amygdala, was normal in both seizure groups. These findings demonstrate that recurrent seizures have significant adverse effects on hippocampal and prefrontal function.
Investigators have used pentylenetetrazol (Holmes et al., 1999; Huang et al., 2002), flurothyl-inhalation (Okada et al., 1986; Sperber and Moshé, 1988; Holmes et al., 1998; Sperber et al., 1999; Huang et al., 1999; Schmid et al., 1999; Sogawa et al., 2001), and hyperthermia (Chang et al., 2003) to induce recurrent seizures in young rats. Investigators have shown that rats subjected to a series of recurrent seizures during the first weeks of life have considerable spatial impairment when the animals are studied during adolescence or adulthood (Neill et al., 1996; Holmes et al., 1998; Huang et al., 1999; Liu et al., 1999; Sogawa et al., 2001; de Rogalski Landrot et al., 2001; Huang et al., 2002; Chang et al., 2003).
We chose to examine rats that had seizures between P0–P10 and P15–P25. The immature rat brain evolves considerably over the two time spans studied here. The time periods evaluated here span major developmental changes including neurogenesis (Altman and Das, 1965; Bayer, 1980; Reznikov, 1991; McCabe et al., 2001), synaptic connectivity (Zimmer and Haug, 1978), apoptosis and synaptic elimination (Simon and O'Leary, 1992; Kim and Kandler, 2003) and myelination (Keyser, 1983; Bockhorst et al., 2008). There are also substantial changes in synaptic physiology during this period of time (Tremblay et al., 1988; Swann et al., 1992; Grantyn et al., 1995; Ben-Ari et al., 2007). A developmental switch in the action of GABA via GABA(A) receptors from excitatory to inhibitory occurs in rat CA3 pyramidal cells at around P8–10, an age that coincides with the transition from immature to mature hippocampal rhythms (Khazipov et al., 2004; Tyzio et al., 2007). In rats, as in children, there are many developmental processes occurring simultaneously and it would not be possible, with this study, to designate which developmental event was responsible for the adverse behavioral outcome.
In this study age of seizure onset did not appear to play a major role in the degree of dysfunction seen in the two seizure groups. While the rats with seizures at an older age showed a trend towards a worse performance, the differences were not robust or consistent. In addition to impaired spatial performance, rats with recurrent seizures, regardless of age of onset, had deficits in synaptic strength as evidenced by decreases in LTP. Repetitive seizures in the neonatal period using kainic acid have been previously been shown to result in result in reduced LTP and enhanced long-term depression (LTD) along with memory impairment (Cornejo et al., 2007). The reduced LTP and increased LTD following neonatal seizures were associated with reductions in the membrane pool of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) subunits (GluR1), decreases in the total amount of N-methyl-D-aspartate subreceptors (NR2A) and increases in the post-synaptic density protein 95 (PSD-95), the primary subsynaptic scaffold protein (Cornejo et al., 2007). Our study indicates that long-standing impaired LTP following seizures is not restricted to a single age as both age groups of rats with seizures had similar degrees of LTP impairment.
The finding that seizures at two distinct age groups during development suggests that seizure-induced cognitive impairment during development is unlikely to be related to one distinct developmental event such as neurogenesis or apoptosis since the rate of these maturational changes vary as a function of age. For example, if recurrent seizures cause cognitive deficits by reducing neurogenesis, it would be expected that seizures in the younger age group would cause more harm since neurogenesis is greater in the first week of life than in the second and third weeks postnatally. Since both age groups were similarly impaired, we surmise that seizure-induced cognitive impairment is likely due to functional brain changes, such as changes in GABA or NMDA subunit configuration, that occur independently of structural developmental changes. However, in this observational study we did not assess pathophysiological mechanisms responsible for the impaired function and our thoughts are speculative at this time.
In summary, recurrent seizures during early development in rodents result in behavioral deficits in learning and memory. These cognitive deficits are paralleled by impaired LTP. Age of seizure onset was not a major factor in outcome suggesting that seizures at any age can result in long-term neurological sequelae. Understanding the mechanism by which seizures cause permanent cognitive impairment across ages remains a major challenge for investigators.
Figure 5.
Paired-pulse inhibition and facilitation. Rats were stimulated at 0.1, 0.5 and 1.0 Hz with an interpulse interval varying from 30 to 100 msec. The ratio of the amplitudes of the second EPSP to the first EPSP is plotted. No significant differences between groups were found for any of the stimulation frequencies.
Acknowledgements
Supported by grant support from NIH (NINDS) NS044295.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Austin KB, Bronzino JD, Morgane PJ. Paired-pulse facilitation and inhibition in the dentate gyrus is dependent on behavioral state. Exp Brain Res. 1989;77:594–604. doi: 10.1007/BF00249612. [DOI] [PubMed] [Google Scholar]
- Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia. 2000;41:426–431. doi: 10.1111/j.1528-1157.2000.tb00184.x. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Berg AT, Levy SR, Novotny EJ, Shinnar S. Predictors of intractable epilepsy in childhood: A case-control study. Epilepsia. 1996;37:24–30. doi: 10.1111/j.1528-1157.1996.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Bjornaes H, Stabell K, Henriksen O, Loyning Y. The effects of refractory epilepsy on intellectual functioning in children and adults. A longitudinal study. Seizure. 2001;10:250–259. doi: 10.1053/seiz.2000.0503. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Morris RG. Introduction. Long-term potentiation and structure of the issue. Philos Trans R Soc Lond B Biol Sci. 2003;358:607–611. doi: 10.1098/rstb.2003.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorst KH, Narayana PA, Liu R, hobila-Vijjula P, Ramu J, Kamel M, Wosik J, Bockhorst T, Hahn K, Hasan KM, Perez-Polo JR. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J Neurosci Res. 2008;86:1520–1528. doi: 10.1002/jnr.21607. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Buresova O, Bures J. Persistence of working memory of rats in an aversively motivated radial maze task. Behav Brain Res. 1985;15:43–49. doi: 10.1016/0166-4328(85)90016-6. [DOI] [PubMed] [Google Scholar]
- Boukhezra O, Riviello P, Fu DD, Lui X, Zhao Q, Akman C, Holmes GL. Effect of the postictal state on visual-spatial memory in immature rats. Epilepsy Res. 2003;55:165–175. doi: 10.1016/s0920-1211(03)00111-6. [DOI] [PubMed] [Google Scholar]
- Bulteau C, Jambaque I, Viguier D, Kieffer V, Dellatolas G, Dulac O. Epileptic syndromes, cognitive assessment and school placement: a study of 251 children. Dev Med Child Neurol. 2000;42:319–327. doi: 10.1017/s0012162200000566. [DOI] [PubMed] [Google Scholar]
- Buresova O, Bolhuis JJ, Bures J. Differential effects of cholinergic blockade on performance of rats in the water tank navigation task and in a radial water maze. Behav Neurosci. 1986;100:476–482. doi: 10.1037//0735-7044.100.4.476. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Long-term prognosis for symptomatic (secondarily) generalized epilepsies: a population-based study. Epilepsia. 2007;48:1128–1132. doi: 10.1111/j.1528-1167.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- Casetta I, Granieri E, Monetti VC, Gilli G, Tola MR, Paolino E, Govoni V, Iezzi E. Early predictors of intractability in childhood epilepsy: a community-based case-control study in Copparo, Italy. Acta Neurol Scand. 1999;99:329–333. doi: 10.1111/j.1600-0404.1999.tb07360.x. [DOI] [PubMed] [Google Scholar]
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res. 2002;51:217–232. doi: 10.1016/s0920-1211(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang AM, Kuo YM, Wang ST, Chang YY, Huang CC. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann Neurol. 2003;54:701–705. doi: 10.1002/ana.10789. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Cormack F, Helen CJ, Isaacs E, Harkness W, Wright I, Vargha-Khadem F, Baldeweg T. The development of intellectual abilities in pediatric temporal lobe epilepsy. Epilepsia. 2007;48:201–204. doi: 10.1111/j.1528-1167.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- de Rogalski Landrot I, Minokoshi M, Silveira DC, Cha BH, Holmes GL. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Brain Res Dev Brain Res. 2001;129:27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- Fastenau PS, Jianzhao S, Dunn DW, Austin JK. Academic underachievement among children with epilepsy: proportion exceeding psychometric criteria for learning disability and associated risk factors. J Learn Disabil. 2008;41:195–207. doi: 10.1177/0022219408317548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetveit A. Assessment of febrile seizures in children. Eur J Pediatr. 2008;167:17–27. doi: 10.1007/s00431-007-0577-x. [DOI] [PubMed] [Google Scholar]
- Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe - a systematic review. Eur J Neurol. 2005;12:245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- French KL, Granholm AC, Moore AB, Nelson ME, Bimonte-Nelson HA. Chronic nicotine improves working and reference memory performance and reduces hippocampal NGF in aged female rats. Behav Brain Res. 2006;169:256–262. doi: 10.1016/j.bbr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Gatt G, Veliskova J, Liu Z, Moshé SL, Holmes GL. Ontogeny of flurothyl-induced seizures: A behavioral and EEG electroencephalographic analysis. Epilepsia. 1993;34 Suppl. 6:63. [Google Scholar]
- Gewiss M, Eclancher F, Poels JF, Van BP, De WP. Prefrontal cortex aspiration in pups and juvenile rats: behavioural changes and recovery of function. Arch Int Physiol Biochim. 1989;97:163–174. doi: 10.3109/13813458909104536. [DOI] [PubMed] [Google Scholar]
- Glosser G, Cole LC, French JA, Saykin AJ, Sperling MR. Predictors of intellectual performance in adults with intractable temporal lobe epilepsy. J Int Neuropsychol Soc. 1997;3:252–259. [PubMed] [Google Scholar]
- Grantyn R, Kraszewski K, Melnick I, Taschenberger H, Warton SS. In vitro development of vertebrate central synapses. Perspect Dev Neurobiol. 1995;2:387–397. [PubMed] [Google Scholar]
- Grossman SP, Grossman L, Walsh L. Functional organization of the rat amygdala with respect to avoidance behavior. J Comp Physiol Psychol. 1975;88:829–850. doi: 10.1037/h0076396. [DOI] [PubMed] [Google Scholar]
- Hauser WA. Seizure disorders: the changes with age. Epilepsia. 1992;33 Suppl 4:S6–S14. doi: 10.1111/j.1528-1157.1992.tb06222.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35 Suppl 2:S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. Epidemiology of epilepsy in children. Neurosurg Clin N Am. 1995;6:419–429. [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog Brain Res. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann AF, Zhao Q, Holmes GL. Cognitive impairment following status epilepticus and recurrent seizures during early development: support for the "two-hit hypothesis". Epilepsy Behav. 2004;5:873–877. doi: 10.1016/j.yebeh.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. J Comp Neurol. 1999;404:537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, Holmes GL. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res Dev Brain Res. 1999;118:99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- Huang LT, Yang SN, Liou CW, Hung PL, Lai MC, Wang CL, Wang TJ. Pentylenetetrazol-induced recurrent seizures in rat pups: time course on spatial learning and long-term effects. Epilepsia. 2002;43:567–573. doi: 10.1046/j.1528-1157.2002.29101.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Hapke RJ. A follow-up study of intractable seizures in childhood. Ann Neurol. 1990;28:699–705. doi: 10.1002/ana.410280516. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Jeltsch H, Bertrand F, Lazarus C, Cassel JC. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol Learn Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A. Effects of chronic epilepsy on intellectual functions. Prog Brain Res. 2002;135:455–463. doi: 10.1016/S0079-6123(02)35042-8. [DOI] [PubMed] [Google Scholar]
- Jones MW. A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med. 2002;2:639–647. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, de Bruin JP, Matthijssen MA, Uylings HB. Ontogeny of open field activity in rats after neonatal lesioning of the mesocortical dopaminergic projection. Behav Brain Res. 1989;32:115–127. doi: 10.1016/s0166-4328(89)80079-8. [DOI] [PubMed] [Google Scholar]
- Keyser A. Basic aspects of development and maturation of the brain: embryological contributions to neuroendocrinology. Psychoneuroendocrinology. 1983;8:157–181. doi: 10.1016/0306-4530(83)90054-9. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Ounsted C, Richards P. Long-term outcome in children with temporal lobe seizures. I: Social outcome and childhood factors. Dev Med Child Neurol. 1979;21:285–298. doi: 10.1111/j.1469-8749.1979.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Muller RU, Huang LT, Kubie JL, Rotenberg A, Rivard B, Cilio MR, Holmes GL. Seizure-induced changes in place cell physiology: relationship to spatial memory. J Neurosci. 2003;23:11505–11515. doi: 10.1523/JNEUROSCI.23-37-11505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang LT, Stafstrom CE, Holmes GL. Consequences of recurrent seizures during early brain development. Neuroscience. 1999;92:1443–1454. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Mikati MA, Holmes GL, Chronopoulos A, Hyde P, Thurber S, Gatt A, Liu Z, Werner S, Stafstrom CE. Phenobarbital modifies seizure-related brain injury in the developing brain. Ann Neurol. 1994;36:425–433. doi: 10.1002/ana.410360314. [DOI] [PubMed] [Google Scholar]
- Morris R. Theories of hippocampal function. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford: Oxford University Press; 2007. pp. 581–713. [Google Scholar]
- Morris R. Development of a water maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982a;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982b;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mortazavi F, Ericson M, Story D, Hulce VD, Dunbar GL. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005;7:629–638. doi: 10.1016/j.yebeh.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Neill J, Liu Z, Sarkisian M, Tandon P, Yang Y, Stafstrom CE, Holmes GL. Recurrent seizures in immature rats: effect on auditory and visual discrimination. Dev Brain Res. 1996;95:283–292. doi: 10.1016/0165-3806(96)00099-5. [DOI] [PubMed] [Google Scholar]
- Okada R, Moshé SL, Wong BY, Sperber EF, Zhao D. Age-related substantia nigra-mediated seizure facilitation. Exp Neurol. 1986;93:180–187. doi: 10.1016/0014-4886(86)90157-3. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Reznikov KY. Cell proliferation and cytogenesis in the mouse hippocampus. Adv Anat Embryol Cell Biol. 1991;122:1–74. doi: 10.1007/978-3-642-76447-9. [DOI] [PubMed] [Google Scholar]
- Rutten A, van Albada M, Silveira DC, Cha BH, Liu X, Hu YN, Cilio MR, Holmes GL. Memory impairment following status epilepticus in immature rats: time-course and environmental effects. Eur J Neurosci. 2002;16:501–513. doi: 10.1046/j.1460-9568.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–1761. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M. Social functioning and seizure status of young adults with onset of epilepsy in childhood. An epidemiological 20-year follow-up study. Acta Neurol Scand Suppl. 1983;96:1–81. [PubMed] [Google Scholar]
- Sillanpaa M. Learning disability: occurrence and long-term consequences in childhood-onset epilepsy. Epilepsy Behav. 2004;5:937–944. doi: 10.1016/j.yebeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Simon DK, O'Leary DD. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa Y, Monokoshi M, Silveira DC, Cha BH, Cilio MR, McCabe BK, Liu X, Hu Y, Holmes GL. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Brain Res Dev Brain Res. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- Soria C, El SS, Escolano S, Bobet R, Bulteau C, Dellatolas G. Quality of life in children with epilepsy and cognitive impairment: a review and a pilot study. Dev Neurorehabil. 2007;10:213–221. doi: 10.1080/13638490601111129. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Romero MT, Stanton PK. Flurothyl status epilepticus in developing rats: behavioral, electrographic, histological and electrophysiological studies. Develop Brain Res. 1999;116:59–68. doi: 10.1016/s0165-3806(99)00075-9. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Moshé SL. Age-related differences in seizure susceptibility to flurothyl. Dev Brain Res. 1988;39:295–297. doi: 10.1016/0165-3806(88)90033-8. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001a;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O'Keefe J. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001b;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Sturniolo MG, Galletti F. Idiopathic epilepsy and school achievement. Arch Dis Child. 1994;70:424–428. doi: 10.1136/adc.70.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Smith KL, Gomez CM, Brady RJ. The ontogeny of hippocampal local circuits and focal epileptogenesis. Epilepsy Res Suppl. 1992;9:115–125. [PubMed] [Google Scholar]
- Tremblay E, Roisin MP, Represa A, Charriaut-Marlangue C, Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988;461:393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48 Suppl 5:96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The open field test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Zhao Q, Hu Y, Holmes GL. Effect of topiramate on cognitive function and activity level following neonatal seizures. Epilepsy Behav. 2005;6:529–536. doi: 10.1016/j.yebeh.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Haug FM. Laminar differentiation of the hippocampus, fascia dentata and subiculum in developing rats, observed with the Timm sulphide silver method. J Comp Neurol. 1978;179:581–617. doi: 10.1002/cne.901790309. [DOI] [PubMed] [Google Scholar]