Abstract
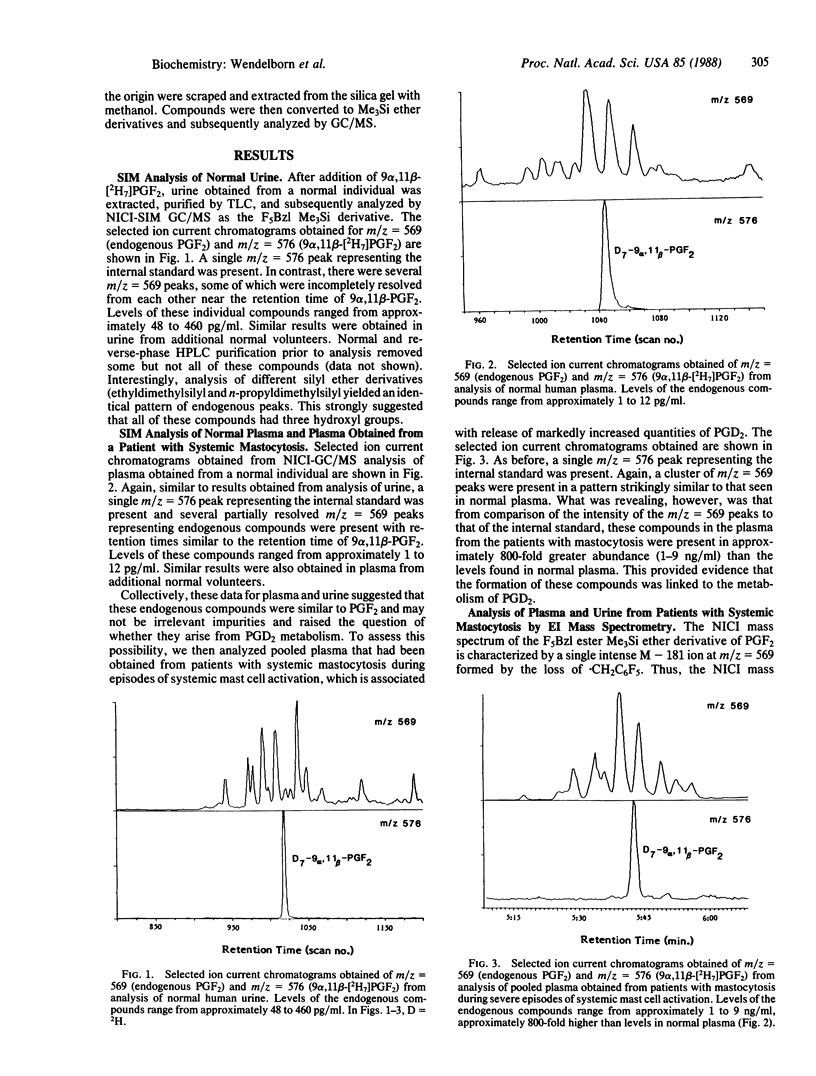
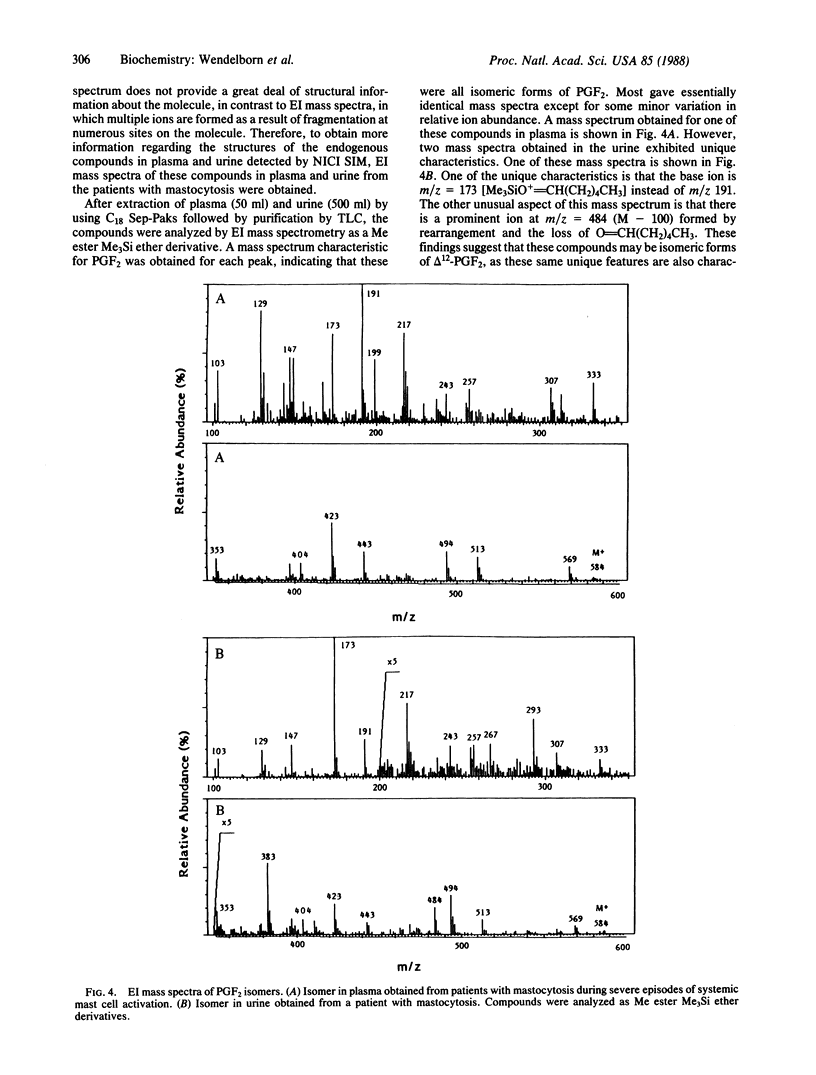
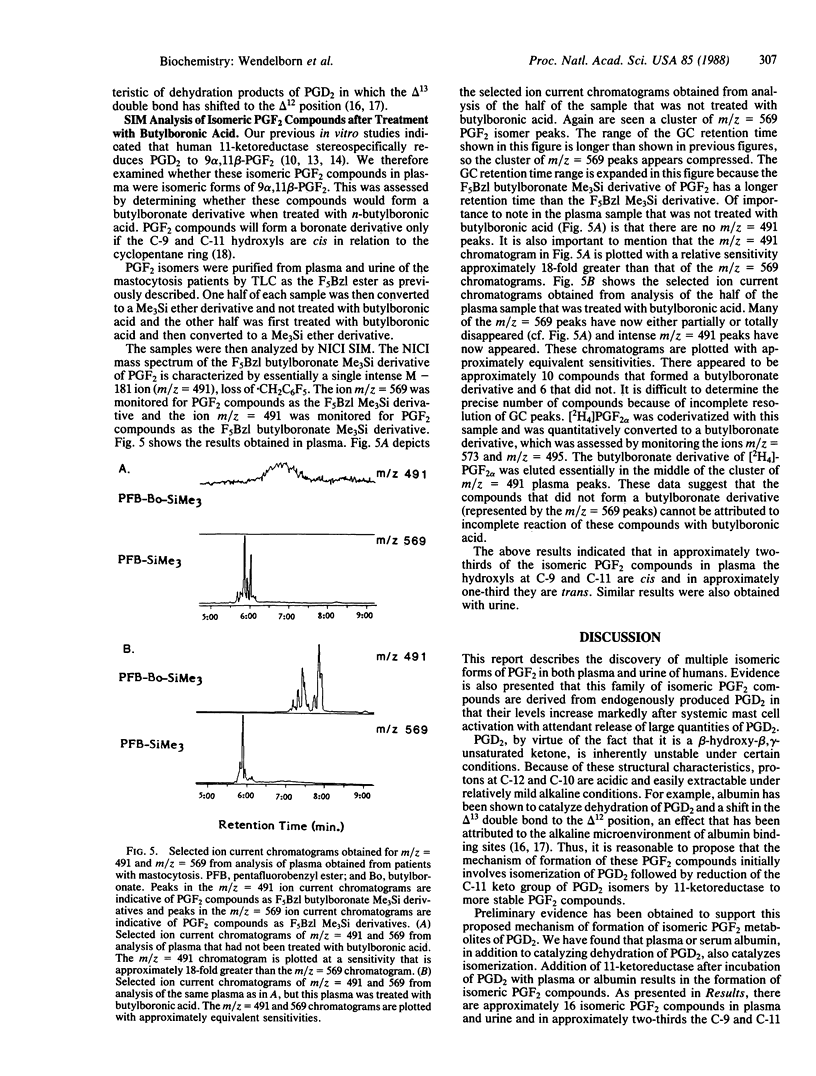
Prostaglandin (PG) D2 has been shown to be transformed by human 11-ketoreductase to 9 alpha,11 beta-PGF2, a biologically active metabolite that is produced in vivo. During the course of developing a mass spectrometric assay for 9 alpha,11 beta-PGF2, several compounds with characteristics similar to PGF2 were detected in both plasma and urine of normal humans by selected ion monitoring. Analysis of pooled plasma obtained from patients with mastocytosis during severe episodes of systemic mast cell activation associated with the release of markedly increased quantities of PGD2 was revealing in that all of these compounds were present in approximately 800-fold greater abundance compared to levels found in normal plasma, suggesting that these compounds arose from PGD2 metabolism. Complete electron impact mass spectra were obtained of these compounds in both plasma and urine; these spectra established that they were all isometric forms of PGF2. Approximately 16 isomeric PGF2 compounds were identified. Treatment with butylboronic acid indicated that the C-9 and C-11 hydroxyls were trans in approximately one-third of the compounds and cis in approximately two-thirds. Preliminary experiments suggest that PGD2 is a very labile compound in vivo and undergoes extensive isomerization, after which reduction by 11-ketoreductase yields a family of more stable isomeric PGF2 compounds. Elucidating the profile of biological activity of these compounds and their mechanism of formation will contribute importantly to our understanding of the biological consequences of PGD2 release in vivo. These results also bring into question the reliability of assays for PGF2 alpha and its metabolites in human biological fluids as a specific index of endogenous PGF2 alpha biosynthesis, as these assays may also measure in part isomeric PGF2 compounds arising from PGD2 metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundy G. L., Morton D. R., Peterson D. C., Nishizawa E. E., Miller W. L. Synthesis and platelet aggregation inhibiting activity of prostaglandin D analogues. J Med Chem. 1983 Jun;26(6):790–799. doi: 10.1021/jm00360a003. [DOI] [PubMed] [Google Scholar]
- Ellis C. K., Smigel M. D., Oates J. A., Oelz O., Sweetman B. J. Metabolism of prostaglandin D2 in the monkey. J Biol Chem. 1979 May 25;254(10):4152–4163. [PubMed] [Google Scholar]
- Fitzpatrick F. A., Wynalda M. A. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J Biol Chem. 1983 Oct 10;258(19):11713–11718. [PubMed] [Google Scholar]
- Hardy C. C., Robinson C., Tattersfield A. E., Holgate S. T. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984 Jul 26;311(4):209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- Kikawa Y., Narumiya S., Fukushima M., Wakatsuka H., Hayaishi O. 9-Deoxy-delta 9, delta 12-13,14-dihydroprostaglandin D2, a metabolite of prostaglandin D2 formed in human plasma. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1317–1321. doi: 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Soter N. A., Diamond P. T., Austen K. F., Oates J. A., Roberts L. J., 2nd Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982 Oct;129(4):1627–1631. [PubMed] [Google Scholar]
- Liston T. E., Roberts L. J., 2nd Metabolic fate of radiolabeled prostaglandin D2 in a normal human male volunteer. J Biol Chem. 1985 Oct 25;260(24):13172–13180. [PubMed] [Google Scholar]
- Liston T. E., Roberts L. J., 2nd Transformation of prostaglandin D2 to 9 alpha, 11 beta-(15S)-trihydroxyprosta-(5Z,13E)-dien-1-oic acid (9 alpha, 11 beta-prostaglandin F2): a unique biologically active prostaglandin produced enzymatically in vivo in humans. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6030–6034. doi: 10.1073/pnas.82.18.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. J., Tonnel A. B., Brash A. R., Roberts L. J., 2nd, Gosset P., Workman R., Capron A., Oates J. A. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986 Sep 25;315(13):800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C., Wolfe L. S. N-butylboronate derivatives of the F prostaglandins. Resolution of prostaglandins of the E and F series by gas-liquid chromatography. J Chromatogr. 1971 Mar 24;56(1):129–133. doi: 10.1016/s0021-9673(00)97786-0. [DOI] [PubMed] [Google Scholar]
- Pugliese G., Spokas E. G., Marcinkiewicz E., Wong P. Y. Hepatic transformation of prostaglandin D2 to a new prostanoid, 9 alpha,11 beta-prostaglandin F2, that inhibits platelet aggregation and constricts blood vessels. J Biol Chem. 1985 Nov 25;260(27):14621–14625. [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Fields J. P., Oates J. A. Mastocytosis without urticaria pigmentosa: a frequently unrecognized cause of recurrent syncope. Trans Assoc Am Physicians. 1982;95:36–41. [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Lewis R. A., Oates J. A., Austen K. F. Prostaglandin thromboxane, and 12-hydroxy-5,8,10,14-eicosatetraenoic acid production by ionophore-stimulated rat serosal mast cells. Biochim Biophys Acta. 1979 Nov 21;575(2):185–192. doi: 10.1016/0005-2760(79)90020-1. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Seibert K., Liston T. E., Tantengco M. V., Robertson R. M. PGD2 is transformed by human coronary arteries to 9 alpha, 11 beta-PGF2, which contracts human coronary artery rings. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:427–429. [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Sweetman B. J., Lewis R. A., Austen K. F., Oates J. A. Increased production of prostaglandin D2 in patients with systemic mastocytosis. N Engl J Med. 1980 Dec 11;303(24):1400–1404. doi: 10.1056/NEJM198012113032405. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Sweetman B. J. Metabolic fate of endogenously synthesized prostaglandin D2 in a human female with mastocytosis. Prostaglandins. 1985 Sep;30(3):383–400. doi: 10.1016/0090-6980(85)90114-5. [DOI] [PubMed] [Google Scholar]
- Seibert K., Sheller J. R., Roberts L. J., 2nd (5Z,13E)-(15S)-9 alpha,11 beta,15-trihydroxyprosta-5,13-dien-1-oic acid (9 alpha,11 beta-prostaglandin F2): formation and metabolism by human lung and contractile effects on human bronchial smooth muscle. Proc Natl Acad Sci U S A. 1987 Jan;84(1):256–260. doi: 10.1073/pnas.84.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Iguchi Y., Iguchi S., Arai Y., Hayaishi O., Roberts L. J., 2nd Stereospecific conversion of prostaglandin D2 to (5Z,13E)-(15S)-9 alpha-11 beta,15-trihydroxyprosta-5,13-dien-1-oic acid (9 alpha,11 beta-prostaglandin F2) and of prostaglandin H2 to prostaglandin F2 alpha by bovine lung prostaglandin F synthase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1583–1587. doi: 10.1073/pnas.83.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]


