Abstract
Cigarette smoking expectancies are systematically related to intention to quit smoking in adult smokers without psychiatric illness, but little is known about these relationships in smokers with serious mental illness. In this study, we compared positive and negative smoking expectancies, and examined relationships between expectancies and intention to quit smoking, in smokers with schizophrenia (n = 46), smokers with schizoaffective disorder (n = 35), and smokers without psychiatric illness (n = 71). In all three groups, reduction of negative affect was rated as the most important smoking expectancy and intention to quit smoking was systematically related to concerns about the health effects and social consequences of smoking. Compared to the other groups of smokers, those with schizoaffective disorder were more concerned with social expectancies and with the immediate negative physical effects of smoking. Results of this study suggest that challenging positive smoking expectancies and providing more tailored information about the negative consequences of smoking might increase motivation to quit smoking in smokers with schizophrenia and schizoaffective disorder, as has been found with non-psychiatric smokers.
Keywords: schizophrenia, tobacco dependence disorder, smoking cessation, motivation, belief, affect
1. Introduction
Smoking outcome expectancies refer to beliefs about the consequences of cigarette smoking, such as beliefs that smoking reduces negative mood, increases stimulation, facilitates some social interactions and curbs appetite (i.e., positive smoking expectancies or “pros” of smoking) and that smoking induces harmful physical effects and social disapproval (negative smoking expectancies or “cons” of smoking). Smoking expectancies have been described in numerous populations, including adolescents (Lewis-Esquerre et al., 2005), college students (Brandon and Baker, 1991) and older adults (Rohsenow et al., 2003; Wetter et al., 1994), including adults with psychiatric disorders (Buckley et al., 2005).
Smoking expectancies are relevant to the treatment of smoking because such expectancies are associated with intention to quit and predict smoking cessation success. Those who intend to quit smoking rate the importance of negative smoking consequences higher than those who do not intend to quit (Brandon et al., 1999; Prochaska and Velicer, 1997). Furthermore, expectancies of harmful health consequences from smoking predicted greater cessation success in the first week of a quit attempt, whereas expectancies that smoking reverses negative mood predicted less cessation success, over and above other predictors such as biological and subjective measures of tobacco dependence, negative affect and perceived stress (Wetter et al., 1994). However, in smokers with serious mental illness, which is associated with low rates of smoking cessation (de Leon and Diaz, 2005; Lasser et al., 2000), less is known about relationships between smoking expectancies and intention to quit smoking.
A number of studies have described smoking outcome expectancies in smokers with schizophrenia, with most finding that reduction of negative affect was rated the most important positive expectancy (Buckley et al., 2005; Esterberg and Compton, 2005; Forchuk et al., 2002; Solty et al., 2009; but see Carosella et al., 1999) and negative health consequences the most important negative expectancy (Buckley et al., 2005; Carosella et al., 1999; Esterberg and Compton, 2005; Lucksted et al., 2004; Solty et al., 2009). However, the few studies that have directly compared smoking expectancies of smokers with schizophrenia with those of a concurrent sample of smokers without psychiatric illness have reported inconsistent findings. Using modified versions of the Reasons for Smoking Scale (Russell et al., 1974), Gurpegui et al. (2007) reported that smokers with schizophrenia were more likely than controls to report that reduction of negative affect was their main reason for smoking; whereas Barr et al. (2008) reported that smokers with schizophrenia or schizoaffective disorder rated the importance of negative affect reduction, pleasure/relaxation, addiction and habit similarly to controls, but endorsed higher importance scores on the sensorimotor manipulation and stimulation scales and lower scores for sociability. Neither study measured negative smoking expectancies, and neither examined whether intention to quit was associated with increases in negative expectancies.
In this study, we used the Smoking Effects Questionnaire (SEQ; Rohsenow et al., 2003) to compare positive and negative smoking outcome expectancies in smokers with schizophrenia, schizoaffective disorder, and equally-nicotine-dependent smokers without psychiatric illness, and to examine relationships between expectancies and intention to quit smoking. Because this measure was validated in a group of general adult smokers (Rohsenow et al., 2003), we also examined the internal consistency reliabilities of the SEQ scales in these study groups. Based on previous research (reviewed in Brandon et al., 1999), we hypothesized that greater intention to quit would be associated with higher importance scores on the negative smoking expectancies scales. A secondary aim was to compare expectancies in smokers with schizophrenia with those of smokers with schizoaffective disorder. Although studies often combine data from people with these diagnoses into one group, mood disturbance is more prominent in schizoaffective disorder (American Psychiatric Association, 1994). Considering previous research on relationships between depression symptom severity and smoking expectancies (Friedman-Wheeler et al., 2007), we hypothesized that smokers with schizoaffective disorder would rate the importance of negative affect reduction higher than the other groups. There were no other specific hypotheses concerning intention to quit and expectancies, but these relationships were explored.
2. Methods
2.1. Participants
Participants were heavy smokers with schizophrenia (SCZ; n = 46), schizoaffective disorder (SCZAFF; n = 35), or no current psychiatric illness (CON; n = 71) who had enrolled in one of four laboratory studies of smoking behavior. Participants in these studies could not be seeking immediate treatment for smoking, but there were no other inclusion or exclusion criteria related to motivation to quit smoking. Participants were at least 18 years of age, smoked at least 20 cigarettes per day and had scores of at least 6 on the Fagerström Test for Nicotine Dependence (FTND), a widely-used measure of nicotine dependence (Heatherton et al., 1991). One question on the FTND queries time to first cigarette after awakening. As responses to this question could be affected by environmental constraints on smoking (Steinberg et al., 2005), participants were asked whether they were allowed to smoke in their residences, and those whose smoking was constrained were asked to respond to this question by estimating how soon they would smoke after awakening if they were permitted to do so. The Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First et al., 1997) was used to confirm diagnoses in SCZ and SCZAFF participants and to rule out psychiatric disorders in CON participants. The Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962) was administered to exclude those with very high psychiatric symptom severity, (i.e., ratings of 6 or higher for uncooperativeness, excitement, conceptual disorganization, tension, posturing, disorientation or emotional withdrawal), as this could interfere with the completion of study procedures, but no potential participants were excluded for this reason. All participants provided written informed consent to participate in research and passed a quiz on their understanding of the critical elements of consent.
2.2. Measures
The following assessments were included in a larger battery administered at study enrollment, prior to any experimental procedures. Breath CO levels were measured using a Smokerlyzer ED50 CO monitor (Bedfont Instruments). Cotinine levels from a subset of participants (n = 79) were measured by a commercial laboratory (Salimetrics, LLC, State College, PA). Samples from the remaining participants were analyzed using a different laboratory and method and are not included in analyses. Psychiatric symptoms in participants with schizophrenia or schizoaffective disorder were assessed by clinically-trained research staff using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). The Scale for Assessment of Negative Symptoms (SANS; Andreasen, 1983) was also administered as it measures negative schizophrenia symptoms in more detail than does the PANSS. The Hamilton Depression Sale (HAM-D; Hamilton, 1960) was used to measure current depression symptom severity in a subsample of participants (n = 62). Intention to quit smoking was assessed according to the Stages of Change algorithm, with Precontemplation defined as not intending to quit within 6 months, Contemplation defined as intending to quit within 6 months but (1) either not intending to quit within 30 days or (2) intending to quit within 30 days but not having made a 24-hr quit attempt in the past year, and Preparation defined as intending to quit within 30 days and having made at least one 24-hr quit attempt in the past year (DiClemente et al., 1991).
Positive and negative smoking outcome expectancies were assessed with the SEQ (Rohsenow et al., 2003). The 33 SEQ items load on seven scales with two higher-order factors (positive and negative). The three negative expectancies scales are Negative Physical Effects (e.g., “Smoking makes me short of breath”), Negative Psychosocial Effects (e.g., “Smoking makes my family or friends respect me less”), and Future Health Concerns (e.g., “Smoking makes me worry about getting or having cancer”). The four positive expectancies scales are Reduce Negative Affect (e.g., “Smoking helps me when I am angry, irritable or frustrated”), Stimulation (e.g., “Smoking stimulates me, perks me up”), Positive Social Effects (e.g., “Smoking makes me feel more relaxed when I am with other people”) and Weight Control (e.g., “Smoking helps me lose weight”). Participants rate the importance of each item from 0-3 (0 = “False, the statement does not apply to me”; 1 = “True, the statement applies to me and is hardly at all important”; 2 = “True, the statement applies to me and is moderately important”; 3 = “True, the statement applies to me and is very important”).
2.3. Statistical Methods
Group comparisons on demographic, clinical and smoking history measures were conducted using one-way analyses of variance tests (ANOVAs) and chi-square tests for categorical variables. Internal consistency reliabilities of the 7 SEQ scales were determined by calculating Cronbach's alpha coefficients for each group (SCZ, SCZAFF, CON). Values greater than 0.70 were considered acceptable (Kaplan and Saccuzzo, 2005).
To examine how positive and negative smoking expectancies were related to intention to quit smoking within each group, between-groups 3×3 analysis of covariance tests (ANCOVAs) were first used to examine the effects of Group (SCZ, SCZAFF, CON) and Stage of Change (Precontemplation, Contemplation, Preparation) on importance scores from the 7 SEQ scales. However, because of the small number of participants in the Preparation stage, and as there were no differences between those in the Contemplation and Preparation stages on SEQ scores, these groups were subsequently combined into one group that was renamed Intention to Quit based on participants’ interest in quitting within the next 6 months. Between-groups 3×2 ANCOVAs were then used to examine the effects of Group (SCZ, SCZAFF, CON) and Intention to Quit (Intention vs. No Intention) on importance scores from the SEQ scales. Gender was covaried in these analyses as the groups differed significantly on gender distribution and as gender is known to affect smoking expectancies (e.g., Rohsenow et al., 2003). Multivariate analyses were not used because we were not interested in the linear combination of SEQ scales; rather, effects on each scale were of interest. Confidence intervals were adjusted using the Bonferroni method. Significant interactions were followed by simple effects tests.
3. Results
3.1. Demographic and clinical characteristics
Participants’ demographic, psychiatric symptom and smoking characteristics are shown in Table 1. Participants were 44.5 ± 10.2 (M ± SD) years old, 68.5% White, 18.8% African American and 7% Hispanic. Sixteen percent were married or living with a romantic partner. There were no significant differences among the groups on these measures. The SCZ group had a higher percentage of men than the SCZAFF or CON groups (X2 (2, N = 152) = 6.17, p < .05). The groups also differed on employment status and income. CON participants were more likely to be employed, either full- or part-time, than SCZAFF participants (X2 (2, N = 152) = 10.88, p < .01) and more likely to earn over US$10,000 per year than SCZ or SCZAFF participants (X2 (2, N = 147) = 16.94, p < .001). SCZ and SCZAFF participants had similar PANSS positive and negative scale scores, but PANSS general scale scores were higher in SCZAFF (F (1, 75) = 7.77, p < .01). There was also a significant effect of Group on HAM-D score (F (2, 59) = 17.79, p < .001). Simple effects tests indicated that HAM-D scores were significantly higher for SCZAFF compared to SCZ participants (p < .01), and significantly higher for SCZ participants compared to CON (p < .05).
Table 1.
Participants’ Demographic and Smoking Characteristics at Enrollment [M (SD) or %].
| Non-Psychiatric (n = 71) | Schizophrenia (n = 46) | Schizoaffective (n = 35) | |
|---|---|---|---|
| Age | 44.5 (12.2) | 45.1 (7.7) | 43.9 (8.5) |
| Male* | 55%a | 76%b | 54%a |
| Race | |||
| White | 70% | 64% | 71% |
| African-American | 21% | 22% | 9% |
| Other | 8% | 13% | 21% |
| Hispanic ethnicity | 4% | 11% | 6% |
| Employed full- or part-time** | 34%a | 20% | 6%b |
| Annual income < $10,000*** | 27%a | 43%a | 69%b |
| Years of education | 12.5 (2.1) | 12.0 (1.8) | 12.7 (2.3) |
| Married or cohabitating | 23% | 13% | 9% |
| Cigarettes per day | 26.0 (9.7) | 27.8 (13.2) | 29.0 (11.3) |
| FTND score | 7.0 (1.5) | 7.1 (1.6) | 7.2 (1.5) |
| Years of daily smoking | 27.8 (12.2) | 25.4 (8.7) | 27.1 (9.5) |
| Breath CO level (ppm)*** | 20.2 (8.4) a | 28.6 (14.9) b | 32.0 (16.6)b |
| Salivary cotinine (ng/ml)1** | 299.0 (154.7)a | 458.8 (214.4)b | 346.0 (142.0)a |
| Smoking stage of change | |||
| Precontemplation | 59% | 53% | 51% |
| Contemplation | 31% | 42% | 29% |
| Preparation | 10% | 4% | 20% |
| PANSS positive scale score | 13.6 (4.7) | 15.6 (5.7) | |
| PANSS negative scale score | 15.2 (5.6) | 16.8 (6.2) | |
| PANSS general scale score* | 27.7 (7.5) a | 32.8 (8.5)b | |
| SANS total score | 29.9 (20.1) | 34.1 (18.4) | |
| HAM-D total score2*** | 3.3 (3.7)a | 6.4 (4.8)b | 12.4 (6.5)c |
| Antipsychotic medication | |||
| Typical | 24% | 11% | |
| Atypical | 76% | 69% | |
| Other psychiatric medication | 33% SSRI; 17% AD, 15% AM, 24% AA | 46% SSRI; 34% AD, 34% AM, 37% AA |
Abbreviations: FTND, Fagerström Test of Nicotine Dependence; CO, carbon monoxide; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; HAM-D, Hamilton Depression Scale; SSRI, selective serotonin reuptake inhibitors; AD, non-SSRI antidepressant; AM, antimanic; AA, antianxiety.
p < .05
p < .01
p < .001
a,b,c superscripts within a row indicate significant differences.
Sample sizes for this measure are 42 non-psychiatric smokers, 18 smokers with schizophrenia, 19 smokers with schizoaffective disorder.
Sample sizes for this measure are 24 non-psychiatric smokers, 19 smokers with schizophrenia, 19 smokers with schizoaffective disorder.
3.2. Tobacco use characteristics
Participants smoked 27.2 ± 11.2 cigarettes per day, had been smoking daily for 26.9 ± 10.6 years, and had FTND scores of 7.1 ± 1.5 at study enrollment. The groups did not differ on these measures. However, SCZ and SCZAFF had higher breath CO levels than CON participants (F (2, 150) = 11.92, p < .001) and, among the subset with analyzable data, SCZ had higher salivary cotinine levels than CON or SCZAFF participants (F (2, 76) = 5.79, p < .01). There were no significant differences among the groups on stage of change for smoking cessation (Table 1).
3.3. Smoking outcome expectancies
In CON participants, internal consistency coefficients (Cronbach's alphas) of the SEQ scales ranged from 0.72 – 0.91 (Table 2). In SCZ participants, internal consistency coefficients exceeded 0.70 for four of the scales but were below 0.70 for three scales: Negative Psychosocial Effects (α = 0.68), Stimulation (α = 0.64) and Negative Physical Effects (α = 0.59). In SCZAFF participants, internal consistency coefficients exceeded 0.70 for all SEQ scales except the Positive Social Effects scale, which was slightly lower (α = 0.68).
Table 2.
Internal consistency coefficients (Chronbach's alphas) of the SEQ scales in smokers with schizophrenia, schizoaffective disorder and non-psychiatric controls.
| |
Positive Smoking Expectancies |
Negative Smoking Expectancies |
|||||
|---|---|---|---|---|---|---|---|
| Reduce Negative Affect | Positive Social Effects | Stimulation | Weight Control | Future Health Concerns | Negative Physical Effects | Negative Psychosocial Effects | |
| Non-Psychiatric | 0.85 | 0.80 | 0.73 | 0.83 | 0.91 | 0.72 | 0.77 |
| Schizophrenia | 0.76 | 0.76 | 0.64 | 0.77 | 0.81 | 0.59 | 0.68 |
| Schizoaffective | 0.90 | 0.68 | 0.73 | 0.83 | 0.73 | 0.74 | 0.77 |
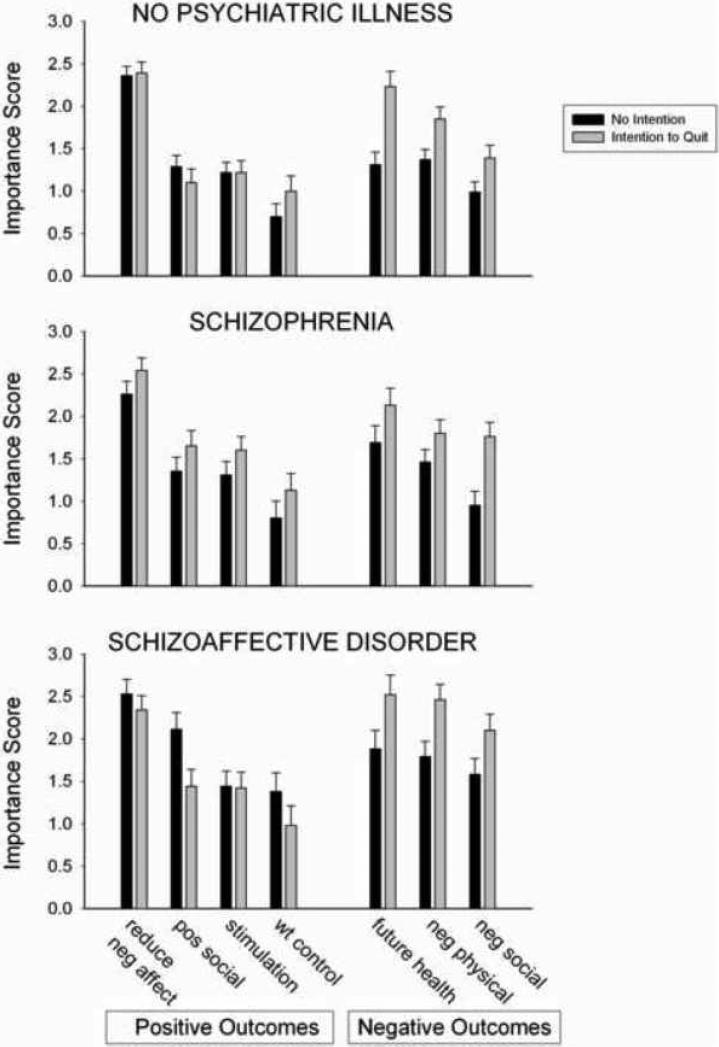
Importance scores for each group on the 7 SEQ expectancy scales are shown in Figure 1. There were significant main effects of Group on importance ratings for the Positive Social Effects scale (F (2, 145) = 5.86, p < .01, the Negative Physical Effects scale (F (2, 145) = 6.28, p < .01) and the Negative Psychosocial scale (F (2, 145) = 7.78, p < .01). Post-hoc tests indicated that SCZAFF participants endorsed higher importance ratings than CON on the Positive Social Effects scale (p < .01) and Negative Psychosocial Effects scale (p < .001), and higher importance ratings than both CON and SCZ on the Negative Physical Effects scale (p < .01 for both comparisons). There was a significant main effect of Intention to Quit on importance ratings on all three negative expectancy scales: the Future Health Concerns scale (F (1, 145) = 16.81, p < .001), the Negative Physical Effects scale (F (1, 145) = 15.54, p < .001) and the Negative Psychosocial Effects scale (F (1, 145) = 17.97, p < .001). Participants who intended to quit within 6 months endorsed higher importance scores these scales (Figure 1). There was a significant interaction between Group and Intention to Quit on importance ratings on the Positive Social Effects scale (F (2, 145) = 3.36, p < .05). Simple effects tests conducted within each group indicated that in the SCZAFF group, participants who did not intend to quit within 6 months endorsed significantly higher importance scores on the Positive Social Effects scale than those who intended to quit (p < .05), but this was not the case for the CON or SCZ groups.
Figure 1.
Positive and negative smoking outcome expectancy importance scores in smokers without psychiatric illness (top), smokers with schizophrenia (middle) and smokers with schizoaffective disorder (bottom) who reported intention (light bars) or no intention (solid bars) to quit smoking within the next 6 months. Bar heights represent M ± SEM.
4. Discussion
Research in smokers with schizophrenia that was conducted in the 1990s suggested these smokers had little interest in quitting, which was thought to contribute to their low rates of smoking cessation success (reviewed in McChargue et al., 2002). Results from the current study are consistent with emerging evidence that percentages of smokers with schizophrenia and schizoaffective disorder who intend to quit smoking are quite similar to those of equally-heavy smokers without psychiatric illness (Siru et al., 2009; Solty et al., 2009). However, the fact that over 50% of the smokers in these samples did not intend to quit smoking within the next 6 months demonstrates that considerably stronger and wider intervention efforts are needed to motivate these quit-resistant smokers.
The profiles of smoking expectancies were quite similar in the three groups of smokers in this study. All groups rated the importance of negative affect reduction as the most important smoking expectancy, one that has long been recognized as highly important to smokers without psychiatric illness (Baker et al., 2004). Although most studies on smoking expectancies in smokers with schizophrenia (reviewed in the Introduction) found the same result, these studies have had at least one of the following limitations: the use of non-validated instruments or methods to measure expectancies, the measurement of only positive expectancies, the use of dichotomous response alternatives that do not provide information on the relative importance of expectancies, the use of a heterogeneous patient group, or the lack of a concurrent group of equally-heavy smokers without psychiatric illness. To our knowledge this is the first study to use a psychometrically-sound questionnaire to measure positive and negative smoking expectancies in smokers with schizophrenia in comparison with a concurrent group of equally-heavy smokers without psychiatric illness, and to include intention to quit as a factor in the analysis. With regard to the expectancies questionnaire used in this study, internal consistency reliabilities of the SEQ scales were found to be comparable and generally acceptable in all three study groups, although somewhat lower in SCZ, particularly on the Negative Physical Effects scale. It should be noted that all participants in this study were heavy (at least 20 cigarettes per day) smokers, and these findings may not generalize to lighter smokers. Future studies may also consider including a measure of intention to quit smoking that offers greater precision than the stage-based classification used in this study.
A striking outcome of this study is that, in each diagnostic group, those who intended to quit rated the importance of negative smoking outcome expectancies higher than those who did not intend to quit, but, with one exception, did not differ in the rated importance of positive expectancies of smoking. This is consistent with previous research with smokers in general (Brandon et al., 1999; Prochaska et al., 1994), and indicates that relationships between intention to quit and concerns about the negative health and social consequences of smoking are as systematic in smokers with schizophrenia and schizoaffective disorder as they are in smokers without these psychiatric disorders. Another novel contribution of this study was its examination of smoking expectancies among smokers with schizoaffective disorder separately from those of smokers with schizophrenia. Results of this comparison suggest that smokers with schizoaffective disorder are particularly concerned with the negative social and physical effects of smoking, and that smokers with schizoaffective disorder with no intention to quit may be more apt to cope with social situations by smoking. These findings are likely related to the higher depression severity in the smokers with schizoaffective disorder relative to the other groups, as previous research has found relationships between depression symptom severity and both stronger positive and negative smoking expectancies (Friedman-Wheeler et al., 2007).
Some aspects of the participants’ demographic and smoking characteristics also merit comment. The findings of significantly higher breath CO and salivary cotinine levels in the schizophrenia group compared to the non-psychiatric control group are consistent with those of numerous previous studies (e.g., Olincy et al., 1997; Strand and Nybäck, 2005). These differences appear to be due to differences in smoking behavior, as smokers with schizophrenia have been found to differ from controls on some smoking topography characteristics (Tidey et al., 2005; 2008), and to not differ from controls in the rate at which they metabolize nicotine and cotinine (Williams et al., 2005). The low employment and income levels of participants with schizophrenia or schizoaffective disorder in this study are consistent with previous findings (reviewed in Bond and Drake, 2008). However, it is also likely that the overall low rates of employment in this study were affected by selection bias as the studies in which these data were gathered were conducted during normal workdays and working hours.
The results of this study support the importance of focusing on the expected pros and cons of smoking in motivation interviewing and other cognitive behavioral interventions for tobacco dependence in people with schizophrenia and schizoaffective disorder (Steinberg et al., 2004). A logical next step for this research would be to examine whether expectancies predict smoking cessation outcomes and withdrawal symptom severity in people with schizophrenia and schizoaffective disorder, as shown in non-psychiatric smokers (Gwaltney et al., 2005; Wetter et al., 1994). These data also suggest that, while challenging positive smoking expectancies may not affect intention to quit, increasing awareness or salience of negative consequences of smoking might increase motivation to quit smoking in smokers with schizophrenia and schizoaffective disorder, as they do in non-psychiatric smokers (Copeland and Brandon, 2000).
Acknowledgements
We thank Amy Adolfo, Elizabeth Cathers, Jeffrey Noble, Vera Mayercik and Netesha Reid for collecting and Laura Dionne for verifying and managing these data. We thank the individuals who participated in this research for their contributions.
Role of Funding Source
This research was supported by NIDA grants R01-DA14002 and R01-DA17566 to J.W.T. and a Senior Research Career Scientist Award from the Department of Veterans Affairs to D.J.R. The funding sources had no other role in the design or conduct of this research. This study was conducted at the Brown University Center for Alcohol and Addiction Studies and the Providence Veterans Affairs Medical Center, Providence, RI, USA. Preliminary data from this study were presented at the 2007 annual meeting of the Society for Research on Nicotine and Tobacco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Neither author has any conflict of interest with this research.
Contributors
Both authors designed the study. The first author conducted the statistical analyses and wrote the first draft of the manuscript. Both authors contributed to and have approved the final manuscript.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: 1994. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:35–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barr AM, Procyshyn RM, Hui P, Johnson JL, Honer WG. Self-reported motivation to smoke in schizophrenia is related to antipsychotic drug treatment. Schizophr. Res. 2008;100:252–260. doi: 10.1016/j.schres.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Bond GR, Drake RE. Predictors of competitive employment among patients with schizophrenia. Curr. Opin. Psychiatry. 2008;21:362–369. doi: 10.1097/YCO.0b013e328300eb0e. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Baker TB. The Smoking Consequences Questionnaire: The subjective utility of smoking in college students. Psychol. Assess. 1991;3:484–491. [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsch I, editor. How expectancies shape experience. American Psychological Association; Washington, DC: 1999. pp. 263–299. Washington D.C. [Google Scholar]
- Buckley TC, Wolfsdorf Kamholz B, Mozley SL, Gulliver SB, Holohan DR, Helstrom AW, Walsh K, Morisette SB, Kassel JD. A psychometric evaluation of the Smoking Consequences Questionnaire-Adult in smokers with psychiatric conditions. Nicotine Tob. Res. 2005;7:739–745. doi: 10.1080/14622200500259788. [DOI] [PubMed] [Google Scholar]
- Carosella AM, Ossip-Klein DJ, Owens CA. Smoking attitudes, beliefs, and readiness to change among acute and long term care inpatients with psychiatric diagnoses. Addict. Behav. 1999;24:331–344. doi: 10.1016/s0306-4603(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH. Testing the causal role of expectancies in smoking motivation and behavior. Addict. Behav. 2000;25:445–449. doi: 10.1016/s0306-4603(99)00003-9. [DOI] [PubMed] [Google Scholar]
- De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO, Fairhurst S, Velicer WF, Rossi JS, Velasquez M. The process of smoking cessation: An analysis of precontemplation, contemplation and contemplation/action. J. Consult. Clin. Psychol. 1991;59:295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc. Sci. Med. 2005;61:293–303. doi: 10.1016/j.socscimed.2004.11.057. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis-I Disorders. Biometrics Research Dept., NY State Psychiatric Inst.; NY NY: 1994. [Google Scholar]
- Forchuk C, Norman R, Malla A, Martin M-L, McLean T, Cheng S, Diaz K, McIntosh E, Rickwood A, Vos S, Gibney C. Schizophrenia and the motivation for smoking. Perspect. Psychiatr. Care. 2002;38:41–49. doi: 10.1111/j.1744-6163.2002.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Friedman-Wheeler DG, Ahrens AH, Haaga DAF, McIntosh E, Thorndike FP. Depressive symptoms, depression proneness, and outcome expectancies for cigarette smoking. Cognit. Ther. Res. 2007;31:547–557. doi: 10.1007/s10608-006-9064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurpegui M, Martínez-Ortega JM, Jurado D, Aguilar MC, Diaz FJ, de Leon J. Subjective effects and the main reason for smoking in outpatients with schizophrenia: a case-control study. Compr. Psychiatry. 2007;48:186–191. doi: 10.1016/j.comppsych.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. J. Abnormal Psychol. 2005;114:661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kaplan D, Saccuzzo DP. Psychological Testing: Principles, Applications and Issues. Wadsworth/Thomsen; Belmont, CA: 2005. [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lewis-Esquerre JM, Rodrigue JR, Kahler CW. Development and validation of an adolescent smoking consequences questionnaire. Nicotine Tob. Res. 2005;7:81–90. doi: 10.1080/14622200412331328475. [DOI] [PubMed] [Google Scholar]
- Lucksted A, McGuire C, Postrado L, Kreyenbuhl J, Dixon LB. Specifying cigarette smoking and quitting among people with serious mental illness. Am. J. Addict. 2004;13:128–138. doi: 10.1080/10550490490436000. [DOI] [PubMed] [Google Scholar]
- McChargue DE, Gulliver SB, Hitsman B. Would smokers with schizophrenia benefit from a more flexible approach to smoking treatment? Addiction. 2002;97:785–793. doi: 10.1046/j.1360-0443.2002.00064.x. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol. Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, Fiore C, Harlow LL, Redding CA, Rosenbloom D, Rossi SR. Stages of change and decisional balance for twelve problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Abrams DB, Monti PM, Colby SM, Martin R, Niaura RS. The Smoking Effects Questionnaire for adult populations. Development and psychometric properties. Addict. Behav. 2003;28:1257–1270. doi: 10.1016/s0306-4603(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Russell MA, Peto J, Patel UA. The classification of smoking by a factorial structure of motives. J. Royal Statist. Society. 1974;137:313–346. [Google Scholar]
- Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104:719–733. doi: 10.1111/j.1360-0443.2009.02545.x. [DOI] [PubMed] [Google Scholar]
- Solty H, Crockford D, White WD, Currie S. Cigarette smoking, nicotine dependence, and motivation for smoking cessation in psychiatric inpatients. Can J. Psychiatry. 2009;54:36–45. doi: 10.1177/070674370905400107. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Steinberg HR, Krejci JA, Ziedonis DM. Applicability of the Fagerstrom Test for Nicotine Dependence in smokers with schizophrenia. Addict. Behav. 2005;30:49–59. doi: 10.1016/j.addbeh.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Ziedonis DM, Krejci JA, Brandon TH. Motivational interviewing with personalized feedback: A brief intervention for motivating smokers with schizophrenia to seek treatment for tobacco dependence. J. Consult. Clin. Psychol. 2004;72:723–728. doi: 10.1037/0022-006X.72.4.723. [DOI] [PubMed] [Google Scholar]
- Strand JE, Nybäck H. Tobacco use in schizophrenia: a study of cotinine concentrations in the saliva of patients and controls. Eur. Psychiatr. 2005;20:50–54. doi: 10.1016/j.eurpsy.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alc. Depend. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Adolfo AB. Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob. Res. 2008;10:1047–1056. doi: 10.1080/14622200802097373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD, Offord KP, Baker TB. Smoking outcome expectancies: Factor structure, predictive validity and discriminative validity. J. Abnorm. Psychol. 1994;103:801–811. doi: 10.1037//0021-843x.103.4.801. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schiz. Res. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]