Abstract
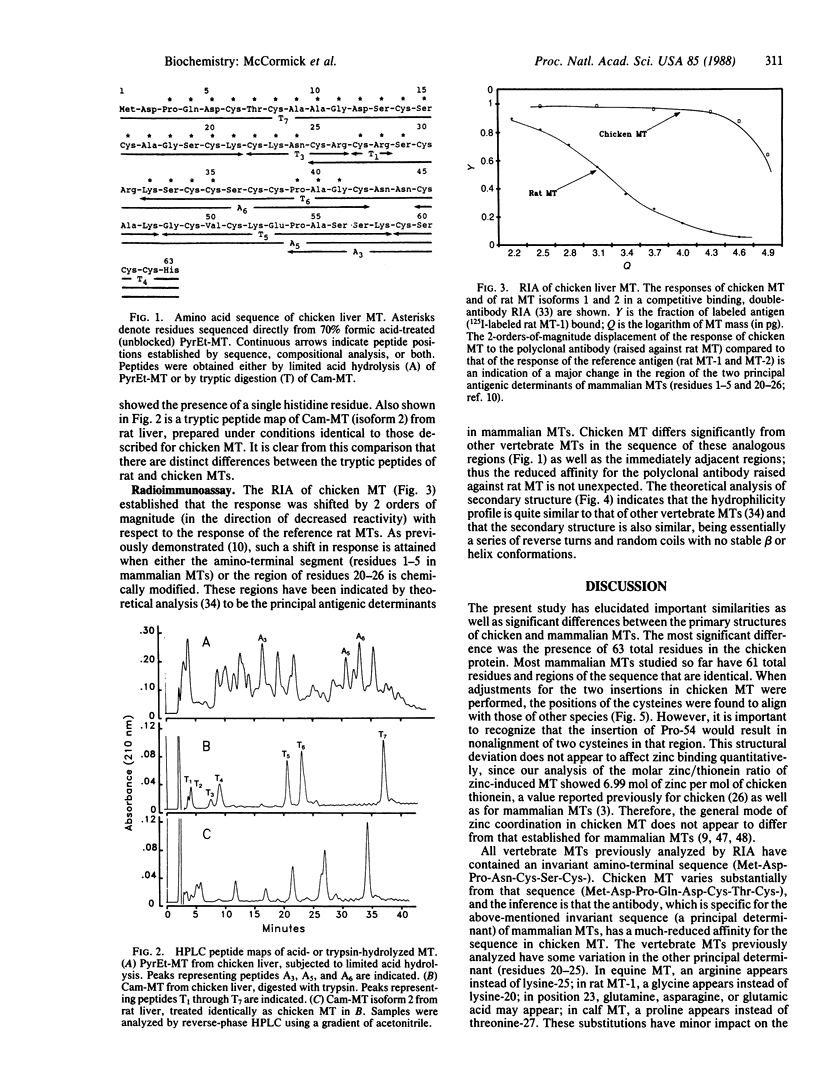
The complete amino acid sequence of metallothionein (MT) from chicken liver is reported. The primary structure was determined by automated sequence analysis of peptides produced by limited acid hydrolysis and by trypsin digestion. The comparative antigenicity of chicken MT was determined by radioimmunoassay using rabbit anti-rat MT polyclonal antibody. Chicken MT consists of 63 amino acids as compared to 61 found in MTs from mammals. One insertion (and two substitutions) occurs in the amino-terminal region, a region considered invariant among mammalian MTs. Eighteen of the 20 cysteines in chicken MT were aligned with cysteines from other mammalian sequences. Two cysteines near the carboxyl terminus are shifted by one residue due to the insertion of proline in that region. Overall, the chicken protein showed approximately equal to 68% sequence identity in a comparison with various mammalian MTs. The affinity of the polyclonal antibody for chicken MT was decreased by 2 orders of magnitude in comparison to that of a mammalian MT (rat MT isoforms). This reduced affinity is attributed to major substitutions in chicken MT in the regions of the principal determinants of mammalian MTs. Theoretical analysis of the primary structure predicted the secondary structure to consist of reverse turns and random coils with no stable beta or helix conformations. There is no evidence that chicken MT differs functionally from mammalian MTs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs R. W., Armitage I. M. Evidence for site-selective metal binding in calf liver metallothionein. J Biol Chem. 1982 Feb 10;257(3):1259–1262. [PubMed] [Google Scholar]
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S. Identification of cysteine-containing peptides in protein digests by high-performance liquid chromatography. Anal Biochem. 1984 Nov 1;142(2):336–339. doi: 10.1016/0003-2697(84)90473-1. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. Analytical peptide mapping by high performance liquid chromatography. Application to intestinal calcium-binding proteins. J Biol Chem. 1979 Aug 10;254(15):7208–7212. [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. The amino acid sequence of bovine intestinal calcium-binding protein. J Biol Chem. 1981 Jun 10;256(11):5669–5674. [PubMed] [Google Scholar]
- Furey W. F., Robbins A. H., Clancy L. L., Winge D. R., Wang B. C., Stout C. D. Crystal structure of Cd,Zn metallothionein. Science. 1986 Feb 14;231(4739):704–710. doi: 10.1126/science.3945804. [DOI] [PubMed] [Google Scholar]
- Garvey J. S. Metallothionein: structure/antigenicity and detection/quantitation in normal physiological fluids. Environ Health Perspect. 1984 Mar;54:117–127. doi: 10.1289/ehp.8454117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey J. S., Vander Mallie R. J., Chang C. C. Radioimmunoassay of metallothioneins. Methods Enzymol. 1982;84:121–138. doi: 10.1016/0076-6879(82)84011-1. [DOI] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Griffith B. B., Walters R. A., Enger M. D., Hildebrand C. E., Griffith J. K. cDNA cloning and nucleotide sequence comparison of Chinese hamster metallothionein I and II mRNAs. Nucleic Acids Res. 1983 Feb 11;11(3):901–910. doi: 10.1093/nar/11.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Huang I. Y., Kimura M., Hata A., Tsunoo H., Yoshida A. Complete amino acid sequence of mouse liver metallothionein-II. J Biochem. 1981 Jun;89(6):1839–1845. doi: 10.1093/oxfordjournals.jbchem.a133385. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- Karin M., Najarian R., Haslinger A., Valenzuela P., Welch J., Fogel S. Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc Natl Acad Sci U S A. 1984 Jan;81(2):337–341. doi: 10.1073/pnas.81.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling M. M., Kägi H. R. Primary structure of human hepatic metallothionein. FEBS Lett. 1977 Oct 15;82(2):247–250. doi: 10.1016/0014-5793(77)80594-2. [DOI] [PubMed] [Google Scholar]
- Klauser S., Kägi J. H., Wilson K. J. Characterization of isoprotein patterns in tissue extracts and isolated samples of metallothioneins by reverse-phase high-pressure liquid chromatography. Biochem J. 1983 Jan 1;209(1):71–80. doi: 10.1042/bj2090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Berger C., Vallee B. L., Kägi J. H. Amino-acid sequence of equine renal metallothionein-1B. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3413–3417. doi: 10.1073/pnas.73.10.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi J. H., Vasák M., Lerch K., Gilg D. E., Hunziker P., Bernhard W. R., Good M. Structure of mammalian metallothionein. Environ Health Perspect. 1984 Mar;54:93–103. doi: 10.1289/ehp.54-1568188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K., Ammer D., Olafson R. W. Crab metallothionein. Primary structures of metallothioneins 1 and 2. J Biol Chem. 1982 Mar 10;257(5):2420–2426. [PubMed] [Google Scholar]
- Lerch K. Copper metallothionein, a copper-binding protein from Neurospora crassa. Nature. 1980 Mar 27;284(5754):368–370. doi: 10.1038/284368a0. [DOI] [PubMed] [Google Scholar]
- Lin L. Y., McCormick C. C. Quantitation of chick tissue zinc-metallothionein by gel electrophoresis and silver stain enhancement. Comp Biochem Physiol C. 1986;85(1):75–84. doi: 10.1016/0742-8413(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Fitch W. M. Evolutionary variability of cytochrome c primary structures. Ann N Y Acad Sci. 1968 Jun 14;151(1):359–381. doi: 10.1111/j.1749-6632.1968.tb11901.x. [DOI] [PubMed] [Google Scholar]
- McCormick C. C. Induction and accumulation of metallothionein in liver and pancreas of chicks given oral zinc: a tissue comparison. J Nutr. 1984 Jan;114(1):191–203. doi: 10.1093/jn/114.1.191. [DOI] [PubMed] [Google Scholar]
- Münger K., Germann U. A., Beltramini M., Niedermann D., Baitella-Eberle G., Kägi J. H., Lerch K. (Cu,Zn)-metallothioneins from fetal bovine liver. Chemical and spectroscopic properties. J Biol Chem. 1985 Aug 25;260(18):10032–10038. [PubMed] [Google Scholar]
- Nemer M., Wilkinson D. G., Travaglini E. C., Sternberg E. J., Butt T. R. Sea urchin metallothionein sequence: key to an evolutionary diversity. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4992–4994. doi: 10.1073/pnas.82.15.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson K. B., Winge D. R. Preferential binding of copper to the beta domain of metallothionein. J Biol Chem. 1984 Apr 25;259(8):4941–4946. [PubMed] [Google Scholar]
- Nordberg G. F., Nordberg M., Piscator M., Vesterberg O. Separation of two forms of rabbit metallothionein by isoelectric focusing. Biochem J. 1972 Feb;126(3):491–498. doi: 10.1042/bj1260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Nakaue H., Deagen J. T., Whanger P. D., Arscott G. H. Accumulation and depletion of zinc in chick tissue metallothioneins. J Nutr. 1979 Oct;109(10):1720–1729. doi: 10.1093/jn/109.10.1720. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Lazdins I., Danks D. M., Mercer J. F. Cloning and sequencing of a sheep metallothionein cDNA. Eur J Biochem. 1984 Sep 17;143(3):507–511. doi: 10.1111/j.1432-1033.1984.tb08399.x. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H. Cloning and sequence analysis of two monkey metallothionein cDNAs. Gene. 1983 Sep;24(1):137–146. doi: 10.1016/0378-1119(83)90139-7. [DOI] [PubMed] [Google Scholar]
- Shaikh Z. A., Lucis O. J. Isolation of cadmium-binding proteins. Experientia. 1971 Sep 15;27(9):1024–1025. doi: 10.1007/BF02138857. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Ebihara Y., Akitomi H., Nishikawa M., Kawamura R. Change in ratio of the two hepatic isometallothioneins with development from prenatal to neonatal rats. Comp Biochem Physiol C. 1983;76(1):33–38. doi: 10.1016/0742-8413(83)90040-3. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Yamamura M. Changes of metal contents and isometallothionein levels in rat tissues after cadmium loading. Biochem Pharmacol. 1980 Sep 15;29(18):2407–2412. doi: 10.1016/0006-2952(80)90342-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Yamamura M. Induction of zinc-thionein in rat liver and kidneys by zinc loading as studied at isometallothionein levels. Toxicol Lett. 1980 Jun;6(1):59–65. doi: 10.1016/0378-4274(80)90103-4. [DOI] [PubMed] [Google Scholar]
- Weser U., Rupp H., Donay F., Linnemann F., Voelter W., Voetsch W., Jung G. Characterization of Cd, Zn-thionein (metallothionein) isolated from rat and chicken liver. Eur J Biochem. 1973 Nov 1;39(1):127–140. doi: 10.1111/j.1432-1033.1973.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Garvey J. S. Antigenicity of metallothionein. Proc Natl Acad Sci U S A. 1983 May;80(9):2472–2476. doi: 10.1073/pnas.80.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge D. R., Gray W. R., Zelazowski A., Garvey J. S. Sequence and antigenicity of calf metallothionein II. Arch Biochem Biophys. 1986 Feb 15;245(1):254–262. doi: 10.1016/0003-9861(86)90212-2. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Differences in the polymorphic forms of metallothionein. Arch Biochem Biophys. 1982 Mar;214(1):80–88. doi: 10.1016/0003-9861(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Domain nature of metallothionein. J Biol Chem. 1982 Apr 10;257(7):3471–3476. [PubMed] [Google Scholar]
- Winge D. R., Nielson K. B., Zeikus R. D., Gray W. R. Structural characterization of the isoforms of neonatal and adult rat liver metallothionein. J Biol Chem. 1984 Sep 25;259(18):11419–11425. [PubMed] [Google Scholar]



