Abstract
In surgery, indications for artificial nutrition comprise prevention and treatment of catabolism and malnutrition. Thus in general, food intake should not be interrupted postoperatively and the re-establishing of oral (e.g. after anastomosis of the colon and rectum, kidney transplantation) or enteral food intake (e.g. after an anastomosis in the upper gastrointestinal tract, liver transplantation) is recommended within 24 h post surgery. To avoid increased mortality an indication for an immediate postoperatively artificial nutrition (enteral or parenteral nutrition (PN)) also exists in patients with no signs of malnutrition, but who will not receive oral food intake for more than 7 days perioperatively or whose oral food intake does not meet their needs (e.g. less than 60–80%) for more than 14 days. In cases of absolute contraindication for enteral nutrition, there is an indication for total PN (TPN) such as in chronic intestinal obstruction with a relevant passage obstruction e.g. a peritoneal carcinoma. If energy and nutrient requirements cannot be met by oral and enteral intake alone, a combination of enteral and parenteral nutrition is indicated. Delaying surgery for a systematic nutrition therapy (enteral and parenteral) is only indicated if severe malnutrition is present. Preoperative nutrition therapy should preferably be conducted prior to hospital admission to lower the risk of nosocomial infections. The recommendations of early postoperative re-establishing oral feeding, generally apply also to paediatric patients. Standardised operative procedures should be established in order to guarantee an effective nutrition therapy.
Keywords: surgery, transplantation, fast track surgery, postoperative nutrition
Abstract
Die Indikationen für eine künstliche Ernährung sind auch in der Chirurgie die Prophylaxe und Behandlung von Katabolie und Mangelernährung. Generell sollte deshalb postoperativ die Nahrungszufuhr nicht unterbrochen werden. Ein oraler (z.B. nach Anastomosen an Kolon und Rektum, Nierentransplantation) bzw. enteraler Kostaufbau (z.B. nach Anastomosen am oberen Gastrointestinaltrakt, Lebertransplantation) wird binnen 24 h nach OP empfohlen. Zur Vermeidung einer erhöhten Letalität, besteht auch bei Patienten ohne Zeichen der Mangelernährung, die perioperativ voraussichtlich mehr als 7 Tage keine orale Nahrungszufuhr oder mehr als 14 Tage oral eine nicht bedarfsdeckende Kost (weniger als 60–80%) erhalten, die Indikation zu einer unverzüglichen postoperativen künstlichen Ernährung. Nur in Fällen einer absoluten Kontraindikation für eine enterale Ernährung wie bei einer chronischen Darmobstruktion mit relevanter Passagestörung, z.B. einer Peritonealkarzinose, besteht die Indikation zur totalen parenteralen Ernährung (TPE). Wenn der Energie- und Nährstoffbedarf durch orale und enterale Zufuhr allein nicht gedeckt werden kann, ist eine kombinierte enterale und parenterale Ernährung indiziert. Die Verschiebung einer Operation zur Durchführung einer gezielten Ernährungstherapie (enteral und parenteral) ist nur bei schwerer Mangelernährung angezeigt. Bei mangelernährten Patienten sollte die präoperative Ernährungstherapie möglichst prästationär durchgeführt werden, um das Risiko nosokomialer Infektionen zu senken. Prinzipiell gelten die Empfehlungen des frühzeitigen postoperativen Kostaufbaus auch für das Kindesalter. Zur Sicherung einer effektiven Ernährungstherapie sollten klinikintern standardisierte Schemata erstellt werden.
Introduction
In surgery, the importance of nutritional status for post-operative morbidity and mortality in various clinical conditions is demonstrated by both retrospective [1], [2], [3], [4], [5], [6] and prospective [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] studies.
The presence of malnutrition is often an expression of the underlying disease i.e. a tumour or chronic organ insufficiency [22], [23], [24], [25], [26], [27], [28], [29], [30], [31] (cf. appropriate chapter). Malnutrition is particularly relevant for outcome after organ transplantation [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. Nutritional status also has a significant influence on morbidity of older patients [42].
Enhanced recovery after surgery (ERAS) is a prerequisite for the desirable reduction of length of hospital stay. This so-called “fast track” system has become a standard in post-operative management, especially after colon operations. The principles of the multimodal process are perioperative limited volume supply, adequate pain therapy (especially by means of epidural anaesthesia), and minimising the administration of opioids, antiemetics and peristaltics. The objective is the re-establishing of oral food intake and full mobilisation of the patient at the earliest possible time.
In surgery, the indications for artificial nutrition are prevention and treatment of catabolism and malnutrition. This mainly affects the perioperative maintenance of nutritional state to prevent malnutrition. Criteria for the success of the “therapeutic” indication for PN are the so-called “outcome” parameters of morbidity, length of hospital stay and mortality, while taking into consideration economic implications. The improvement of nutritional status and quality of life are most important in the post-operative period [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55].
Postoperative re-establishing of food intake
Generally, nutrient intake should not be interrupted post-operatively (A).
The post-operative re-establishing of oral food intake should be adjusted according to the patient’s tolerance (C).
The re-establishing of oral or enteral food intake is recommended within 24 h post surgery (A).
Oral food intake can be reintroduced from the first postoperative day after an anastomosis of the colon and rectum (A).
Enteral intake via a tube with the tip distal to the anastomosis site is recommended for the first few days after an anastomosis in the upper gastrointestinal tract (A).
Commentary
Early re-establishing of oral or enteral food intake lowers the risk of infection and reduces the length of the hospital stay ([56], [57], [58]) (Ia), ([59], [60]) (Ib), [61] (IIa).
Food intake can be reintroduced immediately after a cholecystectomy, because a latency period or oesophagogastric decompression is of no advantage ([62], [63]) (Ib). Early re-establishing of oral food intake, by drinking from the first post operative day, after an anastomosis of the colon and rectum does not result in an increased insufficiency rate or interruption in the healing process ([56], [63], [64]) (Ib), [65] (Ia). The speed at which food is reintroduced should be guided by the gastrointestinal tract function and the patient’s tolerance [57] (Ia), ([63], [64], [65]) (Ib), ([66], [67], [68]) (IIa), ([59], [69]) (IIb).
No comparable data are available for patients with an upper gastrointestinal tract anastomosis e.g. after a gastrectomy or oesophageal resection. In these cases numerous controlled studies have shown the practicability of enteral nutrition via a tube distal to the anastomosis site [70], [71], [72], [73].
In comparison to conventional laparotomies, laparoscopic colonic surgery improves the tolerance to early re-establishing of oral food intake through faster establishment of peristalsis and intestinal passage [74] (Ib), ([68], [75]) (IIa).
Perioperative (pre and postoperative) indications for artificial nutrition
General
Insufficient food intake for more than 14 days is associated with increased mortality (Ib).
Indications for artificial nutrition also exists in patients with no signs of malnutrition, but who will not receive oral food intake for more than 7 days perioperatively or whose oral food intake does not meet their needs (i.e. less than 60–80%) for more than 14 days. In these cases it is recommended that enteral nutrition and, if required, also PN (B) is started immediately post-operatively.
Total PN (TPN) is indicated if there is an absolute contraindication for enteral nutrition, such as in chronic intestinal obstruction with a relevant passage obstruction e.g. a peritoneal carcinoma (A).
If the energy and nutrient requirements cannot be met by oral and enteral intake alone, a combination of enteral and parenteral nutrition is indicated (C).
Standardised operative procedures should be established to secure an effective nutrition therapy (C) (cf. Advice and examples for post-operative PN on general wards, below).
Commentary
The prognostic influence of nutritional state on morbidity, mortality and length of hospital stay (LOS) is prospectively documented for surgical patients, particularly after organ transplantation [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Insufficient food intake over a period of more than 14 days is associated with increased mortality (Ib) [76].
The current guidelines of the American Society for Parental and Enteral Nutrition (ASPEN) recommend post-operative PN for patients who cannot meet their energy needs orally within 7–10 days [77].
The effect of PN in comparison to oral/enteral standard nutrition with regards to the prognosis of surgical patients has been discussed controversially [72], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], (Table 1 (Tab. 1)). Twenty-one randomised studies of patients with abdominal surgery, including patients after liver transplantation and trauma patients, are known to the expert group. In these studies (total) PN was compared with enteral nutrition, or with crystalloid solutions or with a normal hospital diet.
Table 1. Randomised controlled studies on perioperative PN.
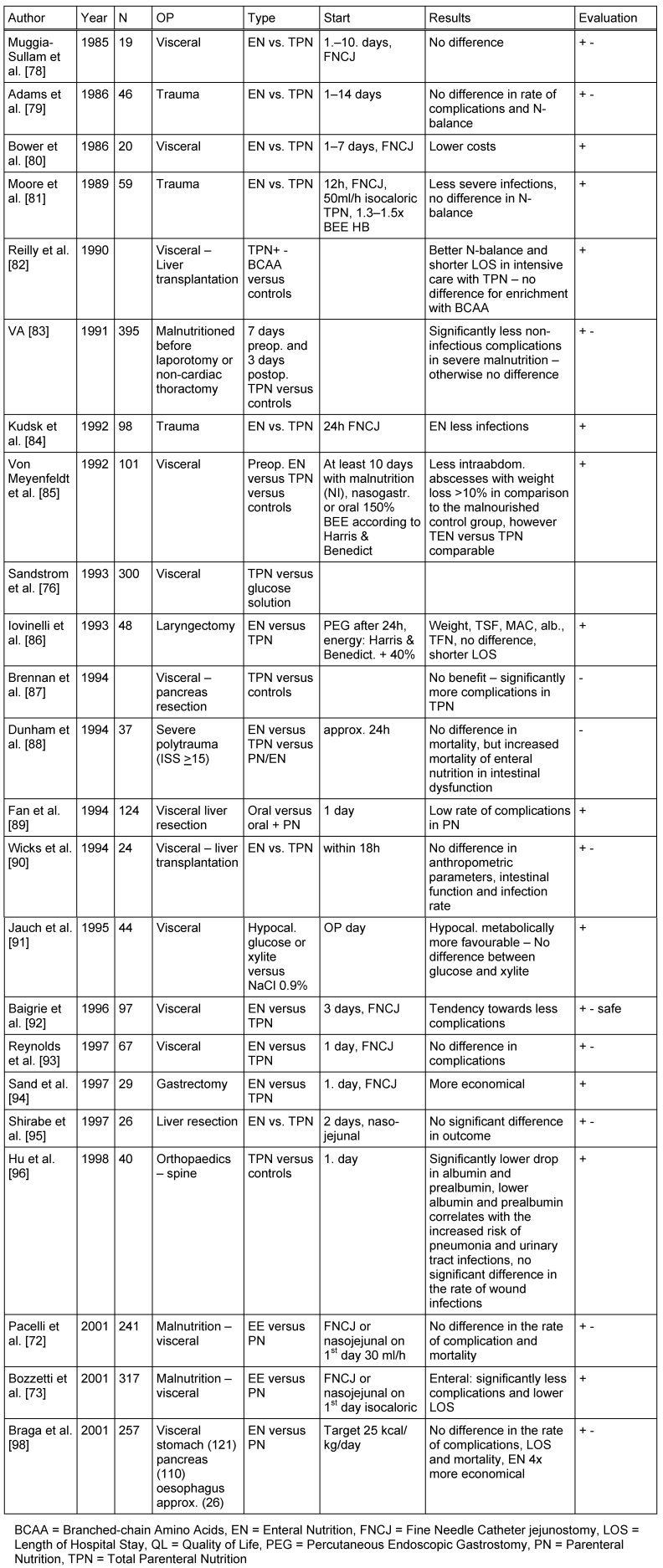
Enteral and parenteral nutrition was compared in 15 studies, of which 6 showed studies significant benefits of enteral nutrition, mainly, a lower incidence of infectious complications, shorter length of stay, and lower costs (Ib). No significant difference, was found in 8 of the 15 studies, which led most authors to favour enteral nutrition because of its lower costs [72], [92], [93], [95] (Ib).
Several authors have pointed out the possible advantages of PN when there is a limited tolerance of enteral nutrition due to intestinal dysfunction especially in the early post-operative phase, which is associated with a lower energy intake [78]. Strict attention, therefore, must be paid to the tolerance of enteral intake especially in patients with severe polytrauma [88] (Ib). An adequate energy intake is better provided by PN when there is a limited gastrointestinal tolerance [99] (IIa).
A meta-analysis by Braunschweig et al. [100] comparing enteral with parenteral nutrition incorporated the results of 27 studies with 1828 patients, (both surgical and non-surgical). It showed a significantly lower risk of infection with oral/enteral nutrition. In malnourished patients, however, PN administration resulted in a significantly lower mortality with a tendency towards lower rates of infection. Heyland et al. [101] incorporated 27 studies in a meta-analysis of PN in surgical patients. Clinical trials comparing enteral versus parenteral nutrition were excluded. An influence of PN on the mortality of surgical patients was not shown. A lower complication rate, especially in those with malnutrition, was observed in the parenterally nourished patients.
These results lead to the recommendation not to enforce a dietary intake covering energy requirements during the first 7–10 post-operative days in well-nourished patients.
Combined enteral/parenteral nutrition
Indication
Combined enteral/parenteral nutrition should always be carried out when artificial nutrition is indicated and the energy requirements cannot be adequately met because of limited enteral tolerance. This is particularly applicable when the energy intake amounts to <60% of the calculated caloric requirements and a central venous catheter for PN is already available (C).
When insertion of a central venous catheter is required for the purpose of artificial nutrition, this indication must be critically considered in relation to the expected time period of PN. Combined nutrition is not necessary if expected time period of PN is <4 days. If the expected PN period is expected to last between 4–7 days, nutrition can be hypocaloric with 2 g carbohydrates and 1 g amino acids/kg body weight administered via a peripheral catheter, and if it is likely to last more than 7–10 days, it is recommended that a central venous catheter should be inserted (C).
Commentary
Combined enteral/parenteral nutrition has not yet been evaluated in prospectively controlled clinical trials with patients undergoing elective surgery. Heyland et al. [102] and Dhaliwal et al. [103] analysed the studies carried out on critically ill patients. Two of these studies from the 80's came from the same study group, and were carried out on patients with bad burns and severe trauma respectively. In the meta-analysis of these studies no advantage was found of combined nutrition regarding mortality, infection, LOS and length of artificial ventilation. Heyland et al. [102], therefore, recommend not to begin with combined enteral and parental nutrition in critically ill patients without signs of malnutrition. They further recommend to decide on parental substrate intake on an individual basis in case of poor tolerance to enteral nutrition.
In major elective surgeries, placement of a central venous catheter is usually a routine . It is the opinion of this expert group that in the presence of a suitable indication this access should be used for PN, especially in malnourished patients, and if necessary also as a part of hypocaloric regime. A randomised controlled study has shown that a hypocaloric PN of 25 kcal/kg and 1.5 g/kg protein presents no increased risk of hyperglycaemia and infectious complications, but results in a significant improvement in nitrogen balance [104] (Ib). Insertion of a central venous catheter exclusively for artificial nutrition should be carefully considered. An increase in energy intake can be achieved in the short-term by lipid administration using peripheral venous access. An increase in enteral intake is the main objective in combined enteral/parenteral nutrition.
A possible approach to combined PN and to tapering PN when reintroducing enteral feeding is shown in plan IV.
Preoperative indications for PN
Delaying surgery for a systematic nutrition therapy (enteral and parenteral) is only indicated if severe malnutrition is present (A).
Preoperative PN is indicated in patients where energy requirement cannot be adequately met by enteral nutrition (C).
An intravenous administration of 200 g glucose preoperatively during the night is recommended in patients who cannot be enterally fed (B).
In malnourished patients, preoperative nutrition therapy should preferably be conducted prior to hospital admission to lower the risk of nosocomial infections (C).
Commentary
Positive effects of PN for 7–10 days were observed post-operatively with regards to the rate of complications [83], [97] and the drop in mortality [83] (Ib). The early post-operative release of cytokines such as IL-6 and IL-8 is, however, significantly higher when PN is administered [105] (Ib). Furthermore, parenteral infusion involves the risk of expanding the extracellular space, thus lowering the albumin concentration and thereby, increasing the risk of pulmonary complications [106] (Ib). Positive effects on postoperative stress adaption were reported after parenteral infusion of 1.5–2 g/kg glucose and 1 g/kg amino acids preoperatively (16–20 h) [107].
There is insufficient data available on the comparison of enteral and parenteral nutrition preoperatively. Therefore oral or enteral feeding should be preferred whenever possible. If parenteral nutrition is necessary to meet energy needs e.g. in stenosis of the upper gastrointestinal tract, it should be combined with oral nutrition (e.g. oral nutritional supplements) whenever possible. The benefits of preoperative PN over 7–10 days are only evident in patients with severe malnutrition (weight loss >15%) prior to major gastrointestinal surgery [83], [97]. When PN is continued for 9 days post-operatively the rate of complications is 30% lower and there is a reduction in mortality (Ib). Questions regarding the type of preoperative nutritional intake have not been clearly resolved in malnourished patients. Preoperative parenteral and enteral nutrition has been compared in one prospective study. Clear advantage of preoperative PN could not be shown [85]. The results of the meta-analysis by Braunschweig [100], however, do favour PN. A significantly lower mortality with a tendency towards lower rates of infection was found in malnourished patients receiving PN.
Glutamine
Indication for glutamine administration
Currently, there is only an indication for post-operative parenteral supplementation of glutamine dipeptide solutions in severely malnourished patients who cannot be adequately fed enterally and, therefore, require PN (C).
A lack of sufficient evidence-based studies deter the expert group from making a general recommendation for parenteral use of glutamine in surgical patients (C).
Commentary
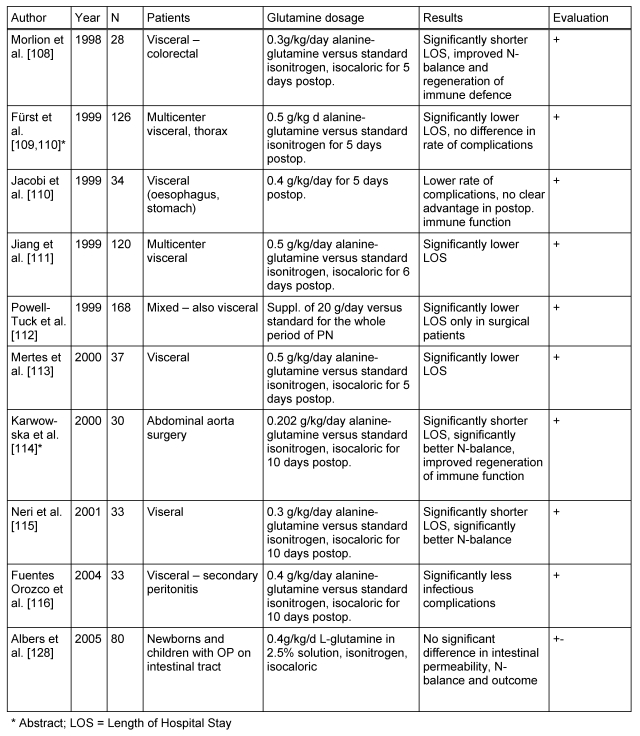
The parenteral supplementation of glutamine dipeptide in 9 controlled randomised trials (Ib) with non-enterally fed surgical patients was reviewed by the working group with regards to the end-points morbidity and outcome (two as abstracts, see Table 2 (Tab. 2) [108], [109], [110], [111], [112], [113], [114], [115], [116]). In eight of these studies, the patients were to undergo elective surgery and in one after emergency visceral surgery. All studies showed significant benefits of glutamine supplementation, seven with respect to post-operative LOS and two with respect to post-operative morbidity. This correlates with the results of an earlier meta-analysis examining elective surgical patients [117] (Ia). A systematic analysis of European and Asian non-enterally nourished surgical patients resulted in 10 studies with the end point of infectious complications and 8 studies of post-operative LOS. Significant benefits of glutamine supplementation were also seen [118] (Ia). Significantly improved regeneration of the post-operative immune function was shown in two current studies with immunological end points [119], [120], [121], [122] (Ib).
Table 2. Randomised controlled studies on glutamine supplementation in the PN of surgical patients.
Based on the current understanding, exclusive PN over 5–7 days is not indicated in surgical patients particularly after elective colorectal surgery with an uncomplicated course [58], [123]. To what extent does parenteral glutamine intake, with oral/enteral nutrition, may have a positive effect, cannot be answered at present due to lack of available data. The possible significance of a short-term perioperative glutamine infusion for a total duration of 72 hours, beginning 24 hours before elective surgery, needs to be further clarified [119].
Specific aspects in paediatric surgery
The recommendations on early post-operative re-establishing of oral feeding generally apply also to infants, children and adolescents (C).
Commentary
In neonates and premature infants, early re-establishing of food (even with the smallest amounts of EN) result in a lower risk of sepsis due to an increase in immune competence [124]. Numerous studies have shown that post-operative energy expenditure increases in newborns after major surgery by 20%, and is normal again within the first 12 to 24 hours [125]. Post-operatively, infants tend to retain water during the first 24 hours due to increased ADH levels and, therefore, fluid intake should be restricted whereas sodium should be given in higher doses [126], [127].
No benefits have been observed when PN is supplemented with glutamine in newborns and children undergoing gastrointestinal surgery [128] (Ib).
Children with short bowel syndrome due to genetic or acquired loss of resorptive surface are dependent on long-term PN. Liver damage and complications like thromboses, embolism and sepsis associated with intravenous nutrition determine the prognosis [129]. An assessment by an intestinal transplantation centre should be considered for PN-dependent paediatric patients with short bowel syndrome who suffer from hyperbilirubinaemia (total bilirubin >3 mg/dl) for more than three months despite adequate therapy [130]. A formula is available to calculate the anticipated duration of PN-dependency in order to determine an early indication of transplantation [131]. An isolated small intestine transplantation is strived for in children with reversible liver damage. PN may usually be terminated over medium-term after the small intestine transplantation has been successful.
Organ transplantation
PN in patients after organ transplants
An early re-establishing of oral feeding should be strived for after successful, uncomplicated heart, liver, and kidney transplantation procedures (C).
Early EN, combined with PN if necessary, is recommended within 24 hours after liver or pancreas transplantations (C).
EN should be increased very carefully within the first week of a small intestine transplantation. Enteral/parenteral nutrition should be combined as well (C).
No recommendation can be made for parenteral supplementation of immune-modulatory substrates due to the lack of data available (C).
No recommendation can be made regarding the parenteral supplementation of glutamine and arginine to precondition against ischemia/reperfusion damage (C).
Commentary
Early oral or enteral feeding should also be strived for transplantation patients [132], [133].
Absorption and blood levels from tacrolism are not impaired by EN [134] (IIb). EN and PN are equally important in patients after liver transplantations [90] (Ib).
Benefits have been reported with administration of MCT/LCT lipid emulsions compared to LCT emulsions, with more favourable regeneration of the function of the reticuloendothelial system after liver transplantation [135]. The metabolism of both lipid solutions shows no difference [136] (Ib).
Advantages of EN are evident when the incidence of viral infections is considered [137] (Ib). In comparison to a standard enteral diet in combination with selective intestinal contamination, a significant drop in the rate of infection was also shown through the use of a high-fibre diet enriched with Lactobacillus plantarum [138] (Ib).
The placement of a fine needle catheter jejunostomy is also feasible in liver transplanted patients [139] (IIb). After small intestine transplantation EN is more difficult because of increased intestinal secretion [140].
The role of pre-conditioning the organ donor or the donor organ i.e. through high-dosage arginine intake for the production of NO and its conversion into glutamine and glutathione is a still open-ended question.
There are no clinical trials on parenteral immunonutrition. Data resulting from animal experiments on parenteral supplementation with glutamine after transplantation of the small intestine show beneficial trophic effects with low mucosa permeability and a low rate of bacterial translocation [141].
Attachment
Advice and examples for post-operative PN on general wards
See also “Safe Practices of PN” [126]
Multi-chamber bags must be mixed according to instructions prior to administration.
Attention should be paid to expiry date, precipitation etc.
Careful labelling of infusion bags (admixtures, patient's name)
Solutions with high osmolarity (>800 mosm/l) should only be infused via central venous access.
The infusion is administered via infusion pumps when feeding paediatric patients and when using hypercaloric nutrition.
Regular checks of the infused solutions should be made during every shift in order to recognise and correct irregularities.
Replacement of the whole infusion system including the three-way valve should take place every 3rd day.
For drug infusion via piggy-bag a separate intravenous line should be used.
Attention should be paid to hygiene rules when injecting admixtures, penetrating a vein or changing the infusion system, or during manipulations at the access etc.
Replacement of additional fluid losses (fever, drainages, diarrhoea, vomiting, stomach tube, etc.).
Exact documentation in the chart (length of infusion, signature)
Regular laboratory tests.
Post-operative infusion and nutrition therapy
Plan I: Fast track with immediate re-establishing of oral food
Indication: Patients who are not suffering from malnutrition and who may receive sufficient oral or enteral nutrition within 4 days, do not require PN irrespective of the type and size of surgery.
Principle: Exclusively electrolyte, fluids and glucose administration irrespective of body weight. Peripheral venous administration is possible. The electrolyte solution can serve as a carrier solution for drugs. Simultaneous increase in oral fluid intake and gradual re-establishing of food.
Application: Peripheral venous, crystalloids – preferred solution: balanced electrolyte solution, NaCl 0.9% in case of increase in serum potassium (dialysis patients).
See example in Table 3 (Tab. 3).
Table 3. Example for fast track.
Plan II: Short-term hypocaloric PN
Indication: Patients who are not malnourished and who probably will not be able to receive sufficient oral or enteral nutrition within 4 days of surgery.
Principle: Hypocaloric PN, i.e. adequate amino acid substitution with limited carbohydrate infusion, only meeting the basic requirements.
Application: Peripheral venous administration is possible. However, it could lead to vein irritation especially with the additional administration of electrolytes, drugs (i.e. antibiotic infusion etc.), complete solutions or two-chamber bags.
See example in Table 4 (Tab. 4).
Table 4. Example for short-term hypocaloric PN.
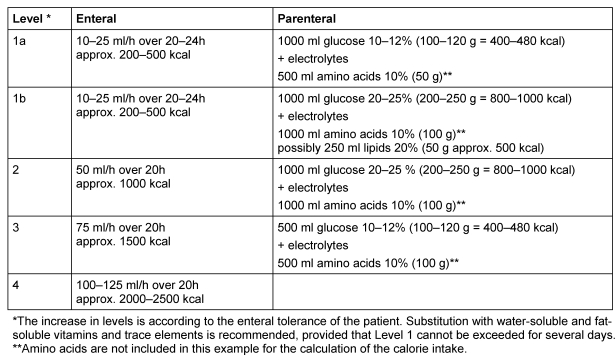
Plan III: PN to meet energy and nutritional requirements
Indications: All patients who are suffering from malnutrition, and those who are not suffering from malnutrition but will not be able to receive sufficient oral or enteral nutrition within 7 days, or those who are not suffering from malnutrition but where it is not anticipated that adequate oral or enteral nutrition can be administered within 14 days.
Principle: Required calorie intake taking into account all substrates as well as adequate substitutions of vitamins and trace elements (total PN). Lipid intake is started on the third day.
There is marked interindividual variance in energy needs for newborns and infants under severe post-operative conditions. Jaksic et al. [142] was not able to detect any increased energy expenditure as a result of massive post-operative stress in newborns. In infants, weight development and fluid balance should be observed to evaluate energy intake. Additionally CO2 production may be measured.
Application: Central venous (catheter via the vena jugularis or vena subclavia), mixed or two-chamber and three-chamber bags. The electrolyte solution can serve as a carrier solution for drugs.
See example in Table 5 (Tab. 5).
Table 5. Example for PN to meet energy and nutritional requirements.
Plan IV: Combined enteral and parenteral nutrition
Indications: All patients, with indications for artificial nutrition, who are unlikely to meet caloric requirements through EN.
Principle: The parenteral substrate intake is adjusted as enteral intake is tolerated with the objective of gradually meeting caloric requirements enterally.
Application: Enteral tube/needle catheter jejunostomy or peripheral venous access, two and three-chamber bags.
See example in Table 6 (Tab. 6).
Table 6. Example for combined enteral and parenteral nutrition.
Notes
This article is part of the publication of the Guidelines on Parenteral Nutrition from the German Society for Nutritional Medicine (overview and corresponding address under http://www.egms.de/en/journals/gms/2009-7/000086.shtml).
English version edited by Sabine Verwied-Jorky, Rashmi Mittal and Berthold Koletzko, Univ. of Munich Medical Centre, Munich, Germany.
References
- 1.Velanovich V. The value of routine preoperative laboratory testing in predicting postoperative complications: a multivariate analysis. Surgery. 1991;109(3 Pt 1):236–243. [PubMed] [Google Scholar]
- 2.Engelman DT, Adams DH, Byrne JG, Aranki SF, Collins JJ, Jr, Couper GS, Allred EN, Cohn LH, Rizzo RJ. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999;118(5):866–873. doi: 10.1016/S0022-5223(99)70056-5. Available from: http://dx.doi.org/10.1016/S0022-5223(99)70056-5. [DOI] [PubMed] [Google Scholar]
- 3.Kama NA, Coskun T, Yuksek YN, Yazgan A. Factors affecting post-operative mortality in malignant biliary tract obstruction. Hepatogastroenterology. 1999;46(25):103–107. [PubMed] [Google Scholar]
- 4.Takagi K, Yamamori H, Toyoda Y, Nakajima N, Tashiro T. Modulating effects of the feeding route on stress response and endotoxin translocation in severely stressed patients receiving thoracic esophagectomy. Nutrition. 2000;16(5):355–360. doi: 10.1016/S0899-9007(00)00231-8. Available from: http://dx.doi.org/10.1016/S0899-9007(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 5.Koval KJ, Maurer SG, Su ET, Aharonoff GB, Zuckerman JD. The effects of nutritional status on outcome after hip fracture. J Orthop Trauma. 1999;13(3):164–169. doi: 10.1097/00005131-199903000-00003. Available from: http://dx.doi.org/10.1097/00005131-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine. 1996;21(22):2676–2682. doi: 10.1097/00007632-199611150-00018. Available from: http://dx.doi.org/10.1097/00007632-199611150-00018. [DOI] [PubMed] [Google Scholar]
- 7.Dannhauser A, Van Zyl JM, Nel CJ. Preoperative nutritional status and prognostic nutritional index in patients with benign disease undergoing abdominal operations - Part I. J Am Coll Nutr. 1995;14(1):80–90. doi: 10.1080/07315724.1995.10718477. [DOI] [PubMed] [Google Scholar]
- 8.Jagoe RT, Goodship TH, Gibson GJ. The influence of nutritional status on complications after operations for lung cancer. Ann Thorac Surg. 2001;71(3):936–943. doi: 10.1016/S0003-4975(00)02006-3. Available from: http://dx.doi.org/10.1016/S0003-4975(00)02006-3. [DOI] [PubMed] [Google Scholar]
- 9.Mazolewski P, Turner JF, Baker M, Kurtz T, Little AG. The impact of nutritional status on the outcome of lung volume reduction surgery: a prospective study. Chest. 1999;116:693–696. doi: 10.1378/chest.116.3.693. Available from: http://dx.doi.org/10.1378/chest.116.3.693. [DOI] [PubMed] [Google Scholar]
- 10.van Bokhorst-de van der Schueren MA, van Leeuwen PA, Sauerwein HP, Kuik DJ, Snow GB, Quak JJ. Assessment of malnutrition parameters in head and neck cancer and their relation to postoperative complications. Head Neck. 1997;19(5):419–425. doi: 10.1002/(sici)1097-0347(199708)19:5<419::aid-hed9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.van Bokhorst-de van der Schueren MA, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86(3):519–527. doi: 10.1002/(SICI)1097-0142(19990801)86:3<519::AID-CNCR22>3.0.CO;2-S. Available from: http://dx.doi.org/10.1002/(SICI)1097-0142(19990801)86:3<519::AID-CNCR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18(3):274–278. doi: 10.1080/07315724.1999.10718863. [DOI] [PubMed] [Google Scholar]
- 13.Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am. 1992;74(2):251–260. [PubMed] [Google Scholar]
- 14.Rey-Ferro M, Castano R, Orozco O, Serna A, Moreno A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition; 1997;13(10):878-81. p. DOI: 10.1016/S0899. Available from: http://dx.doi.org/10.1016/S0899-9007(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 15.Guo CB, Ma DQ, Zhang KH. Applicability of the general nutritional status score to patients with oral and maxillofacial malignancies. Int J Oral Maxillofac Surg. 1994;23(3):167–169. doi: 10.1016/S0901-5027(05)80294-2. Available from: http://dx.doi.org/10.1016/S0901-5027(05)80294-2. [DOI] [PubMed] [Google Scholar]
- 16.Guo CB, Zhang W, Ma DQ, Zhang KH, Huang JQ. Hand grip strength: an indicator of nutritional state and the mix of postoperative complications in patients with oral and maxillofacial cancers. Br J Oral Maxillofac Surg. 1996;34(4):325–327. doi: 10.1016/S0266-4356(96)90012-1. Available from: http://dx.doi.org/10.1016/S0266-4356(96)90012-1. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen NW, Pedersen D. Nutrition as a prognostic indicator in amputations: A prospective study of 47 cases. Acta Orthop Scand. 1992;63(6):675–678. doi: 10.1080/17453679209169734. [DOI] [PubMed] [Google Scholar]
- 18.Mohler JL, Flanigan RC. The effect of nutritional status and support on morbidity and mortality of bladder cancer patients treated by radical cystectomy. J Urol. 1987;137(3):404–407. doi: 10.1016/s0022-5347(17)44049-3. [DOI] [PubMed] [Google Scholar]
- 19.Saluja SS, Kaur N, Shrivastava UK. Enteral nutrition in surgical patients. Surg Today. 2002;32(8):672–678. doi: 10.1007/s005950200125. Available from: http://dx.doi.org/10.1007/s005950200125. [DOI] [PubMed] [Google Scholar]
- 20.Durkin MT, Mercer KG, McNulty MF, et al. Vascular surgical society of great britain and ireland: contribution of malnutrition to postoperative morbidity in vascular surgical patients. Br J Surg. 1999;86(5):702. doi: 10.1046/j.1365-2168.1999.0702a.x. Available from: http://dx.doi.org/10.1046/j.1365-2168.1999.0702a.x. [DOI] [PubMed] [Google Scholar]
- 21.Nezu K, Yoshikawa M, Yoneda T, et al. The effect of nutritional status on morbidity in COPD patients undergoing bilateral lung reduction surgery. Thorac Cardiovasc Surg. 2001;49:216–220. doi: 10.1055/s-2001-16110. Available from: http://dx.doi.org/10.1055/s-2001-16110. [DOI] [PubMed] [Google Scholar]
- 22.Hulsewe KW, Meijerink WJ, Soeters PB, von Meyenfeldt MF. Assessment of outcome of perioperative nutritional interventions. Nutrition. 1997;13(11-12):996–998. doi: 10.1016/S0899-9007(97)00376-6. Available from: http://dx.doi.org/10.1016/S0899-9007(97)00376-6. [DOI] [PubMed] [Google Scholar]
- 23.Butters M, Straub M, Kraft K, Bittner R. Studies on nutritional status in general surgery patients by clinical, anthropometric, and laboratory parameters. Nutrition. 1996;12(6):405–410. doi: 10.1016/S0899-9007(96)00094-9. Available from: http://dx.doi.org/10.1016/S0899-9007(96)00094-9. [DOI] [PubMed] [Google Scholar]
- 24.Correia MI, Caiaffa WT, da Silva AL, Waitzberg DL. Risk factors for malnutrition in patients undergoing gastroenterological and hernia surgery: an analysis of 374 patients. Nutr Hosp. 2001;16(2):59–64. [PubMed] [Google Scholar]
- 25.Lumbers M, New SA, Gibson S, Murphy MC. Nutritional status in elderly female hip fracture patients: comparison with an age-matched home living group attending day centres. Br J Nutr. 2001;85:733–740. doi: 10.1079/BJN2001350. Available from: http://dx.doi.org/10.1079/BJN2001350. [DOI] [PubMed] [Google Scholar]
- 26.Haugen M, Homme KA, Reigstad A, Teigland J. Assessment of nutritional status in patients with rheumatoid arthritis and osteoarthritis undergoing joint replacement surgery. Arthritis Care Res. 1999;12(1):26–32. doi: 10.1002/1529-0131(199902)12:1<26::AID-ART5>3.0.CO;2-#. Available from: http://dx.doi.org/10.1002/1529-0131(199902)12:1<26::AID-ART5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Kuwahara A, Shigemitsu Y, Kinoshita T, Shimoda K, Miyahara M, Kobayashi M, Shimaoka A. Factors related to malnutrition in patients with esophageal cancer. Nutrition. 1991;7(2):117–121. [PubMed] [Google Scholar]
- 28.Weimann A, Meyer HJ, Müller MJ, et al. Bedeutung des präoperativen Gewichtsverlustes für die perioperative Stoffwechseladaptation und das Operationsrisiko bei Patienten mit Tumoren im oberen Gastrointestinaltrakt. Langenbecks Arch Chir. 1992;377(1):45–52. doi: 10.1007/BF00186148. Available from: http://dx.doi.org/10.1007/BF00186148. [DOI] [PubMed] [Google Scholar]
- 29.Bollschweiler E, Schröder W, Hölscher AH, Siewert JR. Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br J Surg. 2000;87(8):1106–1110. doi: 10.1046/j.1365-2168.2000.01474.x. Available from: http://dx.doi.org/10.1046/j.1365-2168.2000.01474.x. [DOI] [PubMed] [Google Scholar]
- 30.Takagi K, Yamamori H, Morishima Y, Toyoda Y, Nakajima N, Tashiro T. Preoperative immunosuppression: its relationship with high morbidity and mortality in patients receiving thoracic esophagectomy. Nutrition. 2001;17(1):13–17. doi: 10.1016/S0899-9007(00)00504-9. Available from: http://dx.doi.org/10.1016/S0899-9007(00)00504-9. [DOI] [PubMed] [Google Scholar]
- 31.Padillo FJ, Andicoberry B, Muntane J, et al. Factors predicting nutritional derangements in patients with obstructive jaundice: multivariate analysis. World J Surg. 2001;25(4):413–418. doi: 10.1007/s002680020043. Available from: http://dx.doi.org/10.1007/s002680020043. [DOI] [PubMed] [Google Scholar]
- 32.Moukarzel AA, Najm I, Vargas J, McDiarmid SV, Busuttil RW, Ament ME. Effect of nutritional status on outcome of orthotopic liver transplantation in pediatric patients. Transplant Proc. 1990;22(4):1560–1563. [PubMed] [Google Scholar]
- 33.Müller MJ, Lautz HU, Plogmann B, Bürger M, Körber J, Schmidt FW. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15(5):782–794. doi: 10.1002/hep.1840150507. Available from: http://dx.doi.org/10.1002/hep.1840150507. [DOI] [PubMed] [Google Scholar]
- 34.Pikul J, Sharpe MD, Lowndes R, Ghent CN. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation. 1994;57(4):469–472. doi: 10.1097/00007890-199402150-00030. Available from: http://dx.doi.org/10.1097/00007890-199402150-00030. [DOI] [PubMed] [Google Scholar]
- 35.Shaw BW, Jr, Wood RP, Gordon RD, Iwatsuki S, Gillquist WP, Starzl TE. Influence of selected patient variables and operative blood loss on six-month survival following liver transplantation. Semin Liver Dis. 1985;5:385–393. doi: 10.1055/s-2008-1040637. Available from: http://dx.doi.org/10.1055/s-2008-1040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selberg O, Böttcher J, Tusch G, Pichlmayr R, Henkel E, Müller MJ. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25(3):652–657. doi: 10.1002/hep.510250327. Available from: http://dx.doi.org/10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 37.Roggero P, Cataliotti E, Ulla L, Stuflesser S, Nebbia G, Bracaloni D, Lucianetti A, Gridelli B. Factors influencing malnutrition in children waiting for liver transplants. Am J Clin Nutr. 1997;65(6):1852–1857. doi: 10.1093/ajcn/65.6.1852. [DOI] [PubMed] [Google Scholar]
- 38.Plöchl W, Pezawas L, Artemiou O, Grimm M, Klepetko W, Hiesmayr M. Nutritional status, ICU duration and ICU mortality in lung transplant recipients. Intensive Care Med. 1996;22(11):1179–1185. doi: 10.1007/BF01709333. Available from: http://dx.doi.org/10.1007/BF01709333. [DOI] [PubMed] [Google Scholar]
- 39.Schwebel C, Pin I, Barnoud D, et al. Prevalence and consequences of nutritional depletion in lung transplant candidates. Eur Respir J. 2000;16(6):1050–1055. doi: 10.1034/j.1399-3003.2000.16f05.x. Available from: http://dx.doi.org/10.1034/j.1399-3003.2000.16f05.x. [DOI] [PubMed] [Google Scholar]
- 40.Figueiredo F, Dickson ER, Pasha T, et al. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000;70(9):1347–1352. doi: 10.1097/00007890-200011150-00014. Available from: http://dx.doi.org/10.1097/00007890-200011150-00014. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson GR, Moretti EW, El Moalem H, Clavien PA, Tuttle-Newhall JE. Malnutrition in liver transplant patients: preoperative subjective global assessment is predictive of outcome after liver transplantation. Transplantation. 2001;72(4):666–670. doi: 10.1097/00007890-200108270-00018. Available from: http://dx.doi.org/10.1097/00007890-200108270-00018. [DOI] [PubMed] [Google Scholar]
- 42.Linn BS, Robinson DS, Klimas NG. Effects of age and nutritional status on surgical outcomes in head and neck cancer. Ann Surg. 1988;207(3):267–273. doi: 10.1097/00000658-198803000-00008. Available from: http://dx.doi.org/10.1097/00000658-198803000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornowski A, Cosnes J, Gendre JP, Quintrec Y. Enteral nutrition in malnutrition following gastric resection and cephalic pancreaticoduodenectomy. Hepatogastroenterology. 1992;39(1):9–13. [PubMed] [Google Scholar]
- 44.Velez JP, Lince LF, Restrepo JI. Early enteral nutrition in gastrointestinal surgery: a pilot study. Nutrition. 1997;13(5):442–445. doi: 10.1016/S0899-9007(97)91283-1. Available from: http://dx.doi.org/10.1016/S0899-9007(97)91283-1. [DOI] [PubMed] [Google Scholar]
- 45.Weimann A, Müller MJ, Adolph M, et al. Kriterien der Überwachung und des Erfolgs einer künstlichen Ernährung – Loccumer Gespräche 1997. Intensivmed; 1997;34(7):744-8. p. DOI: 10.1007/s003900050099. Available from: http://dx.doi.org/10.1007/s003900050099. [DOI] [Google Scholar]
- 46.Hedberg AM, Lairson DR, Aday LA, et al. Economic implications of an early postoperative enteral feeding protocol. J Am Diet Assoc. 1999;99(7):802–807. doi: 10.1016/S0002-8223(99)00191-1. Available from: http://dx.doi.org/10.1016/S0002-8223(99)00191-1. [DOI] [PubMed] [Google Scholar]
- 47.Hamaoui E, Lefkowitz R, Olender L, et al. Enteral nutrition in the early postoperative period: a new semi-elemental formula versus total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1990;14(5):501–507. doi: 10.1177/0148607190014005501. Available from: http://dx.doi.org/10.1177/0148607190014005501. [DOI] [PubMed] [Google Scholar]
- 48.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: The results of a meta-analysis. Ann Surg. 1992;216(2):172–183. doi: 10.1097/00000658-199208000-00008. Available from: http://dx.doi.org/10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mochizuki H, Togo S, Tanaka K, Endo I, Shimada H. Early enteral nutrition after hepatectomy to prevent postoperative infection. Hepatogastroenterology. 2000;47(35):1407–1410. [PubMed] [Google Scholar]
- 50.Shaw-Stiffel TA, Zarny LA, Pleban WE, Rosman DD, Rudolph RA, Bernstein LH. Effect of nutrition status and other factors on length of hospital stay after major gastrointestinal surgery. Nutrition. 1993;9(2):140–145. [PubMed] [Google Scholar]
- 51.Neumayer LA, Smout RJ, Horn HG, Horn SD. Early and sufficient feeding reduces length of stay and charges in surgical patients. J Surg Res. 2001;95(1):73–77. doi: 10.1006/jsre.2000.6047. Available from: http://dx.doi.org/10.1006/jsre.2000.6047. [DOI] [PubMed] [Google Scholar]
- 52.Weimann A, Müller MJ, von Herz U, et al. Lebensqualität als Kriterium des Erfolgs einer künstlichen Ernährung - Loccumer Gespräche 1998. Intensivmed. 1998;35(8):724–726. doi: 10.1007/s003900050200. Available from: http://dx.doi.org/10.1007/s003900050200. [DOI] [Google Scholar]
- 53.Bruning PF, Halling A, Hilgers FJ, et al. Postoperative nasogastric tube feeding in patients with head and neck cancer: a prospective assessment of nutritional status and well-being. Eur J Cancer Clin Oncol. 1988;24(2):181–188. doi: 10.1016/0277-5379(88)90250-7. Available from: http://dx.doi.org/10.1016/0277-5379(88)90250-7. [DOI] [PubMed] [Google Scholar]
- 54.Hammerlid E, Wirblad B, Sandin C, et al. Malnutrition and food intake in relation to quality of life in head and neck cancer patients. Head Neck. 1998;20(6):540–548. doi: 10.1002/(SICI)1097-0347(199809)20:6<540::AID-HED9>3.0.CO;2-J. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199809)20:6<540::AID-HED9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 55.Weimann A. Sinnvolle Ziele für eine Ernährungstherapie beim Tumorpatienten. Aktuel Ernaehr Med. 2001;26:167–169. doi: 10.1055/s-2001-16667. Available from: http://dx.doi.org/10.1055/s-2001-16667. [DOI] [Google Scholar]
- 56.Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus "nil by mouth" after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001;323:773–776. doi: 10.1136/bmj.323.7316.773. Available from: http://dx.doi.org/10.1136/bmj.323.7316.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29(12):2264–2270. doi: 10.1097/00003246-200112000-00005. Available from: http://dx.doi.org/10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Weimann A, Jauch KW, Kemen M, et al. DGEM-Leitlinie Enterale Ernährung: Chirurgie und Transplantation. Aktuel Ernaehr Med; 2003;28:S51-S60. p. DOI: 10.1055/s. Available from: http://dx.doi.org/10.1055/s-2003-36938. [DOI] [Google Scholar]
- 59.Schilder JM, Hurteau JA, Look KY, et al. A prospective controlled trial of early postoperative oral intake following major abdominal gynecologic surgery. Gynecol Oncol. 1997;67(3):235–240. doi: 10.1006/gyno.1997.4860. Available from: http://dx.doi.org/10.1006/gyno.1997.4860. [DOI] [PubMed] [Google Scholar]
- 60.Stewart BT, Woods RJ, Collopy BT, Fink RJ, Mackay JR, Keck JO. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust N Z J Surg. 1998;68(2):125–128. doi: 10.1111/j.1445-2197.1998.tb04721.x. Available from: http://dx.doi.org/10.1111/j.1445-2197.1998.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 61.Moiniche S, Bulow S, Hesselfeldt P, Hestbaek A, Kehlet H. Convalescence and hospital stay after colonic surgery with balanced analgesia, early oral feeding, and enforced mobilisation. Eur J Surg. 1995;161(4):283–288. [PubMed] [Google Scholar]
- 62.Bickel A, Shtamler B, Mizrahi S. Early oral feeding following removal of nasogastric tube in gastrointestinal operations: A randomized prospective study. Arch Surg. 1992;127:287–289. doi: 10.1001/archsurg.1992.01420030049009. [DOI] [PubMed] [Google Scholar]
- 63.Elmore MF, Gallagher SC, Jones JG, Koons KK, Schmalhausen AW, Strange PS. Esophagogastric decompression and enteral feeding following cholecystectomy: a controlled, randomized prospective trial. JPEN J Parenter Enteral Nutr. 1989;13(4):377–381. doi: 10.1177/0148607189013004377. Available from: http://dx.doi.org/10.1177/0148607189013004377. [DOI] [PubMed] [Google Scholar]
- 64.Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Ann Surg. 1995;222(1):73–77. doi: 10.1097/00000658-199507000-00012. Available from: http://dx.doi.org/10.1097/00000658-199507000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeffery KM, Harkins B, Cresci GA, Martindale RG. The clear liquid diet is no longer a necessity in the routine postoperative management of surgical patients. Am Surg. 1996;62(3):167–170. [PubMed] [Google Scholar]
- 66.Choi J, O'Connell TX. Safe and effective early postoperative feeding and hospital discharge after open colon resection. Am Surg. 1996;62(10):853–856. [PubMed] [Google Scholar]
- 67.Detry R, Ciccarelli O, Komlan A, Claeys N. Early feeding after colorectal surgery. Preliminary results. Acta Chir Belg. 1999;99(6):292–294. [PubMed] [Google Scholar]
- 68.Chen HH, Wexner SD, Iroatulam AJ, et al. Laparoscopic colectomy compares favorably with colectomy by laparotomy for reduction of postoperative ileus. Dis Colon Rectum. 2000;43(1):61–65. doi: 10.1007/BF02237245. Available from: http://dx.doi.org/10.1007/BF02237245. [DOI] [PubMed] [Google Scholar]
- 69.Brönnimann S, Studer M, Wagner HE. Frühpostoperative Ernährung nach elektiver Kolonchirurgie. [Early postoperative nutrition after elective colonic surgery]. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:1094–1095. (Ger). [PubMed] [Google Scholar]
- 70.Daly JM, Bonau R, Stofberg P, Bloch A, Jeevanandam M, Morse M. Immediate postoperative jejunostomy feeding: Clinical and metabolic results in a prospective trial. Am J Surg. 1987;153(2):198–206. doi: 10.1016/0002-9610(87)90815-4. Available from: http://dx.doi.org/10.1016/0002-9610(87)90815-4. [DOI] [PubMed] [Google Scholar]
- 71.Kemen M, Senkal M, Homann HH, et al. Early postoperative enteral nutrition with arginine-omega-3 fatty acids and ribonucleic acid-supplemented diet versus placebo in cancer patients: an immunologic evaluation of Impact. Crit Care Med; 1995;23(4):652-9. p. DOI: 10.1097/00003246. Available from: http://dx.doi.org/10.1097/00003246-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Pacelli F, Bossola M, Papa V, et al. Enteral vs parenteral nutrition after major abdominal surgery: an even match. Arch Surg; 2001;136:933-6. p. DOI: 10.1001/archsurg.136.8.933. Available from: http://dx.doi.org/10.1001/archsurg.136.8.933. [DOI] [PubMed] [Google Scholar]
- 73.Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358(9292):1487–1492. doi: 10.1016/S0140-6736(01)06578-3. Available from: http://dx.doi.org/10.1016/S0140-6736(01)06578-3. [DOI] [PubMed] [Google Scholar]
- 74.Schwenk W, Böhm B, Haase O, Junghans T, Müller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg. 1998;383(1):49–55. doi: 10.1007/s004230050091. Available from: http://dx.doi.org/10.1007/s004230050091. [DOI] [PubMed] [Google Scholar]
- 75.Bardram L, Funch-Jensen P, Kehlet H. Rapid rehabilitation in elderly patients after laparoscopic colonic resection. Br J Surg. 2000;87(11):1540–1545. doi: 10.1046/j.1365-2168.2000.01559.x. Available from: http://dx.doi.org/10.1046/j.1365-2168.2000.01559.x. [DOI] [PubMed] [Google Scholar]
- 76.Sandström R, Drott C, Hyltander A, Arfvidsson B, Scherstén T, Wickström I, Lundholm K. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in a randomized study. Ann Surg. 1993;217(2):185–195. doi: 10.1097/00000658-199302000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(1 Suppl):1SA–138SA. [PubMed] [Google Scholar]
- 78.Muggia-Sullam M, Bower RH, Murphy RF, Joffe SN, Fischer JE. Postoperative enteral versus parenteral nutritional support in gastrointestinal surgery: A matched prospective study. Am J Surg; 1985;149(1):106-12. p. DOI: 10.1016/S0002. Available from: http://dx.doi.org/10.1016/S0002-9610(85)80018-0. [DOI] [PubMed] [Google Scholar]
- 79.Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma. 1986;26(10):882–891. doi: 10.1097/00005373-198610000-00004. Available from: http://dx.doi.org/10.1097/00005373-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Bower RH, Talamini MA, Sax HC, Hamilton F, Fischer JE. Postoperative enteral vs parenteral nutrition: A randomized controlled trial. Arch Surg. 1986;121:1040–1045. doi: 10.1001/archsurg.1986.01400090070011. [DOI] [PubMed] [Google Scholar]
- 81.Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma - reduced septic morbidity. J Trauma. 1989;29(7):916–922. doi: 10.1097/00005373-198907000-00003. Available from: http://dx.doi.org/10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Reilly J, Mehta R, Teperman L, et al. Nutritional support after liver transplantation: a randomized prospective study. JPEN J Parenter Enteral Nutr. 1990;14(4):386–391. doi: 10.1177/0148607190014004386. Available from: http://dx.doi.org/10.1177/0148607190014004386. [DOI] [PubMed] [Google Scholar]
- 83.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325(8):525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 84.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–511. doi: 10.1097/00000658-199205000-00013. Available from: http://dx.doi.org/10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.von Meyenfeldt MF, Meijerink WJ, Rouflart MM, Builmaassen MT, Soeters PB. Perioperative nutritional support: a randomised clinical trial. Clin Nutr; 1992;11(4):180-6. p. DOI: 10.1016/0261. Available from: http://dx.doi.org/10.1016/0261-5614(92)90026-M. [DOI] [PubMed] [Google Scholar]
- 86.Iovinelli G, Marsili I, Varrassi G. Nutrition support after total laryngectomy. JPEN J Parenter Enteral Nutr. 1993;17(5):445–448. doi: 10.1177/0148607193017005445. Available from: http://dx.doi.org/10.1177/0148607193017005445. [DOI] [PubMed] [Google Scholar]
- 87.Brennan MF, Pisters PW, Posner M, Quesada O, Shike M. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg. 1994;220(4):436–441. doi: 10.1097/00000658-199410000-00003. Available from: http://dx.doi.org/10.1097/00000658-199410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dunham CM, Frankenfield D, Belzberg H, Wiles C, Cushing B, Grant Z. Gut failure – predictor of or contributor to mortality in mechanically ventilated blunt trauma patients? J Trauma. 1994;37(1):30–34. doi: 10.1097/00005373-199407000-00007. Available from: http://dx.doi.org/10.1097/00005373-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 89.Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331(23):1547–1552. doi: 10.1056/NEJM199412083312303. Available from: http://dx.doi.org/10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
- 90.Wicks C, Somasundaram S, Bjarnason I, et al. Comparison of enteral feeding and total parenteral nutrition after liver transplantation. Lancet. 1994;344(8926):837–840. doi: 10.1016/S0140-6736(94)92824-X. Available from: http://dx.doi.org/10.1016/S0140-6736(94)92824-X. [DOI] [PubMed] [Google Scholar]
- 91.Jauch KW, Kroner G, Hermann A, Inthorn D, Hartl W, Günther B. Postoperative Infusionstherapie: Elektrolytlosung im Vergleich zu hypokalorischen Glukose- bzw. Zuckeraustausch-Aminosäurenlösungen. [Postoperative infusion therapy: electrolyte solution in comparison with hypocaloric glucose and carbohydrate exchange-amino acid solutions]. Zentralbl Chir. 1995;120(9):682–688. (Ger). [PubMed] [Google Scholar]
- 92.Baigrie RJ, Devitt PG, Watkin DS. Enteral versus parenteral nutrition after oesophagogastric surgery: a prospective randomized comparison. Aust N Z J Surg. 1996;66(10):668–670. doi: 10.1111/j.1445-2197.1996.tb00714.x. Available from: http://dx.doi.org/10.1111/j.1445-2197.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 93.Reynolds JV, Kanwar S, Welsh FK, et al. 1997 Harry M. Vars Research Award. Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery? JPEN J Parenter Enteral Nutr. 1997;21(4):196–201. doi: 10.1177/0148607197021004196. Available from: http://dx.doi.org/10.1177/0148607197021004196. [DOI] [PubMed] [Google Scholar]
- 94.Sand J, Luostarinen M, Matikainen M. Enteral or parenteral feeding after total gastrectomy: prospective randomised pilot study. Eur J Surg. 1997;163(10):761–766. [PubMed] [Google Scholar]
- 95.Shirabe K, Matsumata T, Shimada M, Takenaka K, Kawahara N, Yamamoto K, Nishizaki T, Sugimachi K. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection - the results of a randomized prospective study. Hepatogastroenterology. 1997;44(13):205–209. [PubMed] [Google Scholar]
- 96.Hu SS, Fontaine F, Kelly B, Bradford DS. Nutritional depletion in staged spinal reconstructive surgery: The effect of total parenteral nutrition. Spine. 1998;23(12):1401–1405. doi: 10.1097/00007632-199806150-00019. Available from: http://dx.doi.org/10.1097/00007632-199806150-00019. [DOI] [PubMed] [Google Scholar]
- 97.Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24(1):7–14. doi: 10.1177/014860710002400107. Available from: http://dx.doi.org/10.1177/014860710002400107. [DOI] [PubMed] [Google Scholar]
- 98.Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di C, V Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29(2):242–248. doi: 10.1097/00003246-200102000-00003. Available from: http://dx.doi.org/10.1097/00003246-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Woodcock NP, Zeigler D, Palmer MD, Buckley P, Mitchell CJ, MacFie J. Enteral versus parenteral nutrition: a pragmatic study. Nutrition. 2001;17(1):1–12. doi: 10.1016/S0899-9007(00)00576-1. Available from: http://dx.doi.org/10.1016/S0899-9007(00)00576-1. [DOI] [PubMed] [Google Scholar]
- 100.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr; 2001;74(4):534-42. [DOI] [PubMed] [Google Scholar]
- 101.Heyland DK, Montalvo M, MacDonald S, Keefe L, Su XY, Drover JW. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg. 2001;44(2):102–111. [PubMed] [Google Scholar]
- 102.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27(5):355–373. doi: 10.1177/0148607103027005355. Available from: http://dx.doi.org/10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 103.Dhaliwal R, Jurewitsch B, Harrietha D, Heyland DK. Combination enteral and parenteral nutrition in critically ill patients: harmful or beneficial? A systematic review of the evidence. Intensive Care Med. 2004;30(8):1666–1671. doi: 10.1007/s00134-004-2345-y. Available from: http://dx.doi.org/10.1007/s00134-004-2345-y. [DOI] [PubMed] [Google Scholar]
- 104.McCowen KC, Friel C, Sternberg J, et al. Hypocaloric total parenteral nutrition: effectiveness in prevention of hyperglycemia and infectious complications - a randomized clinical trial. Crit Care Med. 2000;28(11):3606–3611. doi: 10.1097/00003246-200011000-00007. Available from: http://dx.doi.org/10.1097/00003246-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 105.Lin MT, Saito H, Fukushima R, et al. Preoperative total parenteral nutrition influences postoperative systemic cytokine responses after colorectal surgery. Nutrition. 1997;13(1):8–12. doi: 10.1016/S0899-9007(97)90871-6. Available from: http://dx.doi.org/10.1016/S0899-9007(97)90871-6. [DOI] [PubMed] [Google Scholar]
- 106.Gil MJ, Franch G, Guirao X, et al. Response of severely malnourished patients to preoperative parenteral nutrition: a randomized clinical trial of water and sodium restriction. Nutrition. 1997;13(1):26–31. doi: 10.1016/S0899-9007(97)90875-3. Available from: http://dx.doi.org/10.1016/S0899-9007(97)90875-3. [DOI] [PubMed] [Google Scholar]
- 107.Bolder U, Ebers M, Tacke J, Löhlein D. Effekte einer unmittelbaren präoperativen Substratzufuhr auf das postoperative Stoffwechselverhalten. Aktuel Ernaehr Med. 1995;20:98–103. [Google Scholar]
- 108.Morlion BJ, Stehle P, Wachtler P, et al. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg. 1998;227(2):302–308. doi: 10.1097/00000658-199802000-00022. Available from: http://dx.doi.org/10.1097/00000658-199802000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fürst P. Effects of supplemental parenteral L-alanyl-L-glutamine (Ala-Gln) following elective operation - a European multicentre study. Clin Nutr. 1999;18:16. [Google Scholar]
- 110.Jacobi CA, Ordemann J, Zuckermann H, Docke W, Volk HD, Müller JM. Einfluss von Alanyl-Glutamin bei der postoperativen totalen parenteralen Ernährung auf die Morbidität unter besonderer Berücksichtigung der Immunfunktion: erste Ergebnisse einer prospektiv randomisierten Studie. [The influence of alanyl-glutamine on immunologic functions and morbidity in postoperative total parenteral nutrition: preliminary results of a prospective randomized trial]. Zentralbl Chir. 1999;124(3):199–205. (Ger). [PubMed] [Google Scholar]
- 111.Jiang ZM, Cao JD, Zhu XG, et al. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double-blind, controlled study of 120 patients. JPEN J Parenter Enteral Nutr. 1999;23(5):S62–S66. doi: 10.1177/014860719902300516. Available from: http://dx.doi.org/10.1177/014860719902300516. [DOI] [PubMed] [Google Scholar]
- 112.Powell-Tuck J. Total parenteral nutrition with glutamine dipeptide shortened hospital stays and improved immune status and nitrogen economy after major abdominal surgery. Gut. 1999;44(2):155. doi: 10.1136/gut.44.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mertes N, Schulzki C, Goeters C, et al. Cost containment through L-alanyl-L-glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective randomized double-blind controlled study. Clin Nutr. 2000;19(6):395–401. doi: 10.1054/clnu.2000.0142. Available from: http://dx.doi.org/10.1054/clnu.2000.0142. [DOI] [PubMed] [Google Scholar]
- 114.Karwowska KA, Szulc R, Dworacki G, Zeromski J. Influence of glutamine-enriched parenteral nutrition on nitrogen balance and immunologic status in patients undergoing elective aortic aneurysm repair. Clin Nutr. 2000;19:22. doi: 10.1016/s0899-9007(01)00537-8. [DOI] [PubMed] [Google Scholar]
- 115.Neri A, Mariani F, Piccolomini A, Testa M, Vuolo G, Di Cosmo L. Glutamine-supplemented total parenteral nutrition in major abdominal surgery. Nutrition. 2001;17(11-12):968–969. doi: 10.1016/S0899-9007(01)00693-1. Available from: http://dx.doi.org/10.1016/S0899-9007(01)00693-1. [DOI] [PubMed] [Google Scholar]
- 116.Fuentes-Orozco C, Anaya-Prado R, Gonzalez-Ojeda A, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin Nutr. 2004;23(1):13–21. doi: 10.1016/S0261-5614(03)00055-4. Available from: http://dx.doi.org/10.1016/S0261-5614(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 117.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30(9):2022–2029. doi: 10.1097/00003246-200209000-00011. Available from: http://dx.doi.org/10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 118.Jiang ZM, Jiang H, Fürst P. The impact of glutamine dipeptides on outcome of surgical patients: systematic review of randomized controlled trials from Europe and Asia. Clin Nutr Suppl. 2004;1(1):17–23. doi: 10.1016/j.clnu.2004.07.009. Available from: http://dx.doi.org/10.1016/j.clnu.2004.07.009. [DOI] [Google Scholar]
- 119.Exner R, Tamandl D, Goetzinger P, et al. Perioperative GLY-GLN infusion diminishes the surgery-induced period of immunosuppression: accelerated restoration of the lipopolysaccharide-stimulated tumor necrosis factor-alpha response. Ann Surg. 2003;237(1):110–115. doi: 10.1097/00000658-200301000-00015. Available from: http://dx.doi.org/10.1097/00000658-200301000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jing-Xiang S, Xiao-Huang T, Lie W, Chen-Jing L. Glutamine dipeptide-supplemented parenteral nutrition in patients with colorectal cancer. Clin Nutr Suppl. 2004;1(1):49–53. doi: 10.1016/j.clnu.2004.07.010. Available from: http://dx.doi.org/10.1016/j.clnu.2004.07.010. [DOI] [Google Scholar]
- 121.Yao GX, Xue XB, Jiang ZM, Yang NF, Wilmore DW. Effects of perioperative parenteral glutamine-dipeptide supplementation on plasma endotoxin level, plasma endotoxin inactivation capacity and clinical outcome. Clin Nutr. 2005;24(4):510–515. doi: 10.1016/j.clnu.2005.04.002. Available from: http://dx.doi.org/10.1016/j.clnu.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 122.Lin MT, Kung SP, Yeh SL, Liaw KY, Wang MY, Kuo ML, Lee PH, Chen WJ. Glutamine-supplemented total parenteral nutrition attenuates plasma interleukin-6 in surgical patients with lower disease severity. World J Gastroenterol. 2005;11(39):6197–6201. doi: 10.3748/wjg.v11.i39.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, Jauch KW, Kemen M, Hiesmayr JM, Horbach T, Kuse ER, Vestweber KH. ESPEN Guidelines on Enteral Nutrition: Surgery including Organ Transplantation. Clin Nutr. 2006;25(2):224–244. doi: 10.1016/j.clnu.2006.01.015. Available from: http://dx.doi.org/10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 124.Marin VB, Rebollo MG, Castillo-Duran CD, et al. Controlled study of early postoperative parenteral nutrition in children. J Pediatr Surg. 1999;34(9):1330–1335. doi: 10.1016/S0022-3468(99)90005-2. Available from: http://dx.doi.org/10.1016/S0022-3468(99)90005-2. [DOI] [PubMed] [Google Scholar]
- 125.Shulman RJ, Phillips S. Parenteral nutrition in infants and children. J Pediatr Gastroenterol Nutr; 2003;36(5):587-607. p. DOI: 10.1097/00005176. Available from: http://dx.doi.org/10.1097/00005176-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 126.Mirtallo J, Canada T, Johnson D, et al. Safe practices for parenteral nutrition. JPEN J Parenter Enteral Nutr. 2004;28(6):S39–S70. doi: 10.1177/0148607104028006S39. Available from: http://dx.doi.org/10.1177/0148607104028006S39. [DOI] [PubMed] [Google Scholar]
- 127.Deutsche Arbeitsgemeinschaft für künstliche Ernährung (DAKE); Österreichische Arbeitsgemeinschaft für künstliche Ernährung (AKE) Empfehlungen zur parenteralen Infusions- und Ernährungstherapie im Kindesalter. Klin Padiatr. 1987;199(4):315–317. [PubMed] [Google Scholar]
- 128.Albers MJ, Steyerberg EW, Hazebroek FW, et al. Glutamine supplementation of parenteral nutrition does not improve intestinal permeability, nitrogen balance, or outcome in newborns and infants undergoing digestive-tract surgery: results from a double-blind, randomized, controlled trial. Ann Surg. 2005;241(4):599–606. doi: 10.1097/01.sla.0000157270.24991.71. Available from: http://dx.doi.org/10.1097/01.sla.0000157270.24991.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amii LA, Moss RL. Nutritional support of the pediatric surgical patient. Curr Opin Pediatr; 1999;11:237-40. p. DOI: 10.1097/00008480. Available from: http://dx.doi.org/10.1097/00008480-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 130.Kaufman SS. Prevention of parenteral nutrition-associated liver disease in children. Pediatr Transplant. 2002;6:37–42. doi: 10.1034/j.1399-3046.2002.1o061.x. [DOI] [PubMed] [Google Scholar]
- 131.Sondheimer JM, Cadnapaphornchai M, Sontag M, Zerbe GO. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr. 1998;132(1):80–84. doi: 10.1016/S0022-3476(98)70489-5. Available from: http://dx.doi.org/10.1016/S0022-3476(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 132.Weimann A, Kuse ER, Bechstein WO, Neuberger JM, Plauth M, Pichlmayr R. Perioperative parenteral and enteral nutrition for patients undergoing orthotopic liver transplantation: Results of a questionnaire from 16 European transplant units. Transpl Int. 1998;11 Suppl 1:S289–S291. doi: 10.1007/s001470050481. [DOI] [PubMed] [Google Scholar]
- 133.Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16(2):43–55. doi: 10.1016/S0261-5614(97)80022-2. Available from: http://dx.doi.org/10.1016/S0261-5614(97)80022-2. [DOI] [PubMed] [Google Scholar]
- 134.Murray M, Grogan TA, Lever J, Warty VS, Fung J, Venkataramanan R. Comparison of tacrolimus absorption in transplant patients receiving continuous versus interrupted enteral nutritional feeding. Ann Pharmacother. 1998;32(6):633–636. doi: 10.1345/aph.17181. Available from: http://dx.doi.org/10.1345/aph.17181. [DOI] [PubMed] [Google Scholar]
- 135.Kuse ER, Kotzerke J, Muller S, Nashan B, Luck R, Jaeger K. Hepatic reticuloendothelial function during parenteral nutrition including an MCT/LCT or LCT emulsion after liver transplantation - a double-blind study. Transpl Int. 2002;15(6):272–277. doi: 10.1111/j.1432-2277.2002.tb00165.x. Available from: http://dx.doi.org/10.1111/j.1432-2277.2002.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 136.Delafosse B, Viale JP, Pachiaudi C, Normand S, Goudable J, Bouffard Y, Annat G, Bertrand O. Long- and medium-chain triglycerides during parenteral nutrition in critically ill patients. Am J Physiol. 1997;272(4 Pt 1):E550–E555. doi: 10.1152/ajpendo.1997.272.4.E550. [DOI] [PubMed] [Google Scholar]
- 137.Hasse JM, Blue LS, Liepa GU, et al. Early enteral nutrition support in patients undergoing liver transplantation. JPEN J Parenter Enteral Nutr. 1995;19(6):437–443. doi: 10.1177/0148607195019006437. Available from: http://dx.doi.org/10.1177/0148607195019006437. [DOI] [PubMed] [Google Scholar]
- 138.Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation – a randomized, double-blind trial. Am J Transplant. 2005;5(1):125–130. doi: 10.1111/j.1600-6143.2004.00649.x. Available from: http://dx.doi.org/10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 139.Pescovitz MD, Mehta PL, Leapman SB, Milgrom ML, Jindal RM, Filo RS. Tube jejunostomy in liver transplant recipients. Surgery. 1995;117(6):642–647. doi: 10.1016/S0039-6060(95)80007-7. Available from: http://dx.doi.org/10.1016/S0039-6060(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 140.Rovera GM, Graham TO, Hutson WR, et al. Nutritional management of intestinal allograft recipients. Transplant Proc. 1998;30(6):2517–2518. doi: 10.1016/S0041-1345(98)00706-4. Available from: http://dx.doi.org/10.1016/S0041-1345(98)00706-4. [DOI] [PubMed] [Google Scholar]
- 141.Li YS, Li JS, Jiang JW, et al. Glycyl-glutamine-enriched long-term total parenteral nutrition attenuates bacterial translocation following small bowel transplantation in the pig. J Surg Res. 1999;82(1):106–111. doi: 10.1006/jsre.1998.5525. Available from: http://dx.doi.org/10.1006/jsre.1998.5525. [DOI] [PubMed] [Google Scholar]
- 142.Jaksic T, Shew SB, Keshen TH, Dzakovic A, Jahoor F. Do critically ill surgical neonates have increased energy expenditure? J Pediatr Surg. 2001;36(1):63–67. doi: 10.1053/jpsu.2001.20007. Available from: http://dx.doi.org/10.1053/jpsu.2001.20007. [DOI] [PubMed] [Google Scholar]