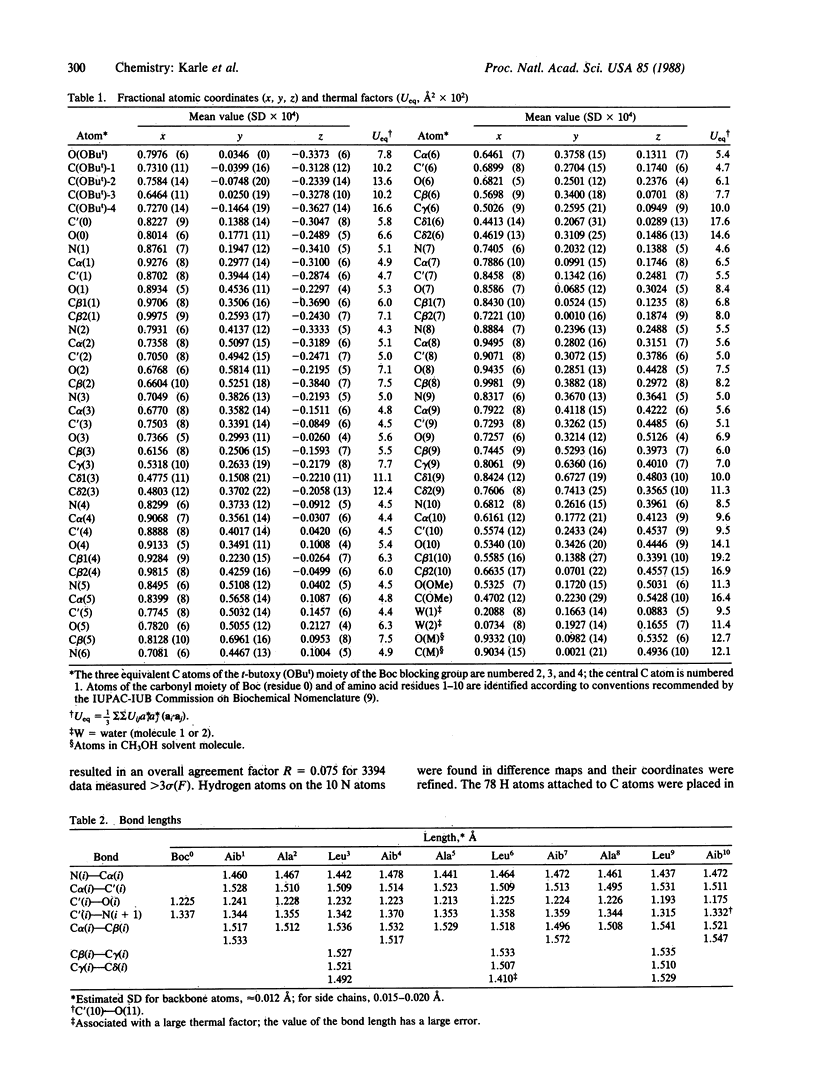
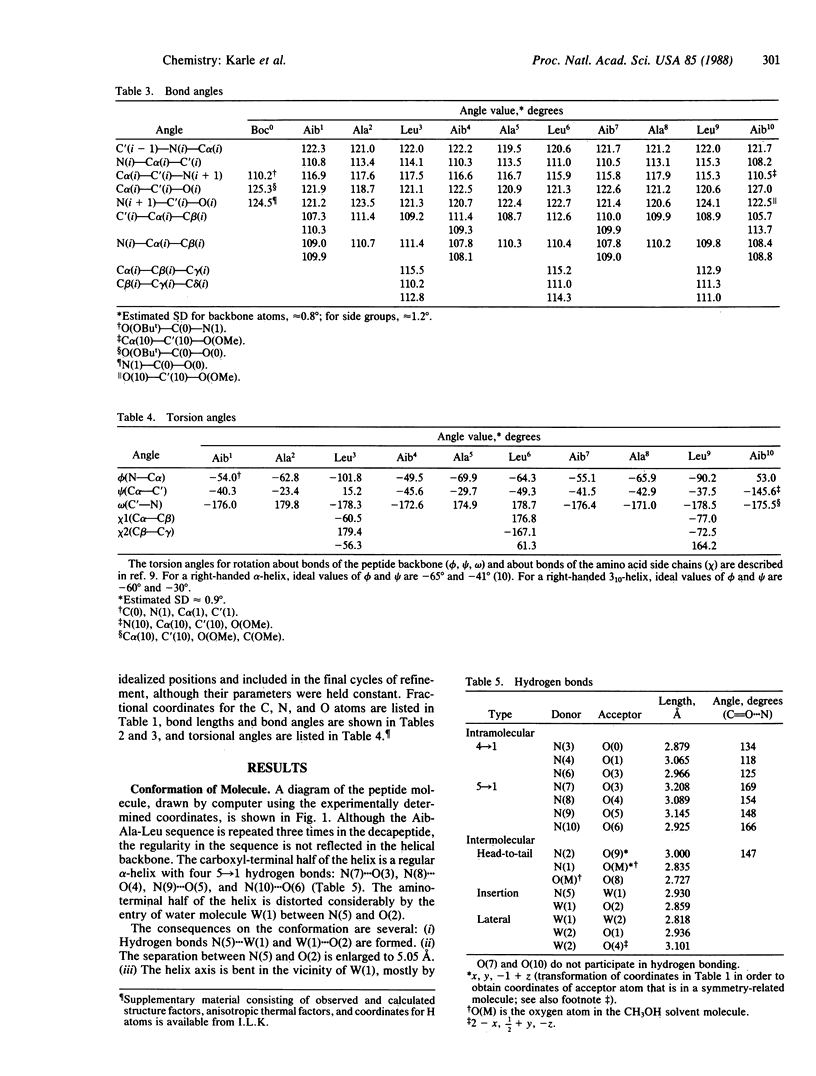
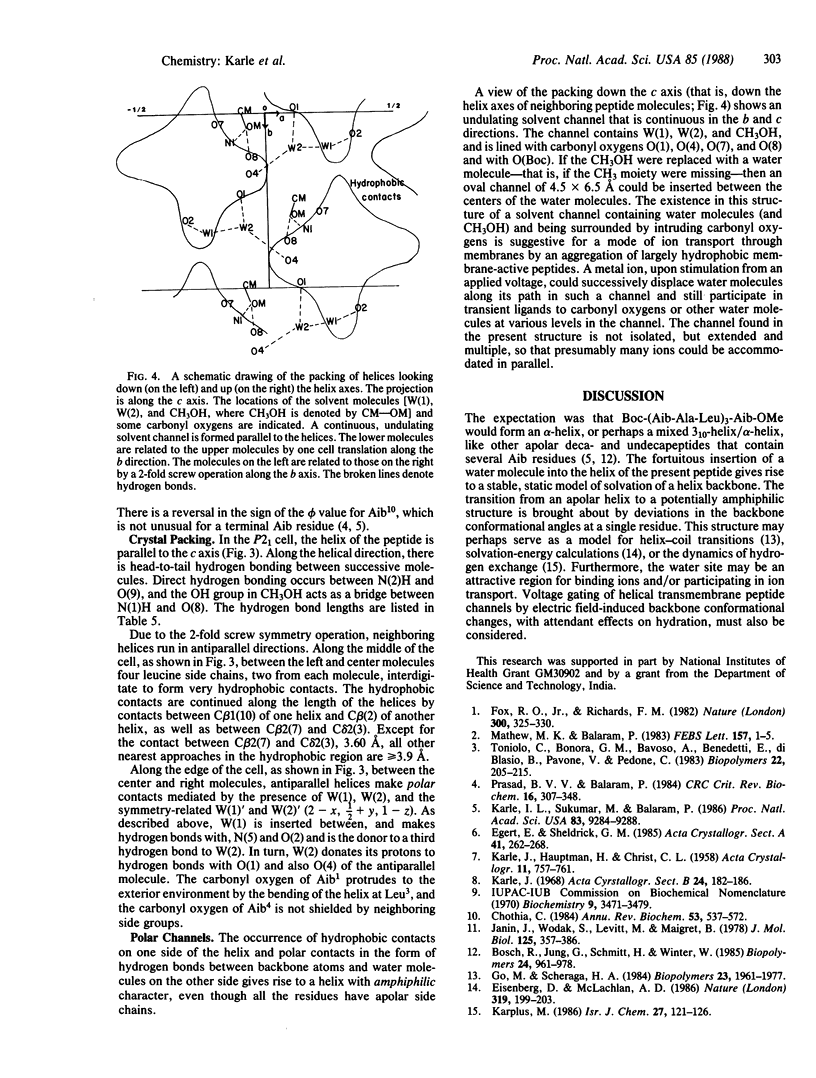
Abstract
Although the peptide Boc-Aib1-Ala2-Leu3-Aib4-Ala5-Leu6-Aib7-Ala8-L eu9-Aib10-OME [with a t-butoxycarbonyl (Boc) blocking group at the amino terminus, a methyl ester (OMe) at the carboxyl terminus, and four alpha-amino-isobutyric (Aib) residues] has a 3-fold repeat of residues, the helix formed by the peptide backbone is irregular. The carboxyl-terminal half assumes an alpha-helical form with torsion angles phi and psi of approximately -60 degrees and -45 degrees, respectively, whereas the amino-terminal half is distorted by an insertion of a water molecule between the amide nitrogen of Ala5 [N(5)] and the carbonyl oxygen of Ala2 [O(2)]. The water molecule W(1) acts as a bridge by forming hydrogen bonds N(5)...W(1) (2.93 A) and W(1)...O(2) (2.86 A). The distortion of the helix exposes the carbonyl oxygens of Aib1 and Aib4 to the outside environment, with the consequence that the helix assumes an amphiphilic character despite having all apolar residues. Neighboring helices in the crystal run in antiparallel directions. On one side of a helix there are only hydrophobic contacts with efficient interdigitation of leucine side chains with those from the neighboring helix. On the other side of the helix there are hydrogen bonds between protruding carbonyl oxygens and four water molecules that separate two neighboring helices. Along the helix axis the helices bind head-to-tail with a direct hydrogen bond N(2)...O(9) (3.00 A). Crystals grown from methanol/water solution are in space group P21 with a = 15.778 +/- 0.004 A, b = 11.228 +/- 0.002 A, c = 18.415 +/- 0.003 A, beta = 102.10 +/- 0.02 degrees, and two formula units per cell for C49H88N10O13.2H2O.CH3OH. The overall agreement factor R is 7.5% for 3394 reflections observed with intensities greater than 3 sigma (F), and the resolution is 0.90 A.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chothia C. Principles that determine the structure of proteins. Annu Rev Biochem. 1984;53:537–572. doi: 10.1146/annurev.bi.53.070184.002541. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Go M., Scheraga H. A. Molecular theory of the helix-coil transition in polyamino acids. V. Explanation of the different conformational behavior of valine, isoleucine, and leucine in aqueous solution. Biopolymers. 1984 Oct;23(10):1961–1977. doi: 10.1002/bip.360231012. [DOI] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Sukumar M., Balaram P. Parallel packing of alpha-helices in crystals of the zervamicin IIA analog Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-OMe.2H2O. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9284–9288. doi: 10.1073/pnas.83.24.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Balaram P. The stereochemistry of peptides containing alpha-aminoisobutyric acid. CRC Crit Rev Biochem. 1984;16(4):307–348. doi: 10.3109/10409238409108718. [DOI] [PubMed] [Google Scholar]



