Abstract
Partial EN (enteral nutrition) should always be aimed for in patients with renal failure that require nutritional support. Nevertheless PN (parenteral nutrition) may be necessary in renal failure in patient groups with acute or chronic renal failure (ARF or CRF) and additional acute diseases but without extracorporeal renal replacement therapy, or in patients with ARF or CRF with additional acute diseases on extracorporeal renal replacement therapy, haemodialysis therapy (HD), peritoneal dialysis (PD) or continuous renal replacement therapy (CRRT), or in patients on HD therapy with intradialytic PN. Patients with renal failure who show marked metabolic derangements and changes in nutritional requirements require the use of specifically adapted nutrient solutions. The substrate requirements of acutely ill, non-hypercatabolic patients with CRF correspond to those of patients with ARF who are not receiving any renal replacement patients therapy (utilisation of the administered nutrients has to be monitored carefully). In ARF patients and acutely ill CRF patients on renal replacement therapy, substrate requirements depend on disease severity, type and extent/frequency of extracorporeal renal replacement therapy, nutritional status, underlying disease and complications occurring during the course of the disease. Patients under HD have a higher risk of developing malnutrition. Intradialytic PN (IDPN) should be used if causes of malnutrition cannot be eliminated and other interventions fail. IDPN should only be carried out when modifiable causes of malnutrition are excluded and enhanced oral (like i.e. additional energy drinks) or enteral supply is unsuccessful or cannot be carried out.
Keywords: acute renal failure, chronic renal failure, haemodialysis, peritoneal dialysis
Abstract
Patienten mit Nierenversagen sollten, wenn möglich, eine zumindest partielle enterale Ernährung erhalten. Trotzdem kann bei bestimmten Patientengruppen mit Nierenversagen eine PE (parenterale Ernährung) notwendig werden, wie z.B. bei Patienten mit akutem bzw. chronischem Nierenversagen (ANV bzw. CNV) und zusätzlichen akuten Erkrankungen ohne extrakorporalen Nierenersatz, bei Patienten mit ANV und Patienten mit CNV und zusätzlichen akuten Erkrankungen unter extrakorporaler Nierenersatztherapie, Hämodialysetherapie (HD), Peritonealdialyse (PD) oder kontinuierlicher Nierenersatztherapie (CRRT) sowie bei Patienten unter HD Therapie mit intradialytischer parenteraler Ernährung. Bei Patienten mit Nierenversagen machen umfangreiche metabolische Störungen und Änderungen des Nährstoffbedarfes die Verwendung von spezifisch adaptierten Nährlösungen notwendig. Bei akut-kranken nicht-hyperkatabolen Patienten mit CNV entspricht der Substratbedarf dem der Patienten mit ANV ohne Nierenersatztherapie (die Verwertung der zugeführten Nährstoffe muss sorgfältig überprüft werden). Bei Patienten mit ANV und akut-kranken Patienten mit CNV unter Nierenersatztherapie wird der Substratbedarf vom Schweregrad der Erkrankung, Art und Dosis der extrakorporalen Nierenersatzverfahren, dem Ernährungszustand, der Grunderkrankung und von im Krankheitsverlauf auftretenden Komplikationen bestimmt. Patienten unter einer HD haben ein hohes Risiko, eine Mangelernährung auszubilden. Eine intradialytische PE (IDPE) sollte nur dann durchgeführt werden, wenn „beseitigbare” Ursachen einer Mangelernährung ausgeschlossen wurden und wenn eine diätetische/orale (z.B. Trinknahrung) oder enterale Therapie nicht vorgenommen werden kann bzw. nicht erfolgreich ist.
Preliminary remark
In patients with renal failure, enteral nutrition (EN) should be the primary choice for nutrition therapy, and administered whenever possible (see also recommendations by the German Society of Nutrition (DGEM) on enteral nutrition [1]). However, patients with renal failure often have limitations in enteral intake, and therefore, the administration of a quantitatively sufficient EN becomes impossible. Dysfunction of gastric and intestinal motility is observed in patients even with moderately abnormal renal function and often restricts the a amount of EN tolerated. Therefore, in clinical practice many acutely ill patients with renal failure require temporary and/or supplementary parenteral nutrition (PN). Even if PN is required, one should always aim for establishind at least a minimal enteral nutrition to enhance intestinal integrity [1].
The nutritional status has a massive impact on the prognosis of patients with renal failure [2]. In acutely ill patients, however, the degree of malnutrition is not the only indication to start PN/EN, but also when the patient can not be sufficiently orally/enterally nourished, and the degree of severity of the underlying illness and the associated catabolism [3].
Only few systematic studies have performed on parenterally fed patients with renal failure, and only very few controlled studies with an acceptable study design have been published. Therefore, recommendations for practice for this patient group only reach the level of an expert opinion (C).
General recommendations for nutrition and nutritional requirements of patients with renal diseases have been published by the National Kidney Foundation (NKF) [4], as well as by the European Society for Parenteral and Enteral Nutrition (ESPEN) [5], [6].
Parenteral nutrition in patients with renal failure
At least partial EN should always be aimed for in patients with renal failure that require nutritional support (C).
PN may be necessary in renal failure in the following patient groups (C).
Patients with acute or chronic renal failure (ARF or CRF) and additional acute diseases but without extracorporeal renal replacement therapy.
Patients with ARF or CRF with additional acute diseases on extracorporeal renal replacement therapy, haemodialysis therapy (HD), peritoneal dialysis (PD) or continuous renal replacement therapy (CRRT).
Patients on HD therapy with intradialytic PN.
Commentary
PN is administered almost solely to patients with acute (ARF) or chronic renal failure (CRF), or those on chronic renal replacement therapy (HD or PD), who also have acute diseases. ARF or CRF is not a crucial factor for PN administration in intensive care patients, but rather it is important whether the patient requires extracorporeal therapy [HD] or continuous renal replacement therapy (CRRT), as well as the presence of hypercatabolism depending on the severity of disease.
Aims of PN in patients with renal failure
PN in patients with renal failure aims at reduction of the hypercatabolic state, and the prevention or elimination of malnutrition and related functions, such as immunology, wound healing, antioxidative potential, inflammation. While delaying the progress of CRF through protein or phosphate restriction is the aim of chronic dietary therapy, this is not the goal of short-term PN, which is usually administered only in acute situations.
Metabolic situation in patients with renal failure
Patients with renal failure who show marked metabolic derangements and changes in nutritional requirements require the use of specifically adapted nutrient solutions (C).
Commentary
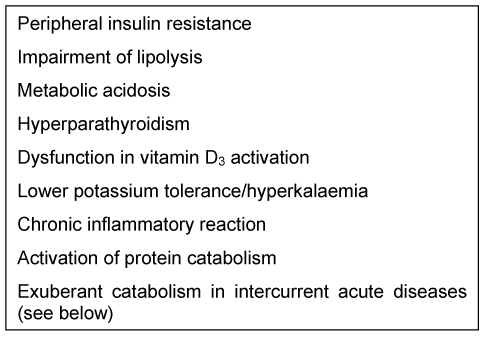
Metabolism in patients with renal failure is not only influenced by abnormal renal function, but also by the underlying disease, emerging complications and additional organ failure (Table 1 (Tab. 1)). Metabolism and nutrient balance are also influenced by the type and intensity of extracorporeal renal replacement therapy [7]. Distinctive changes in metabolism and substrate requirements of renal failure patients make it necessary to adapt PN according to their needs [7] (Table 1 (Tab. 1)).
Table 1. Metabolic disorders in patients with renal failure.
Energy metabolism is not markedly influenced by renal dysfunction but may be altered by underlying disease and accompanying complications. In multiple organ failure, energy expenditure is only about 30% above the calculated resting metabolic rate [8]. An increase in energy intake of more than 30 kcal/kg/day was not associated with any further improvement in nitrogen balance, but resulted in increased metabolic complications [9].
The metabolic changes in ARF are mainly characterised by protein catabolism. There are alterations of the metabolism of individual amino acids, including the utilisation of exogenously administered amino acids is altered, and various non-essential amino acids, e.g. tyrosine, may become indispensible.
Dysfunctions in carbohydrate metabolism in ARF usually manifest clinically through hyperglycaemia. This is mainly caused by peripheral insulin resistance, and in addition by an activation of hepatic gluconeogenesis that cannot be suppressed by exogenous nutrient intake, other than in stable CRF and healthy persons (“obligatory” negative nitrogen balance).
Altered lipid metabolism is characterised by hypertriglyceridemia explained by the suppressed lipolysis. Fat clearance is delayed after enteral or parenteral intake [10].
Patients with ARF or patients with CRF and acute diseases have a markedly reduced antioxidative potential [11].
Activation of vitamin D3 is also impaired in ARF resulting in secondary hyperparathyroidism [12].
Influence of renal replacement therapy on metabolic and nutrient balances
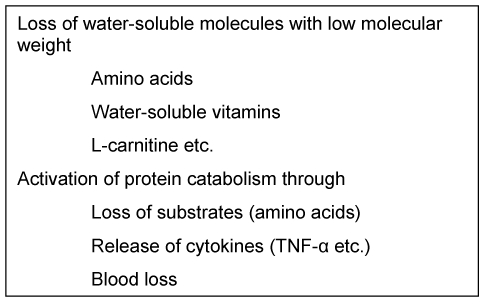
Metabolic changes due to haemodialysis are shown in Table 2 (Tab. 2). Currently continuous renal replacement therapy (CRRT), especially continuous venovenous haemofiltration (CVVH), is usually used in intensive care patients. The continuous therapy mode and the usually high filtration rates result in significant influences on electrolyte and nutrient balance, especially when losses are not sufficiently replaced [13]. The loss of amino acids is approximately 0.2 g per litre of filtrate. Other substances such as water-soluble vitamins are also lost. Complications can also occur through excessive lactate or citrate intake through the dialysate fluid (hyperlactaemia, metabolic alkalosis).
Table 2. Effects of haemodialysis.
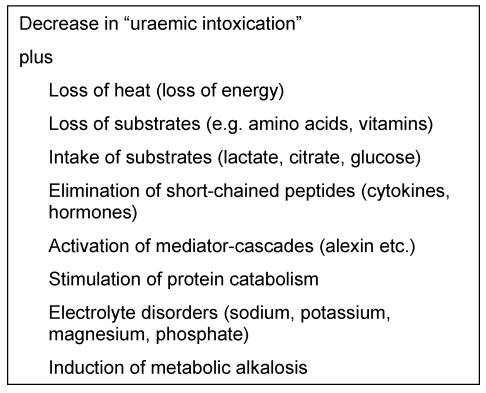
Electrolyte metabolic disorders such as hypophosphataemia, hypomagnesaemia and/or hyponatraemia are frequently observed due to the high fluid turnover required for CRRT [13] (Table 3 (Tab. 3)).
Table 3. Effects of continuous renal replacement therapy.
Parenteral nutrition substrates for patients with renal failure
Amino acid solutions
Special amino acid solutions for patients with renal failure (so-called “nephro-solutions”) show beneficial effects on some surrogate parameters, but effects on clinical end points are not documented (IV).
Solutions providing exclusively essential amino acids should no longer be used (A).
Commentary
Renal failure influences the amino acid or protein metabolism, and changes the utilisation of intravenously administered amino acids [14]. However, the question as to which parenteral amino acid solution should be used in these patients remains controversial. Potential advantages of specially adapted amino acid solutions (“nephro-solutions”) include assured intake of an adequate (higher) dose of amino acids without urea increase, partial/total correction of imbalances in plasma aminograms and the intake of amino acids that become essential due to renal failure (i.e. tyrosine as dipeptide) [15], [16]. Previously used solutions providing exclusively essential amino acids should not be used [7].
Clinical advantages such as improved survival rates have not been documented with the use of “nephro-solutions” (cf. chapter “Amino acids” http://www.egms.de/en/journals/gms/2009-7/000083.shtml). Benefits are most likely in patients who do not require dialysis and whose urea increase can be reduced. In patients who require renal replacement therapy, the advantages are expeceted to be less relevant because haemodialysis and haemofiltration have a “smoothing” effect on the plasma aminogram. There are no advantages in using solutions enriched with branched-chain amino acids in intensive care patients or patients with ARF [17].
In 1982 Mirtallo et al. administered either a standard amino acid solution (AA intake 33 g/day) or a solution comprised exclusively of essential amino acids (AA intake 29 g/day) to patients with non-dialysis CRF. Various parameters in the nitrogen balance tended to a slight improvement when the standard solution was used [18].
In a controlled study, Smolle et al. compared a conventional amino acid solution with a “nephro-solution” in patients with ARF [16]. The modified solution resulted in a normalisation of plasma amino acid concentrations and the phenylalanine/tyrosine ratio.
Lipid emulsions
Patients with renal failure should receive lipid emulsions with a triglyceride dose of up to 1 g/kg body weight/day in PN with regular montoring of plasma triglycerides (C).
Commentary
The first step in lipid utilisation is the lipolysis of the infused triglycerides which is reduced in renal failure, with impaired fat clearance in patients with ARF and CRF [10]. Oxidation of the released fatty acids is not impaired. Lipid emulsions can be used in PN but the dose should not exceed ~1 g/kg body weight/day. Regular monitoring of plasma triglycerides should be performed. At present there are no studies documenting any advantages of specific lipid emulsions over others in patients with renal failure (cf. chapter “Lipid emulsions” http://www.egms.de/en/journals/gms/2009-7/000081.shtml).
Carbohydrates
Parenteral carbohydrates should be provided by glucose in patients with renal failure (C).
Normoglycaemia should be the goal during PN. Insulin is frequently required for maintaining normoglycaemia in these patients who often show insulin resistance (A).
Commentary
Advantages of using parenteral glucose substitutes patients with renal failure are not shown, but their use might be associated with significant disadvantages. Glucose substitutes are partly metabolised in the kidney and increase renal oxygen consumption [19].
Normoglycaemia should be maintained in patients with renal failure when using PN [20]. Insulin is frequently required to maintain normoglycaemia as these patients often show insulin resistance.
L-carnitine
The administration of L-carnitine (500 mg/day) is justified in malnourished and critically-ill patients on renal replacement therapy (and thus increased loss) (C).
Commentary
Whether L-carnitine should be regarded as an essential substrate in patients with renal failure has not yet been clarified. “Carnitine responders” are mainly malnourished patients [4]. Good prospective studies and evidence-based dosing specifications are not available.
Vitamins
Fat-soluble vitamins
Patients with chronic renal failure require an individually dosed pharmacological therapy with vitamin D3 or its analogues in addition to the standard intake with fat-soluble vitamins in PN (C).
Patients with CRF and acute concomitant diseases as well as patients with ARF have an increased requirement of vitamin E (C).
Commentary
Patients with renal failure have dysfunctional activation of vitamin D. For this reason activated vitamin D3 or its analogues should be administered. The substitution of vitamin K exceeding daily basal requirements is not necessary. In contrast to stable patients with CRF, the levels of vitamin E and vitamin A are lower in acutely ill patients with renal failure, which results in the need for substitution [12]. Systematic substitution studies are, however, not available.
Water-soluble vitamins
Patients on renal replacement therapy and malnourished patients without renal replacement therapy should receive approximately double the normal daily requirements of water-soluble vitamins with PN (C).
An increased intake of vitamin C (>250 mg/day) can be disadvantageous and result in increased oxalate formation (B).
Commentary
Patients with renal failure require an increased dose of water-soluble vitamins. In patients on renal replacement therapy, the dosage should amount to approximately 2-fold the daily requirements due to additional losses through therapy [21], [22], [23].
The vitamin C supplementation should also be higher than the recommended daily intake of healthy persons, but should not exceed 250 mg/day, in order to prevent possible secondary oxalosis. Excessive vitamin C intake/supplementation can even cause ARF itself [24].
Trace elements
Patients on renal replacement therapy and malnourished patients without renal replacement therapy should receive the recommended daily intake of trace elements in PN (C).
Selenium intake should be >200 µg/day in patients on renal replacement therapy (B).
Commentary
Renal balance studies of trace elementsare not available except for selenium, hence, in accordance with other patient groups supplementation should correspond to the recommended daily intake of healthy persons [3].
Selenium is an exception, as approximately double the daily intake is lost through continuous renal therapy, despite its high protein binding properties. Therefore, an increased supplementation should be given [21] (B).
Electrolytes
Electrolyte intake should be individually determined in patients with renal failure (C).
Commentary
Potassium and phosphate restriction is generally recommended in patients with renal failure. The requirements are, however, extremely different for acutely ill patients. Hypokalaemia or hypophosphataemia may occur initially in the disease course. A fast decline in potassium or phosphate levels may also occur in patients with renal failure after commencing PN (“Refeeding hypophosphataemia or hypokalaemia”). The individual electrolyte requirement can vary tremendously during the course of disease and is crucially influenced by residual diuresis.
PN in patients with ARF/CRF without renal replacement therapy
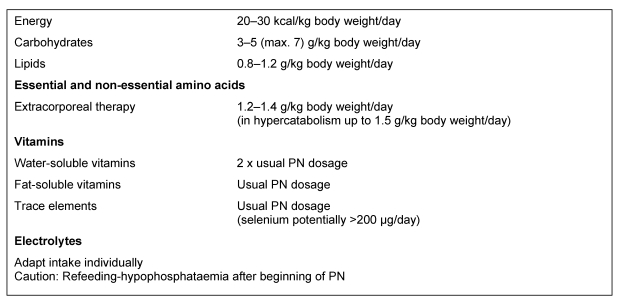
The substrate requirements of acutely ill, non-hypercatabolic with CRF correspond to those of patients with ARF who are not receiving any renal replacement patients therapy (C) (Table 4 (Tab. 4)).
In patients with renal failure, PN should be started slowly (approximately 50% of the requirements) in order to monitor the utilisation of the administered nutrients and prevent metabolic imbalances (C).
Table 4. Recommended parenteral nutrient intakes in ARF/CRF without renal replacement therapy (see [1], [3], [4], [5], [6]).
Commentary
Patients with CRF or ARF, who do not require renal replacement therapy, are rarely catabolic, whereas dialysis is necessary in increased catabolism due to the associated increase of urea. Only a few of these patients require PN. The substrate requirements of acutely ill, non-hypercatabolic patients with CRF correspond to those of patients with ARF without renal replacement therapy (Table 4 (Tab. 4)).
Renal failure patients with uraemia develop malnutrition, related to the stage of the disease. The reasons for this are manifold and comprise lower oral food intake, restrictive diet regime, the toxic effects of uraemia, inflammation, metabolic acidosis, endocrine factors such as insulin resistance, hyperparathyroidism, altered leptin levels, as well as gastroplegia, malassimilation and other gastrointestinal alterations [25].
PN in patients with ARF/CRF and renal replacement therapy
In ARF patients and acutely ill CRF patients on renal replacement therapy, substrate requirements depend on disease severity, type and extent/frequency of extracorporeal renal replacement therapy, nutritional status, underlying disease and complications occurring during the course of the disease (for recommendations for medium substrate intake see Table 5 (Tab. 5)) (C).
Table 5. Recommended parenteral substrate intakes in patients with ARF and in acutely ill patients on renal replacement therapy.
Commentary
Critically ill patients with ARF are by far, the largest group of patients with renal failure who require parenteral nutrition therapy. ARF is rarely a mono-organ failure; usually, additional complications like severe infections, sepsis or multi-organ failure also occur. In these patients, ARF is only one factor that determines the need for and type of nutritional therapy (cf. chapter “Intensive medicine” http://www.egms.de/en/journals/gms/2009-7/000073.shtml).
Altered water and electrolyte balance, specific metabolic disorders, gastrointestinal motility and influence of extracorporeal therapies on metabolic and substrate balances should be considered during planning, execution and monitoring of PN in renal failure patients.
Patients on intermittent haemodialysis therapy with accompanying acute diseases should be assessed metabolically like patients with ARF, and treated similarly with regards to nutritional therapy. The specifications made here for ARF can, therefore, be used for both these patient groups.
Indications for nutritional therapy
The same recommendations apply for PN therapy of patients with ARF as for other intensive care patients. Nutritional state is a significant determinant of outcome in ARF patients, smilar to patients with other acute diseases. PN is indicated in patients with ARF without pre-existing malnutrition who will not be able receive sufficient oral or enteral nutrition for approximately 5 days. This often applies to ARF patients because underlying diseases resulting in ARF tend to impair intestinal motility which often limits quantitatively sufficient enteral nutrition [26]. In addition to duration of not meeting needs by oral or enteral supply, the extent of malnutrition and the severity of underlying diseases are main determinants of the indication and timing for starting PN therapy.
Parenteral substrate requirements in patients with ARF on renal replacement therapy
The requirement of amino acids/protein is increased (A).
The requirement of water-soluble vitamins is increased (A).
The requirement of other micro nutrients (fat-soluble vitamins, trace elements) should be met (A).
Electrolyte intake should be dosed individually for patients with renal failure (C).
Commentary
The substrate requirements are determined less by presence or absence of ARF, but more by the severity of the disease, type and dose of the extracorporeal renal replacement therapy, underlying diseases, and complications occurring during the course of the disease. Tolerance to excessive substrate intakes (i.e. amino acids, trace elements, vitamins) is reduced and overdoses should thus be avoided because the regulatory functions of the kidneys are missing in ARF.
(See section on “Influence of renal replacement therapy on metabolism and nutrient balances” for vitamin and trace element requirements.)
Amino acid requirements in patients with ARF on renal replacement therapy
The amino acid requirements of patients with ARF on renal replacement therapy depend on the extent of the underlying disease and the intensity of renal replacement therapy (B).
Sufficient amino acid intake must be assured (B).
Commentary
The ideal amino acid intake in these patients is controversial and has not been properly clarified through studies. There are no randomised studies with an adequate study design.
There is, however, consensus that protein restriction, which was previously recommended in analogy to CRF with a minimal intake rate of approximately 0.6 g of AA/ kg/day, is not indicated in acutely ill patients.
Studies from the 1990s have dealt with the extent of catabolism or the optimal intake. In numerous studies a “protein catabolic rate” of 1.4 to 1.75 g/kg/day was found in patients with ARF under CRRT [17], [27], [28], [29]. In these studies, a protein intake of this magnitude was recommended which is consistent with the available information. Taking into account the amino acid loss through CRRT of approximately 0.2 g/kg/day, this intake is similar to the recommended intake of other intensive care patients (cf. chapter “Intensive medicine” http://www.egms.de/en/journals/gms/2009-7/000073.shtml).
Recent studies from Australia [30], [31], [32] have suggested that the amino acid/protein intake in intensive care patients with ARF on CRRT should reach up to 2.5 g/kg/day. The authors found an increase in the plasma amino acid levels and an enhanced nitrogen balance which correlated with an improves outcome. The study design, however, did not permit any conclusions on causality regarding improved outcomes through increased amino acid intake.
Protein catabolism cannot be suppressed by an increased amino acid intake in acute diseases. Potential dangers of increased intake in patients with ARF are enhanced uraemic toxicity and increased requirements of extracorporeal therapy. It unclear why patients with ARF should have much higher amino acid requirements than other intensive care patients, after adjustment of therapy-related loss.
Admixture of glutamine to PN in critically ill patients with ARF
Glutamine intake should be avoided in non-dialysis patients due to its high nitrogen content (C).
In patients with ARF on renal replacement therapy, glutamine intake may be considered (C).
Commentary
The question of whether glutamine should be added to the PN solution in critically ill patients with ARF has not been adequately answered. A post-hoc analysis of the study of Griffith et al. has determined that the benefits of glutamine supplementation were particularly marked in patients with ARF on renal replacement therapy [33]. The administration of glutamine should be calculated into the amino acid intake, and the dose of renal replacement therapy adapted.
Metabolic monitoring in patients with ARF
Metabolic monitoring of nutritional therapy in ARF patients should be performed similar to monitoring in other intensive care patients, but more stringently (C).
In particular electrolyte balance must be checked (frequent source of error!) (C).
Commentary
In patients with ARF there is a restricted tolerance to volume intake and electrolytes, and impairment in the metabolism of various substrates resulting in a high risk of complications in nutrition therapy. More stringent metabolic monitoring of nutrition therapy is, therefore, necessary for this patient group as compared to other patients. Slow introduction of nutritional therapy reduces the risk of occurrence of metabolic complications.
Influence of parenteral nutrition on the regeneration of renal function
Various nutrients can influence renal reparation and different aspects of renal function. Both parenterally and enterally administered amino acids increase renal blood flow as well as creatinine clearance (“renal reserve”). However, amino acids have been found to be toxic for the kidney.
There are three aspects to consider:
An unfavourable effect of AA on renal function and the course of ARF (“AA paradox”) has been described in animal experiments [34]. This is only relevant when amino acids are infused in a higher dose at the time of the insult, thus increasing renal oxygen requirements, but appears insignificant in the clinical situation. In contrast, it has been shown that various amino acids like alanine, glycine, taurine and, particularly, arginine have a protective effect on the kidneys, prevent ARF or may delay the progression of CRF [7]. The question as to what extent PN improves renal reparation has not yet been determined. Abel et al. suggested an improvement but this has not been confirmed in further studies [35]. However, experimentally substrate deficiency aggravates renal damage in ARF.
Both parenterally and enterally administered amino acids increase renal blood flow and also creatinine clearance (“renal reserve”). An influence of this effect on renal function in ARF has only been studied in animal experiments [36], whereas clinical observations have only been reported in abstract form.
Intradialytic parenteral nutrition (IDPN)
Patients under haemodialysis (HD) have a higher risk of developing malnutrition (A).
Commentary
The close connection between nutritional status and complications or outcome is well documented for patients receiving chronic haemodialysis therapy. Malnutrition in dialysis patients has numerous causes; a significant factor is anorexia leading to intakes below requirements [25], [37].
Light to moderate malnutrition is found in approximately 30% of dialysis patients, and severe malnutrition in 5–10% [38]. Dietary interventions alone seem to have only limited effect. Dialysis itself is a catabolic state caused not only by losses of nutrients such as amino acids, but also by activation of protein catabolism which lasts for a few hours after the end of dialysis. Isotops studies indicated that the catabolic state of HD can be converted to an anabolic state through intradialytic nutrient supply (Table 2 (Tab. 2)) [39], [40].
Strategies for the treatment of malnutrition in HD patients
Strategies for the treatment of malnutrition are summarised in Table 6 (Tab. 6). In malnourished HD-patients with inadequate oral food intake, attempts can be made to motivate the patients to accept energy drinks during the HD therapy which leads to an increased nutrient intake in some patients (see recommendation in “Enteral Nutrition Guidelines” [1]). Intradialytic PN (IDPN) should be used if causes of malnutrition cannot be eliminated and other interventions fail.
Table 6. Malnutrition in HD patients – possibilities for intervention.
Indications for intradialytic PN (IDPN)
IDPN should only be carried out when modifiable causes of malnutrition are excluded and enhanced oral or enteral supply is unsuccessful or cannot be carried out (C).
Commentary
The following international criteria for malnutrition have been suggested, even though they are not based on firm evidence [41]:
Middle predialysis serum albumin 3 months
Middle predialysis serum creatinine 3 months
Weight loss >10% of ideal body weight or >20% of normal body weight (no time limit)
Clinical examination indicates moderate to severe malnutrition
Dietary history indicating protein intake <0.8 g/kg, reduced calorie intake <25 kcal/kg
Subjective Global Assessment (SGA) “C”= severe malnutrition
IDPN should be considered when three of the above mentioned criteria are associated with the following conditions:
Aborted attempts to increase oral/enteral food intake
Refusal of enteral gavage
Compounding and completion of IDPN
The nutrient solution should be continuously infused into the venous drip chamber of the tube system throughout the complete duration of dialysis (B).
Depending on the compounding of the nutrient solution, blood glucose, triglycerides and possibly even the phosphate/potassium concentrations should be checked during the first treatment (C).
Commentary
In many studies, only amino acid solutions have been administered as IDPN, although some studies have infused only slightly higher amounts than the 2 g/h loss due to dialysis. Recent studies have coinfused amino acids, glucose and lipids together with pre-mixed solutions from the pharmacy or commercial ready-made solutions. In some countries the concept of a complete nutrient solution is practised, where water-soluble vitamins, carnitine and, if necessary, electrolytes are added to an all-in-one bag containing the three basic nutrients of amino acids/glucose/lipids. Preparation of a nutrient solution on an individual basis, for every single patient, as occurs in some countries, is extremely expensive and has no documented advantages compared to standardised admixtures.
IDPN necessitates a compromise between the desire to infuse an adequate amount of nutrients and a limited time-frame in which these can be infused. The following issues should be considered when deciding about the infusion amount, even though evidence-based recommendations are not available as of now (C).
Amino acids: Desirable intake >0.5 g/kg/dialysis. “Nephro” solutions have been used in more recent studies [42].
Glucose: Limits are set due to the short infusion time and the frequently existing glucose intolerance, recommended intake 50–100 g/dialysis. Insulin must be given with higher doses of glucose or to diabetics.
Lipids: Limitations exist due to dysfunction in lipolysis. An intake between 20 and 40 g of lipids/dialysis appears appropriate in order to prevent hypertriglyceridemia, in contrast to higher dose recommendations by French authors.
Double the usual daily dose of water-soluble vitamins should be given, and in severely malnourished patients carnitine should also be given.
The dialysis-related substrate loss is not considerably increased by the infusion [43].
Studies on IDPN
Approximately 25 studies have been published on IDPN, however, with very different indications, nutrient solutions and nutrient inatkes, and lengths of therapy. Most studies were cohort studies without a control group; some were retrospective and others used “run-in” periods prior to the intervention. Only the study by Cano et al. was controlled but did not include a placebo [44].
Almost all studies have shown significant improvements in different parameters; including anthropometry (body weight, mid arm circumference, triceps skinfolds), serum proteins, (albumin, total protein, transferrin, prealbumin), plasma amino acid concentrations, lymphocyte cells and immune reactivity. An influence on survival was determined only in two studies, but firm conclusions cannot be drawn because of limitaions in study design [45], [46]. A multicenter study from France is currently being completed [47].
Abbreviations
ARF: acute renal failure
CAPD: chronic ambulant peritoneal dialysis
CRF: chronic compensated renal failure
CRRT: continuous renal replacement therapy
HD: chronic haemodialysis therapy
IDPN: intradialytic parenteral nutrition
MAC: mid-arm circumference
TSF: triceps skinfold
BW: body weight
PN: parenteral nutrition
Notes
This article is part of the publication of the Guidelines on Parenteral Nutrition from the German Society for Nutritional Medicine (overview and corresponding address under http://www.egms.de/en/journals/gms/2009-7/000086.shtml).
English version edited by Sabine Verwied-Jorky, Rashmi Mittal and Berthold Koletzko, Univ. of Munich Medical Centre, Munich, Germany.
References
- 1.Lochs HL, Weimann A. DGEM-Leitlinie Enterale Ernährung. [DGEM Guidelines Enteral Nutrition]. Akt Ernahrungsmed. 2003;28:S1–S120. (Ger). [Google Scholar]
- 2.Fiaccadori E, Lombardi M, Leonardi S, Rotelli CF, Tortorella G, Borghetti A. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10(3):581–593. doi: 10.1681/ASN.V103581. [DOI] [PubMed] [Google Scholar]
- 3.Druml W. Nutritional management of acute renal failure. J Ren Nutr. 2005;15(1):63–70. doi: 10.1053/j.jrn.2004.09.012. Available from: http://dx.doi.org/10.1053/j.jrn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Clinical practice guidelines for nutrition in chronic renal failure; K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35(6 Suppl 2):S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 5.Toigo G, Aparicio M, Attman PO, Cano N, Cianciaruso B, Engel B, Fouque D, Heidland A, Teplan V, Wanner C. Expert working group report on nutrition in adult patients with renal insufficiency (Part 2 of 2) Clin Nutr. 2000;19(4):281–291. doi: 10.1054/clnu.2000.0129. Available from: http://dx.doi.org/10.1054/clnu.2000.0129. [DOI] [PubMed] [Google Scholar]
- 6.Toigo G, Aparicio M, Attman PO, Cano N, Cianciaruso B, Engel B, Fouque D, Heidland A, Teplan V, Wanner C. Expert Working Group report on nutrition in adult patients with renal insufficiency (part 1 of 2) Clin Nutr. 2000;19(3):197–207. doi: 10.1054/clnu.1999.0130. Available from: http://dx.doi.org/10.1054/clnu.1999.0130. [DOI] [PubMed] [Google Scholar]
- 7.Druml W. Nutritional support in patients with acute renal failure. In: Molitoris BA, Finn WF, editors. Acute renal failure: A Companion to Brenner & Rector's "The Kidney". Philadelphia: WB Saunders; 2001. pp. 465–489. [Google Scholar]
- 8.Schneeweiss B, Graninger W, Stockenhuber F, Druml W, Ferenci P, Eichinger S, Grimm G, Laggner AN, Lenz K. Energy metabolism in acute and chronic renal failure. Am J Clin Nutr. 1990;52(4):596–601. doi: 10.1093/ajcn/52.4.596. [DOI] [PubMed] [Google Scholar]
- 9.Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Picetti E, Parenti E, Meschi T, Borghi L, Tagliavini D, Cabassi A. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant. 2005;20(9):1976–1980. doi: 10.1093/ndt/gfh956. Available from: http://dx.doi.org/10.1093/ndt/gfh956. [DOI] [PubMed] [Google Scholar]
- 10.Druml W, Fischer M, Sertl S, Schneeweiss B, Lenz K, Widhalm K. Fat elimination in acute renal failure: long-chain vs medium-chain triglycerides. Am J Clin Nutr. 1992;55(2):468–472. doi: 10.1093/ajcn/55.2.468. [DOI] [PubMed] [Google Scholar]
- 11.Metnitz GH, Fischer M, Bartens C, Steltzer H, Lang T, Druml W. Impact of acute renal failure on antioxidant status in multiple organ failure. Acta Anaesthesiol Scand. 2000;44(3):236–240. doi: 10.1034/j.1399-6576.2000.440304.x. Available from: http://dx.doi.org/10.1034/j.1399-6576.2000.440304.x. [DOI] [PubMed] [Google Scholar]
- 12.Druml W, Schwarzenhofer M, Apsner R, Hörl WH. Fat-soluble vitamins in patients with acute renal failure. Miner Electrolyte Metab. 1998;24(4):220–226. doi: 10.1159/000057374. Available from: http://dx.doi.org/10.1159/000057374. [DOI] [PubMed] [Google Scholar]
- 13.Druml W. Metabolic aspects of continuous renal replacement therapies. Kidney Int Suppl. 1999;72:S56–S61. doi: 10.1046/j.1523-1755.56.s72.1.x. Available from: http://dx.doi.org/10.1046/j.1523-1755.56.s72.1.x. [DOI] [PubMed] [Google Scholar]
- 14.Druml W, Fischer M, Liebisch B, Lenz K, Roth E. Elimination of amino acids in renal failure. Am J Clin Nutr. 1994;60(3):418–423. doi: 10.1093/ajcn/60.3.418. [DOI] [PubMed] [Google Scholar]
- 15.Druml W, Roth E, Lenz K, Lochs H, Kopsa H. Phenylalanine and tyrosine metabolism in renal failure: dipeptides as tyrosine source. Kidney Int Suppl. 1989;27:S282–S286. [PubMed] [Google Scholar]
- 16.Smolle KH, Kaufmann P, Fleck S, Lueger A, Mausser G, Pölz W, Kleinberger G, Krejs GJ. Influence of a novel amino acids solution (enriched with the dipeptide glycyl-tyrosine) on plasma amino acid concentration of patients with acute renal failure. Clin Nutr. 1997;16(5):239–246. doi: 10.1016/S0261-5614(97)80035-0. Available from: http://dx.doi.org/10.1016/S0261-5614(97)80035-0. [DOI] [PubMed] [Google Scholar]
- 17.Kierdorf HP. The nutritional management of acute renal failure in the intensive care unit. New Horiz. 1995;3(4):699–707. [PubMed] [Google Scholar]
- 18.Mirtallo JM, Schneider PJ, Mavko K, Ruberg RL, Fabri PJ. A comparison of essential and general amino acid infusions in the nutritional support of patients with compromised renal function. JPEN J Parenter Enteral Nutr. 1982;6(2):109–113. doi: 10.1177/0148607182006002109. Available from: http://dx.doi.org/10.1177/0148607182006002109. [DOI] [PubMed] [Google Scholar]
- 19.Kehrer G, Blech M, Kallerhoff M, Kleinert H, Bretschneider HJ. Intraischemic metabolic effects of different disaccharides on protected canine kidneys. Urol Res. 1989;17(6):371–376. doi: 10.1007/BF00510529. Available from: http://dx.doi.org/10.1007/BF00510529. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. Available from: http://dx.doi.org/10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 21.Berger MM, Shenkin A, Revelly JP, Roberts E, Cayeux MC, Baines M, Chioléro RL. Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am J Clin Nutr. 2004;80(2):410–416. doi: 10.1093/ajcn/80.2.410. [DOI] [PubMed] [Google Scholar]
- 22.Story DA, Ronco C, Bellomo R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med. 1999;27(1):220–223. doi: 10.1097/00003246-199901000-00057. Available from: http://dx.doi.org/10.1097/00003246-199901000-00057. [DOI] [PubMed] [Google Scholar]
- 23.Fortin MC, Amyot SL, Geadah D, Leblanc M. Serum concentrations and clearances of folic acid and pyridoxal-5-phosphate during venovenous continuous renal replacement therapy. Intensive Care Med. 1999;25(6):594–598. doi: 10.1007/s001340050908. Available from: http://dx.doi.org/10.1007/s001340050908. [DOI] [PubMed] [Google Scholar]
- 24.Mashour S, Turner JF, Jr, Merrell R. Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest. 2000;118:561–563. doi: 10.1378/chest.118.2.561. Available from: http://dx.doi.org/10.1378/chest.118.2.561. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80(2):299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 26.Druml W, Mitch WE. Enteral nutrition in renal disease. In: Rombeau JL, Rolandelli RH, editors. Clinical nutrition: Enteral and tube feeding. Philadelphia: WB Saunders; 1997. pp. 439–461. [Google Scholar]
- 27.Leblanc M, Garred LJ, Cardinal J, Pichette V, Nolin L, Ouimet D, Geadah D. Catabolism in critical illness: estimation from urea nitrogen appearance and creatinine production during continuous renal replacement therapy. Am J Kidney Dis. 1998;32(3):444–453. doi: 10.1053/ajkd.1998.v32.pm9740161. Available from: http://dx.doi.org/10.1053/ajkd.1998.v32.pm9740161. [DOI] [PubMed] [Google Scholar]
- 28.Chima CS, Meyer L, Hummell AC, Bosworth C, Heyka R, Paganini EP, Werynski A. Protein catabolic rate in patients with acute renal failure on continuous arteriovenous hemofiltration and total parenteral nutrition. J Am Soc Nephrol. 1993;3(8):1516–1521. doi: 10.1681/ASN.V381516. [DOI] [PubMed] [Google Scholar]
- 29.Macias WL, Alaka KJ, Murphy MH, Miller ME, Clark WR, Mueller BA. Impact of the nutritional regimen on protein catabolism and nitrogen balance in patients with acute renal failure. JPEN J Parenter Enteral Nutr. 1996;20(1):56–62. doi: 10.1177/014860719602000156. Available from: http://dx.doi.org/10.1177/014860719602000156. [DOI] [PubMed] [Google Scholar]
- 30.Bellomo R, Seacombe Bapplsci J, Daskalakis M, Farmer M, Wright C, Parkin G, Boyce N. A prospective comparative study of moderate versus high protein intake for critically ill patients with acute renal failure. Ren Fail. 1997;19(1):111–120. doi: 10.3109/08860229709026265. Available from: http://dx.doi.org/10.3109/08860229709026265. [DOI] [PubMed] [Google Scholar]
- 31.Scheinkestel CD, Adams F, Mahony L, Bailey M, Davies AR, Nyulasi L, Tuxen DV. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition. 2003;19(9):733–740. doi: 10.1016/S0899-9007(03)00107-2. Available from: http://dx.doi.org/10.1016/S0899-9007(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 32.Scheinkestel CD, Kar L, Marshall K, Bailey M, Davies A, Nyulasi L, Tuxen DV. Prospective randomized trial to assess caloric and protein needs of critically ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition. 2003;19(11-12):909–916. doi: 10.1016/S0899-9007(03)00175-8. Available from: http://dx.doi.org/10.1016/S0899-9007(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13(4):295–302. [PubMed] [Google Scholar]
- 34.Zager RA. Amino acid hyperalimentation in acute renal failure: a potential therapeutic paradox. Kidney Int Suppl. 1987;22:S72–S75. [PubMed] [Google Scholar]
- 35.Abel RM, Beck CH, Jr, Abbott WM, Ryan JA, Jr, Barnett GO, Fischer JE. Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose: Results of a prospective, double-blind study. N Engl J Med. 1973;288(14):695–699. doi: 10.1056/NEJM197304052881401. [DOI] [PubMed] [Google Scholar]
- 36.Pons M, Plante I, LeBrun M, Gourde P, Simard M, Grenier L, Thibault L, Labrecque G, Beauchamp D. Protein-rich diet attenuates cyclosporin A-induced renal tubular damage in rats. J Ren Nutr. 2003;13(2):84–92. doi: 10.1053/jren.2003.50027. Available from: http://dx.doi.org/10.1053/jren.2003.50027. [DOI] [PubMed] [Google Scholar]
- 37.Kopple JD. Therapeutic approaches to malnutrition in chronic dialysis patients: the different modalities of nutritional support. Am J Kidney Dis. 1999;33(1):180–185. doi: 10.1016/S0272-6386(99)70280-5. Available from: http://dx.doi.org/10.1016/S0272-6386(99)70280-5. [DOI] [PubMed] [Google Scholar]
- 38.Aparicio M, Cano N, Chauveau P, Azar R, Flory A, Laville M, Leverve X. Nutritional status of haemodialysis patients: a French national cooperative study; French Study Group for Nutrition in Dialysis. Nephrol Dial Transplant. 1999;14(7):1679–1686. doi: 10.1093/ndt/14.7.1679. Available from: http://dx.doi.org/10.1093/ndt/14.7.1679. [DOI] [PubMed] [Google Scholar]
- 39.Pupim LB, Flakoll PJ, Brouillette JR, Levenhagen DK, Hakim RM, Ikizler TA. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J Clin Invest. 2002;110(4):483–492. doi: 10.1172/JCI15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veeneman JM, Kingma HA, Boer TS, Stellaard F, De Jong PE, Reijngoud DJ, Huisman RM. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2003;284(5):E954–E965. doi: 10.1152/ajpendo.00264.2002. [DOI] [PubMed] [Google Scholar]
- 41.Lazarus JM. Recommended criteria for initiating and discontinuing intradialytic parenteral nutrition therapy. Am J Kidney Dis. 1999;33(1):211–216. doi: 10.1016/S0272-6386(99)70287-8. Available from: http://dx.doi.org/10.1016/S0272-6386(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 42.Czekalski S, Hozejowski R. Intradialytic amino acids supplementation in hemodialysis patients with malnutrition: results of a multicenter cohort study. J Ren Nutr. 2004;14(2):82–88. doi: 10.1053/j.jrn.2004.01.007. Available from: http://dx.doi.org/10.1053/j.jrn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Wolfson M, Jones MR, Kopple JD. Amino acid losses during hemodialysis with infusion of amino acids and glucose. Kidney Int. 1982;21:500–506. doi: 10.1038/ki.1982.52. Available from: http://dx.doi.org/10.1038/ki.1982.52. [DOI] [PubMed] [Google Scholar]
- 44.Cano N, Labastie-Coeyrehourq J, Lacombe P, Stroumza P, di Costanzo-Dufetel J, Durbec JP, Coudray-Lucas C, Cynober L. Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr. 1990;52(4):726–730. doi: 10.1093/ajcn/52.4.726. [DOI] [PubMed] [Google Scholar]
- 45.Chertow GM, Ling J, Lew NL, Lazarus JM, Lowrie EG. The association of intradialytic parenteral nutrition administration with survival in hemodialysis patients. Am J Kidney Dis. 1994;24(6):912–920. doi: 10.1016/s0272-6386(12)81060-2. [DOI] [PubMed] [Google Scholar]
- 46.Capelli JP, Kushner H, Camiscioli TC, Chen SM, Torres MA. Effect of intradialytic parenteral nutrition on mortality rates in end-stage renal disease care. Am J Kidney Dis. 1994;23(6):808–816. doi: 10.1016/s0272-6386(12)80134-x. [DOI] [PubMed] [Google Scholar]
- 47.Cano N. Intradialytic parenteral nutrition: where do we go from here? J Ren Nutr. 2004;14(1):3–5. doi: 10.1053/j.jrn.2003.09.004. Available from: http://dx.doi.org/10.1053/j.jrn.2003.09.004. [DOI] [PubMed] [Google Scholar]