Abstract
BACKGROUND
Autism is a heterogeneous neurodevelopmental disorder of unknown etiology. The amygdala has long been a site of intense interest in the search for neuropathology in autism, given its role in emotional and social behavior. An interesting hypothesis has emerged that the amygdala undergoes an abnormal developmental trajectory with a period of early overgrowth in autism; however this finding has not been well established at young ages nor analyzed with boys and girls independently.
METHODS
We measured amygdala volumes on MRI scans from eighty-nine toddlers at 1 to 5 years of age (mean 3 yrs). Each child returned at ~5 years of age for final clinical evaluation.
RESULTS
Toddlers who later received a confirmed autism diagnosis (32 males, 9 females) had a larger right (p<.01) and left (p<.05) amygdala compared to typically-developing toddlers (28 males, 11 females) with and without covarying for total cerebral volume. Amygdala size in toddlers with autism spectrum disorder correlated with the severity of their social and communication impairments as measured on the ADI-R and Vineland. Strikingly, females more robustly differed from typical in amygdala volume whereas males accounted for the significant relationship of amygdala size with severity of their clinical impairments.
CONCLUSIONS
This study provides evidence that the amygdala is enlarged in young children with autism; the overgrowth must begin before 3 years of age and is associated with the severity of clinical impairments. However, neuroanatomical phenotypic profiles differ between males and females, which critically impacts future studies on the genetics and etiology of autism.
Keywords: MRI, temporal, volume, neuroanatomy, development, brain growth
INTRODUCTION
The amygdala has long been a site of intense interest in the search for neuropathological markers for autism, given its well-established role in the production and recognition of emotions and modulatory role in social behavior (1). Initial signs of autism in toddlers include unusual affective behavior, reduced social interest, and poor eye contact (2, 3), which are all suggestive of aberrant amygdala function. In addition, the core behavioral features of the autism diagnosis at four years of age are similarly suggestive of amygdala dysfunction, including impairments in reciprocal social interaction and abnormal development of non-verbal communication (4). In addition, common co-morbid neurological disorders (5, 6) such as epilepsy and anxiety may be attributed to an abnormally functioning amygdala (7, 8).
The heterogeneity and unknown etiologies of autism, variability of analyses employed, and different age groups studied have limited the consistency of results across structural MRI studies of amygdala volume (9). In particular, consideration of subject age has emerged as a critical factor (10). Only three studies have been published on the size of the amygdala in children with autism less than ten years of age, finding the amygdala to be enlarged by ~15% in children with confirmed autism relative to age-matched controls (10–12). However, studies of older adolescents, adults, or a wide age range of subjects, have found either no difference in (13) or even smaller (14–16) amygdala volumes in individuals with autism. In addition, amygdala volume increases throughout adolescence in typically-developing male children (17, 18), but not in male children with autism (10). Thus, an intriguing hypothesis has emerged that the amygdala is initially larger than normal in children with autism, and then does not undergo the same age-related increase in volume that takes place in typically-developing children. This hypothesis parallels the general theory of early brain overgrowth in young children with autism (19, 20), although the aberrant growth trajectory of the amygdala may extend to a later age of development relative to cortical regions (10). Several questions remain: At what age in development does the amygdala become enlarged in children with autism? Is the enlargement related to the degree of social and emotional impairments? Do males and females with autism differ in their neuroanatomical phenotype?
The objectives of the current study were to measure the volume of the amygdala in toddlers at risk for autism compared to age-matched typically-developing children at the age of first clinical detection, to evaluate the potential relationship between amygdala size and the severity of behavioral impairments at final clinical outcome, and to characterize the neuropathological and behavioral profiles of males and females independently.
METHODS
Diagnostic Assessment
Eighty nine toddlers (n= 66 males, 23 females) between the ages of 18–60 months were included in amygdala volumetric analyses as part of a longitudinal MRI study of early brain development in autism (Table 1). A parent or guardian gave informed consent for participation in this research program as approved by the Institutional Review Board of Rady Children’s Hospital San Diego and the University of California, San Diego.
TABLE 1.
Subject and diagnostic data at final clinical visit.
| Autism (n= 41) | PDD-NOS (n= 9) | Typical (n= 39) | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Number of subjects | 32 | 9 | 6 | 3 | 28 | 11 |
| Mean Age at MRI (mo) | 36 ± 7.2 | 36 ± 4.7 | 36 ± 9.1 | 56 ± 6.1 | 34 ± 7.1 | 37 ± 6.4 |
| Age range at MRI (mo) | 22–54 | 26–44 | 25–50 | 49–61 | 20–51 | 23–44 |
|
Mean Age at Final Clinical Visit (mo) |
49 ± 1.1 | 49 ± 9.7 | 59 ± 9.8 | 50 ± 7.9 | 46 ± 1.3 | 46 ± 1.2 |
| Full Scale IQ | 58 ± 20 | 57 ± 23 | 93 ± 32 | 63 ± 19 | 111 ± 17 | 115 ± 15 |
| Verbal IQ | 50 ± 20 | 50 ± 29 | 82 ± 27 | 56 ± 13 | 113 ± 19 | 115 ± 15 |
| Performance IQ | 65 ± 19 | 62 ± 21 | 104 ± 35 | 72 ± 31 | 109 ± 20 | 113 ± 16 |
| ADI Social | 19 ± 6 | 18 ± 6 | 14 ± 5 | 15 ± 2 | n/a | n/a |
| ADI NV Comm. | 10 ± 3 | 9 ± 3 | 6 ± 3 | 7 ± 5 | n/a | n/a |
| ADI RnR | 6 ± 2 | 5 ± 2 | 5 ± 3 | 4 ± 2 | n/a | n/a |
| ADOS Soc | 10 ± 3 | 11 ± 2 | 5 ± 2 | 5 ± 2 | n/a | n/a |
| ADOS Comm. | 6 ± 2 | 7 ± 2 | 4 ± 2 | 5 ± 1 | n/a | n/a |
| ADOS SBRI | 3 ± 1 | 3 ± 2 | 1 ± 1 | 4 ± 2 | n/a | n/a |
| Vineland Soc | 66 ± 9 | 64 ± 7 | 78 ± 9 | 71 ± 8 | 97 ± 8 | 102 ± 15 |
| Vineland Comm. | 66 ± 11 | 62 ± 16 | 84 ± 16 | 69 ± 11 | 108 ± 11 | 105 ± 9 |
| Vineland ABC | 63 ± 8 | 64 ± 11 | 74 ± 14 | 67 ± 15 | 102 ± 10 | 105 ± 12 |
Provisionally autistic 12–36 month old children were initially recruited by clinician referral, through presentations at local autism support group meetings, and by letter distributed at agencies (directed to parents of children with autism who may have younger siblings). Typically-developing young children were recruited via notices given to local preschools, magazine advertisements, and referral from parents already participating.
At entry into the study between 12–36 months of age, children received a diagnostic evaluation by a research psychologist (CCB) with extensive clinical experience with autism. During the initial visit, the Mullen Scales of Early Learning (21) was administered as a standardized measure of early cognition. Toddlers (n= 63 males, 18 females) who displayed symptoms of autism were assigned to a “provisional autism spectrum disorder” (p-ASD) group, and administered the Autism Diagnostic Observation Schedule (ADOS) (22, 23); their parents completed the Autism Diagnostic Interview (ADI-R) (24). The ADOS is a semi-structured standardized assessment of communication, social interaction, and play for young children to evaluate presence of autism spectrum disorder. It provides a diagnostic classification of autism, autism spectrum, or non-spectrum based on the child’s presentation during the assessment. The ADI-R is a standardized comprehensive parent interview designed to assess, confirm, or deny an autism spectrum disorder through queries closely associated with the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (4). Toddlers (n= 28 males, 11 females) who presented age-equivalent performance on the Mullen Scales and showed no characteristics of autism were judged as typically-developing and placed in the “control” group. Toddlers who showed delays on the standardized cognitive assessment but did not meet criteria for autism spectrum were not included in the current analyses. Each child returned at ~36 months of age for an MRI scan and again at ~48 months of age for the final clinical evaluation. At the final visit, the ADOS, ADI-R, either the Mullen or the Wechsler Preschool and Primary Scale of Intelligence-III (25) (depending on child’s age), and the Vineland Adaptive Behavioral Scales Survey Interview (26) were administered. The Vineland is a standardized parent or caregiver interview to asses a child’s functional skills in four different developmental domains; this was administered to both the control and p-ASD groups. Only the exit ADI-R (standardized for age 4) and Vineland scores were used for statistical and correlation analyses in this study.
At 48 months of age or older, children in the p-ASD group were given a final diagnosis (by CCB and CL) of Autistic Disorder (n= 32 males, 9 females), Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) (n= 6 males, 3 females), or Non-Spectrum based on the exit ADI-R and final ADOS (Table 1). Fifteen males and 2 females with a provisional autism diagnosis had at least one successful MRI scan but dropped from the study before final diagnosis could be determined. Ten males and 4 females in the p-ASD group that completed the final diagnostic assessment were considered non-spectrum at final diagnosis. Of these, 4 males and 2 females were given a developmental delay diagnosis and the others were mixed non-spectrum (e.g. ADHD, receptive-expressive language disorder). Neither participants that dropped prior to final diagnosis nor those that did not meet criteria for autism spectrum disorder at final evaluation were included in Table 1 or in amygdala volumetric analyses.
Neuroimaging
All MRI scans were collected at the UCSD Medical Center, Hillcrest on a 1.5 Tesla Siemens Symphony system. Thirty-three p-ASD (25 autism, 8 PDD-NOS) study participants received sedation (Propofol) to undergo MRI. None of the typically-developing controls underwent anesthesia. All non-sedation scans were performed at night during natural sleep. The protocols for scanning each participant included a three-dimensional T1-Weighted MPRAGE (128 coronal slices, matrix 224×256, 0.859375 mm×0.859375 mm in-plane×1.5 mm slice thickness, TE = 3.67 ms, TR = 2730 ms). Although this study was part of a larger longitudinal study of brain development in autism and many subjects in this cohort received scans at multiple time points, only the scan collected closest to 36 months of age (Table 1) was used for the current analysis of amygdala volume.
Scans were transferred to the laboratory where each MPRAGE sequence was first used to measure total cerebral volume (TCV), which was comprised of total cortical gray and white matter volumes and excluded subcortical structures. The boundaries of the cerebrum were manually defined as previously described in detail (27, 28) using the software program AREA (29).
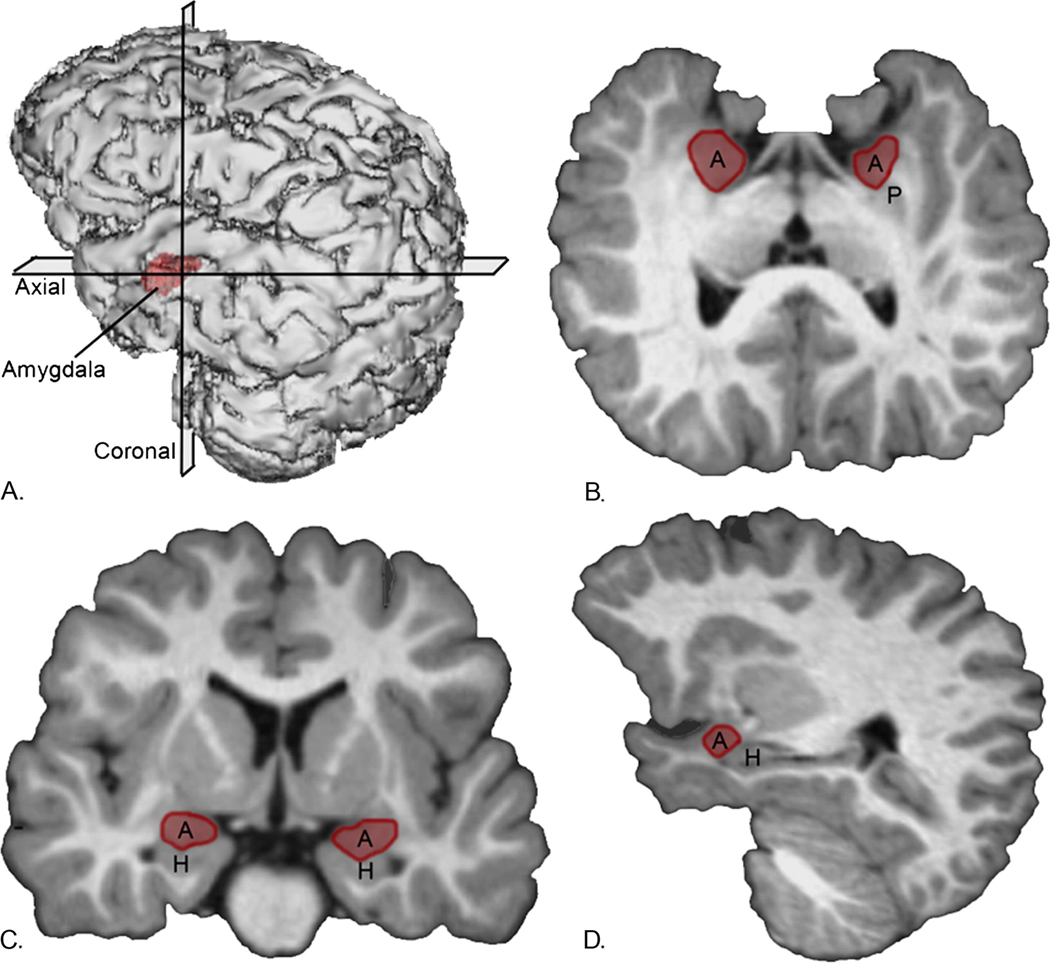
To measure amygdala volume, each raw MPRAGE sequence was imported into the program Analyze 8.1 (30) and processed in an identical manner to procedures described in Schumann et al. (10). Briefly, images were converted to .5mm cubic voxel dimensions and reoriented along the horizontal axis from rostral to caudal pole of the hippocampus to aid in distinguishing amygdala from the hippocampus (Figure 1a). Two blinded raters manually traced the amygdala after establishing reliability on the MRI scans of 20 subjects with an inter-rater reliability correlation of >.94 and an intra-rater reliability correlation of >.95. The initial tracing process involved defining the borders in coronal sections starting with the most caudal level in which the amygdala was visible to the approximate most rostral section in which the amygdala was present (Figure 1b). Outlines were then verified and edited in the horizontal (axial) and sagittal views that were simultaneously available to the rater while tracing (Figure 1c, d).
Figure 1.
Three-dimensional reconstruction of images (a) in which lines indicate the position though the cerebrum of the coronal plane (b), sagittal plane (c), and transverse (horizontal) plane (d). A, amygdala; H, hippocampus; P, putamen.
Statistics
Statistical analyses were performed with Statistical Program for the Social Sciences Edition 16.0 software (SPSS, Chicago, IL). Differences between study diagnostic groups for age of the participant at the time of the MRI scan and clinical scores were tested using ANOVA. An ANCOVA was performed to compare amygdala volume between the autism and control groups after adjusting for the covariates of gender and TCV. The ANCOVA was then repeated for males and females separately. In order to detect a potential relationship of amygdala volume with clinical diagnostic scores; partial correlation analyses were carried out independently for two participant groups: 1) autism spectrum disorder (ASD), which included subjects with a confirmed diagnosis of autism and those with PDD-NOS, and 2) typically-developing age-matched controls. The age of the participant at the time of scan and at the final clinical visit were considered covariates. Males and females were also analyzed independently.
RESULTS
Clinical diagnostic and behavioral measures collected at final clinical visit are listed in Table 1. There was no difference in the age of the groups at the time of MRI acquisition. There was, as expected, a significant group effect for all IQ and Vineland measures (p<.001).
Mean volumetric data for all participants are given in Table 2. Amygdala volumes in all groups significantly correlated with age at time of scan (r=.49, p<.05); however there was no age×group effect for autism and controls and therefore age was not included as a covariate in further volumetric comparisons. ANCOVA with gender as a covariate revealed increased right (F(1,77)=12.39, p=.001) and left (F(1,77)=6.83, p=.01) amygdala volume in the autism group relative to controls. There was no significant difference in TCV between the autism and control groups and no TCV×group interaction. When gender and TCV were included as covariates, right (F(1,76)=10.19, p=.002) and left (F(1,76)=4.95, p=.03) amygdala volumes were significantly enlarged in the autism group relative to controls. Amygdala volumes in subjects with PDD-NOS were not analyzed independently since there were too few subjects to justify a separate group.
TABLE 2.
Volumetric measures (in cubic centimeters) ± standard deviations for all participants by diagnostic group and gender
| Autism (n= 41) | PDD-NOS (n= 9) | Typical (n= 39) | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Right Amygdala | 1.84 ± .19* | 1.47 ± .09** | 1.62 ± .06 | 1.45 ± .14 | 1.73 ± .16 | 1.31 ± .07 |
| Left Amygdala | 1.77 ± .21 | 1.40 ± .14** | 1.62 ± .12 | 1.39 ± .16 | 1.69 ± .15 | 1.26 ± .07 |
| Total Cerebral | 1090 ± 81 | 1046 ± 67 | 1079 ± 88 | 1015 ± 104 | 1071 ± 88 | 1003 ± 78 |
(p<0.05;
p<0.01 autism subjects significantly differed from gender-matched controls). PDD-NOS subjects were not included in volumetric comparisons due to limited sample size.
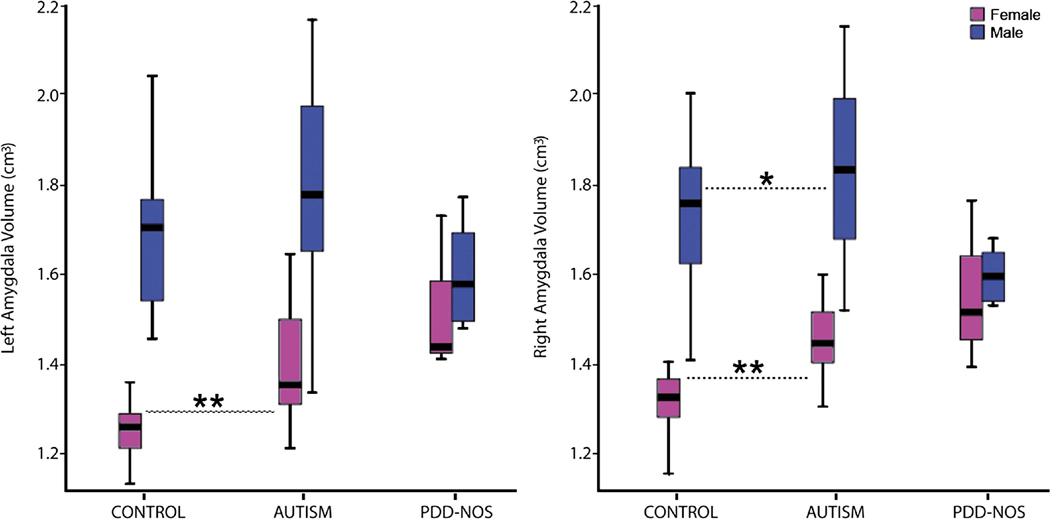
When males and females were analyzed separately (Figure 2), males with autism had a significantly larger right (F(1,58)=6.46, p=.014) and a trend toward a larger left (F(1,58)=3.12, p=.08) amygdala volume compared to age-matched typically-developing males. There was a medium effect size of .63 and .44 for left and right amygdala volume, respectively, indicating sufficient power to detect a difference between autism and control male groups. When TCV was included as a covariate, right (F(1,57)=5.65, p=.021) amygdala volume remained significantly enlarged in males with autism compared to control.
Figure 2.
Amygdala volume (in cubic centimeters) for all participants by diagnostic group (*p<0.05; **p<0.01 significantly different from gender matched controls). Length of box is the interquartile range computed from Tukey’s hinges. Line inside box is median and whiskers represent entire value range.
Females with autism had a significantly larger right (F(1,18)=16.71, p=.001) and left (F(1,18)=8.98, p=.008) amygdala volume compared to age-matched typically-developing females. When TCV was included as a covariate, right (F(1,17)=13.23, p=.002) and left (F(1,17)=6.74, p=.019) amygdala volume remained significantly enlarged in females with autism compared to control.
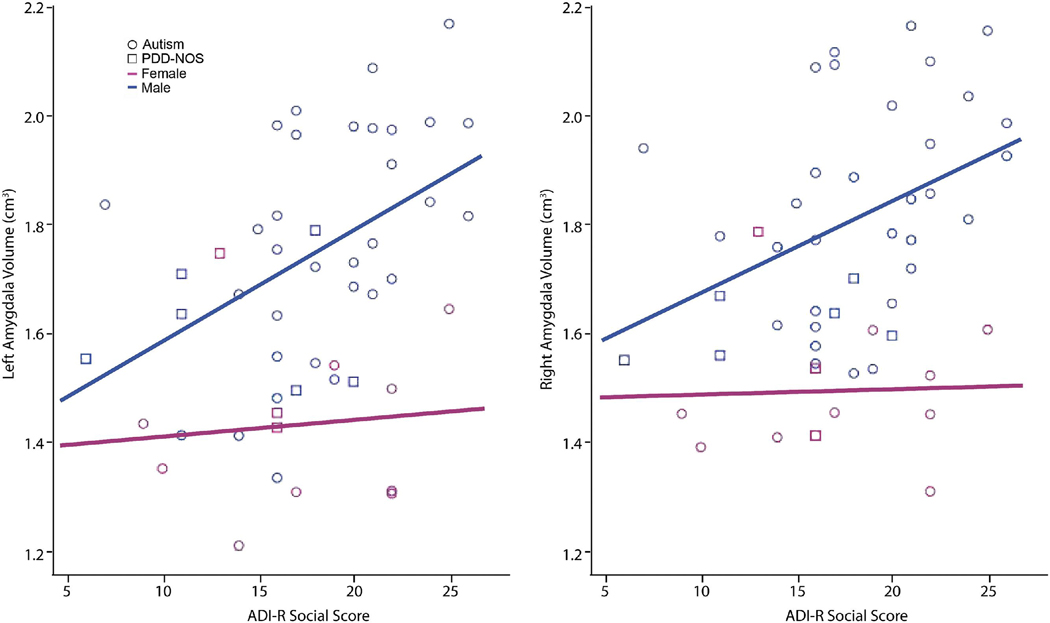
For analyses of the relationship of amygdala volume to clinical scores, the autism and PDD-NOS groups were combined to form an autism spectrum disorder (ASD) group. There were significant age at scan×group and age at final clinical evaluation×group interactions for ASD relative to control; therefore ages at scan and at the time of clinical evaluation were included as covariates. Partial correlation analyses, with gender and ages as covariates, revealed a significant correlation of the ADI-R Social (A) score of ASD participants with their right (r=.44, p=.002) and left (r=.48, p=.001) amygdala volumes. When gender was analyzed separately, right (r=.52, p=.001) and left (r=.57, p<.001) amygdala volumes were correlated with the ADI-R Social scores of ASD males (Figure 3). The ADI-R Nonverbal Communication (BNV) score also significantly correlated with the left amygdala volume (r=.48, p=.004) in ASD males. ASD females did not show detectable correlations between amygdala volume and any clinical measures. ASD amygdala volumes, either combined or for males and females independently, did not correlate with the ADI-R Restricted, Repetitive, and Stereotyped Patterns of Behavior score. Total cerebral volume did not significantly correlate with any of the measures in any groups, but showed a trend toward correlating with the ADI Social score (p=.08) and Repetitive Behavior score (p=.07) for ASD subjects covaried for age at scan, age at diagnosis, and gender.
Figure 3.
Linear regression scatter plots showing a positive correlation for right (r=.52, p=.001) and left (r=.57, p<.001) amygdala volume (in cubic centimeters) and ADI-R Social score in ASD males.
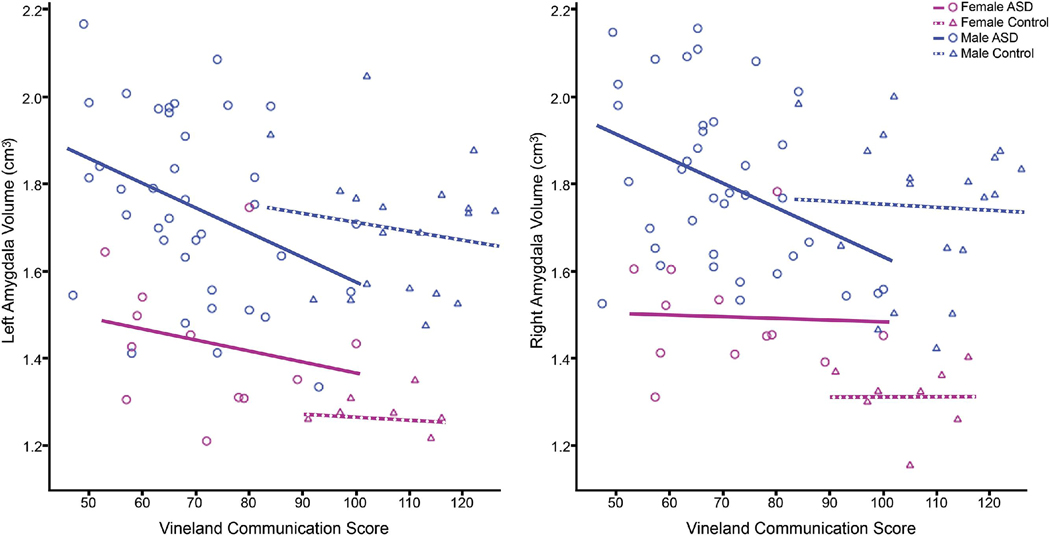
When all subjects were analyzed together and covaried for gender, age at scan, and age at final clinical visit on the Vineland, there was a significant negative correlation of communication score with right (r=−.31, p<.01) and left (r=−.30, p<.01) amygdala volume (Figure 4) and the social score with right (r=−.31, p<.01) and left (r=−.31, p<.01) amygdala volume. When controls were analyzed independently, they did not show significant correlations between amygdala volume and any clinical measures. When ASD subjects were analyzed independently, they did show a significant negative correlation in Vineland Communication and Social scores and right (r=−.31, p<.05) and left (r=−.30, p<.05) amygdala volumes. In ASD males, but not ASD females, there was a significant negative correlation in Vineland Communication score and right (r=−.38, p<.05) and left (r=.34, p<.05) amygdala volume. The Vineland Social score also negatively correlated with right (r=−.35, p<.05) and left (r=−.34, p=.05) amygdala volume in ASD males, but not ASD females.
Figure 4.
Linear regression scatter plots showing a positive correlation for right (r=-.38, p<.05) and left (r=.34, p<.05) amygdala volumes (in cubic centimeters) and Vineland Communication score in ASD males.
DISCUSSION
This study provides evidence that the amygdala is enlarged in young children with autism and that the overgrowth must begin before 3 years of age at about the time symptoms become clinically evident. This finding is consistent with early overgrowth found in cortical regions (19, 20), but differs in that the amygdala may remain enlarged later into childhood than the rest of the brain (10). Additionally, the enlargement in right amygdala volume in males and females and left amygdala volume in females is disproportionate to total cerebral volume at 3 years of age. In male toddlers who eventually receive a diagnosis of autism spectrum disorder, the degree of amygdala enlargement at that early age is associated with the severity of their social and communication impairments at final clinical evaluation at ~5 years of age. This is also similar to findings from other brain regions, in which enlargement at 3–5 years of age is associated with the severity of symptoms at 5–8 years of age (31).
Our study is the first to report the striking finding that amygdala enlargement in females with autism is more severe, compared to age- and gender-matched typical-developing counterparts, than in males with autism. Indeed, a significant difference was present despite a group size of only nine females with autism. Interestingly, unlike ASD males, the enlargement in ASD females was not related to the severity of social and communication impairments, although this may simply reflect the modest sample size. Previous studies (32) have also found more robust differences in cortical volumes in females than males. Although speculative at present, this suggests that autistic males may be a more heterogeneous group in amygdala volume, which varies with the degree of clinical impairment, compared to autistic females who may have a more homogeneous neuropathological profile of an enlarged amygdala regardless of the degree of their behavioral impairment. This finding awaits confirmation from a larger study on females with autism, but also raises further questions regarding gender differences: Does amygdala enlargement in females represent a completely different neuropathological process or is the same process altered by other components of the social brain in females with autism? Might pathology in different brain regions be associated with the social behavior impairments in females with ASD?
Our findings of an enlarged amygdala are similar to that of Sparks and colleagues (12) and the recently published study by Mosconi and colleagues (11), which to date are the only other published studies on amygdala volume in young children under 5 years of age with a confirmed diagnosis of autism. In the Sparks study, the 4 year-old participants with autism had enlarged amygdala volumes bilaterally compared to age-matched typically-developing children. In the Mosconi study, bilateral amygdala enlargement was observed in children with autism at both 2 and 4 years of age compared to a control group that consisted of typical and developmentally-delayed children. However neither study analyzed males and females with a confirmed diagnosis of autism independently; Sparks et al. did analyze females with ASD and did not find a difference in amygdala volume compared to controls, but this small group of n=7 females included only three with autism. These differences limit the viability of a direct comparison between studies. Munson et al. (33) examined the relationship between amygdala volume and the severity of clinical outcome in the Sparks et al (12) cohort. Similar to our findings, they reported that a larger right amygdala volume at 3–4 years of age was associated with more severe social and communication impairments on the ADI and Vineland and poorer outcome at 6 years of age. However, they did not evaluate males and females independently.
One inherent limitation of this study is that the autism group had significantly lower cognitive abilities as demonstrated with lower IQ scores than the control group. It is possible that pathology in the amygdala is not specific to autism but common to mental retardation. This is doubtful, though, since studies that include subjects with mental retardation without autism (12, 34, 35) indicate that the amygdala is not enlarged, as we find in our autism sample, but instead are similar in size to typically-developing controls. Therefore the findings of the current study are unlikely to be driven solely by low cognitive ability in children with autism.
The current study taken together with similar previously published studies confirms that the amygdala is enlarged in young children with autism (10, 12, 36) and that the period of enlargement appears to be limited to early development, due to the continued growth of the amygdala which increases in size by 40% from 5–18 years of age in typically-developing males (17, 18). Therefore, by adolescence, amygdala size in the typically-developing child has caught up with, and likely surpassed, amygdala size in the child with autism (10). Studies of older populations have found that amygdala volume is smaller in autism relative to age-matched typical controls (14–16). In one study by Nacewicz and colleagues (15), a smaller amygdala volume was associated with gaze avoidance and more severe behavioral impairments as measured with the ADI-R. This relationship is the opposite pattern of that observed in younger children in the current study as well as Munson et al (33). It remains unknown if autism subjects that demonstrate early overgrowth also demonstrate reduced amygdala size in adulthood, as preliminarily suggested by these findings; this awaits confirmation from a longitudinal study on brain growth throughout development in people with autism.
How might pathology in the amygdala relate to specific behavioral abnormalities? We and others (15, 33) have found that the volume of the amygdala in individuals with ASD is associated with the severity of their social and non-verbal impairments as measured on the ADI and Vineland. The correlation analyses carried out in the current study are considered exploratory and few specific inferences may be drawn linking amygdala pathology to specific behaviors scored on these tests, as each score is comprised of several behavioral measures. Additionally, there is considerable overlap between the behaviors that comprise the social and non-verbal communication scores on both algorithms. Both scores include amygdala-associated behaviors such as observing social smiling, gaze direction, facial expressions, imaginative and imitative social play and seeking to share enjoyment with others. Other lines of research, such as functional imaging in older children and adults with autism, provide some evidence of an abnormal pattern of amygdala activation in response to social stimuli.
In Pierce and colleagues (37), and Schultz (38), it was suggested that abnormalities in the amygdala in autism, and diminished attention to faces early on (39), may be the first in a cascade of problems that lead to later emotional and social impairments. An emerging hypothesis is that the amygdala may play a role in mediating or directing visual attention to the eye region of the face to detect emotion or danger (1). However, autism subjects show abnormal visual scan paths during eye-tracking studies when viewing faces, typically spending little time on core social features such as the eyes (40, 41). As mentioned earlier, Nacewicz et al. (15) found that individuals with autism (8–25 years of age) who had a smaller amygdala also spent the least amount of time fixating on the eye region of the face. It is unclear whether this finding in people with autism reflects a lack of motivation and/or anxiety to look at the eyes for social and emotional cues. Pierce and colleagues (37) found that when autistic subjects viewed familiar faces, they were able to activate the amygdala appropriately in response to both familiar and unfamiliar faces, suggesting that the familiar faces may have enhanced motivation or attention to all of the stimuli. Dalton and colleagues (42) utilized functional imaging and eye-tracking technology simultaneously while showing subjects familiar and unfamiliar faces; the amount of time persons with autism spent looking at the eye region of the face was strongly positively correlated with amygdala activation, but not in typically-developing control subjects. This suggests a heightened emotional, or even fearful, response when autistic individuals look at another person’s eyes, regardless of whether they are familiar or a stranger. Spezio et al. (43) confirmed that participants with autism show less fixation on the eyes and mouth, but also a greater tendency to saccade away from the eyes when information was present in those regions. Recently, Kleinhans and colleagues (44) found evidence of reduced habituation to faces in their autism spectrum group; the degree of reduction was related to the severity of social impairments, which lends further evidence for the theory that the amygdala is hyper-aroused in people with autism in response to socially relevant stimuli.
The amygdala, with its dense reciprocal connections with the visual stream (45), modulates many levels of visual processing that in turn may influence the development of early preference for faces seen in typical newborns (46). However, the amygdala is just one of several structures that work in parallel to produce normal social cognition, one of the core impairments in autism. The amygdala, when presented with a socially demanding situation, evaluates and allocates resources to those stimuli that pose a potential threat. Structures such as the fusiform gyrus, frontal cortex, cingulate cortex, somatosensory cortex, and the superior temporal gyrus also have specialized roles in the broader system of social behavior (47) and the detection of potential neuropathology in these regions, as well as how each might be influenced by the amygdala, is an important direction for future study.
To conclude, we found an enlarged amygdala volume in toddlers with autism; the degree of amygdala enlargement at that age was associated with the severity of social and communication impairments at five years of age. The amygdala was disproportionately enlarged relative to total brain volume in the toddlers with autism. Males and females differed in their neuropathological and behavioral profiles, where females more robustly differed from typical in amygdala volume and males showed a significant relationship of amygdala size with the severity of their social and communication impairments. However, many factors contribute to the volume of a structure, including the number and size of neurons and glia, dendritic arborization, myelination, vasculature, and so forth, and therefore more research is required to explain the unusual amygdala growth trajectory that has been further supported by our findings. It is clear, though, that the age and gender of the participants should be considered essential factors in evaluating and interpreting findings of all future volumetric studies of autism. These findings will have a major impact on defining the neural phenotypes of males and females with autism that is critical for the next phase of etiological and genetic phenotyping research.
Acknowledgements
We are grateful to the participants and their families for years of dedication to this research program. In addition, we appreciate the technical assistance of Mindy Kim, Trudy Kao and Jarnet Han in image collection and processing, as well as manuscript preparation. We also thank Dr. Natasha Akshoomoff and Dr. Ruth Carper for initial recruitment and screening of the toddlers and Graham Wideman for technological support. This research program was supported by an ROI grant to Eric Courchesne (NIH/NINDS # R01-NS-019855) and the Autism Center of Excellence (NIH/NICHD # 1P50MH081755-01).
Footnotes
Disclosures
Dr. Lord receives royalties from the ADI-R and ADOS; as per agreement with the University of Michigan, all proceeds go to charity. The other authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Development. 1998;69:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- 3.Werner E, Dawson G, Osterling J, Dinno N. Brief report: Recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. J Autism Dev Disord. 2000;30:157–162. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 5.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Pitkanen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 9.Schumann CM, Amaral DG. The Human Amygdala and Autism. In: Whalen P, Phelps E, editors. The Human Amygdala. New York: Guilford Press; 2009. [Google Scholar]
- 10.Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of neuroscience. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparks BF, Friedman SD, Shaw DW, Aylward E, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 13.Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 14.Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 15.Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce K, Müller R-A, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform 'face area' in autism: Evidence from fMRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 17.Giedd JN. Normal development. Child and Adolescent Psychiatric Clinics of North America. 1997;6:265–282. [Google Scholar]
- 18.Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 19.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 20.Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Mullen EM. Mullen Scales of Early Learning. AGS ed. MN: American Guidance Service Inc.; 1995. [Google Scholar]
- 22.DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. Journal of Autism and Developmental Disorders. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- 23.Lord C, Risi S, Lambrecht L, Cook E, Jr, Leventhal B, DiLavore P, et al. The Autism Diagnostic Observation Schedule-Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-III. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- 26.Sparrow S, Balla D, Cicchetti D. Vineland scales of adaptive behavior: survey form manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- 27.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 28.Courchesne E, Karns C, Davis HR, Ziccardi R, Carper R, Tigue Z, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 29.Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Archives of Neurology. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- 30.Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- 31.Akshoomoff N, Lord C, Lincoln A, Courchesne RY, Carper R, Townsend J, et al. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry. 2004;43:349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2007;46:515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- 33.Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, et al. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- 34.Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. Amygdala and hippocampal volumes in children with Down syndrome: a high-resolution MRI study. Neurology. 2001;56:972–974. doi: 10.1212/wnl.56.7.972. [DOI] [PubMed] [Google Scholar]
- 35.Zeegers M, Pol HH, Durston S, Nederveen H, Schnack H, Daalen EV, et al. No differences in MR-based volumetry between 2- and 7-year-old children with autism spectrum disorder and developmental delay. Brain Dev. 2008 doi: 10.1016/j.braindev.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Mosconi MW, Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. A longitudinal study of amygdala volume and joint attention in 2–4 year old children with autism. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2009.19. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 38.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- 40.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 41.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 42.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal Use of Facial Information in High-Functioning Autism. J Autism Dev Disord. 2006 doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- 44.Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 45.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 46.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns' preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 47.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]