Abstract
Compared to enteral or hypocaloric oral nutrition, the use of PN (parenteral nutrition) is not associated with increased mortality, overall frequency of complications, or longer length of hospital stay (LOS). The risk of PN complications (e.g. refeeding-syndrome, hyperglycaemia, bone demineralisation, catheter infections) can be minimised by carefully monitoring patients and the use of nutrition support teams particularly during long-term PN. Occuring complications are e.g. the refeeding-syndrome in patients suffering from severe malnutrition with the initiation of refeeding or metabolic, hypertriglyceridemia, hyperglycaemia, osteomalacia and osteoporosis, and hepatic complications including fatty liver, non-alcoholic fatty liver disease, cholestasis, cholecystitis, and cholelithiasis. Efficient monitoring in all types of PN can result in reduced PN-associated complications and reduced costs. Water and electrolyte balance, blood sugar, and cardiovascular function should regularly be monitored during PN. Regular checks of serum electrolytes and triglycerides as well as additional monitoring measures are necessary in patients with altered renal function, electrolyte-free substrate intake, lipid infusions, and in intensive care patients. The metabolic monitoring of patients under long-term PN should be carried out according to standardised procedures. Monitoring metabolic determinants of bone metabolism is particularly important in patients receiving long-term PN. Markers of intermediary, electrolyte and trace element metabolism require regular checks.
Keywords: refeeding syndrome, hyperglycaemia, hypertriglyceridemia, liver disease, catheter infection
Abstract
Im Vergleich zu einer enteralen bzw. hypokalorisch oralen Ernährung ist die PE (parenterale Ernährung) nicht mit einer erhöhten Letalität, Komplikationshäufigkeit insgesamt und Krankenhausverweildauer verbunden. Das Risiko durch PE verursachter Komplikationen (wie z.B. Refeeding-Syndrom, Hyperglykämie, Knochendemineralisierung, Katheterinfektionen) kann durch eine sorgfältige Überwachung des Patienten und durch den Einsatz von Ernährungsteams, besonders während einer Langzeit-PE, minimiert werden. Auftretende Komplikationen sind z.B. das Refeeding-Syndrom bei Patienten mit ausgeprägter Mangelernährung, bei denen eine erneute Ernährung begonnen wird. Weitere schwere Nebenwirkungen können metabolische und hepatische Komplikationen sein, wie eine Hypertriglyzeridämie, Hyperglykämie, Fettleber, Steatohepatitis, Cholestase, Cholezystitis, Cholelithiasis sowie Osteomalazie und Osteoporose. Die Effizienzkontrolle bei jeder Art von PE kann zur Reduktion von PE-assoziierten Komplikationen und zur Kostensenkung führen. Unter PE sollte Wasser,- Elektrolyt- und Zuckerhaushalt sowie die kardiozirkuläre Funktion engmaschig überwacht werden. Regelmäßige Kontrollen der Serumelektrolyte und der Triglyzeride sind unter PE erforderlich sowie zusätzliche Überwachungsmaßnahmen bei Patienten mit Nierenfunktionsstörungen, bei elektrolytfreier Substratzufuhr, Fettinfusion bzw. bei Intensivpatienten. Das metabolische Monitoring von Patienten unter längerfristiger PE sollte nach standardisierten Schemata erfolgen. Unter Langzeit-PE sind zusätzlich die metabolischen Determinanten des Knochenstoffwechsels überwachungspflichtig. Variablen des Intermediär-, Elektrolyt- und Spurenelementstoffwechsels bedürfen einer Kontrolle in regelmäßigen Abständen.
Complications
Preliminary remarks and general information
Parenteral Nutrition (PN) is beneficial and life-saving in a variety of clinical conditions, but can also result in numerous, potentially serious, side-effects [1], [2]. The risk of such complications can be minimised by carefully monitoring patients and the use of nutrition support teams.
One of the most dramatic side effects of PN is the so-called refeeding syndrome, which can occur in severely malnourished patients who are receiving aggressive PN. In addition, hyperglycaemia can occur due to parenteral carbohydrate intake in diabetic patients, in response to postaggression metabolism or the systemic inflammatory response syndrome (SIRS), or due to systemic steroid therapy. In extreme cases PN can result in a hyperosmolar, hyperglycaemic non-ketotic coma.
When suddenly discontinuing parenteral intake, rebound hypoglycaemia, although rare, may occur. Abnormalities in the acid-base-balance can occur due to PN and may result in significant electrolyte shifts. Hypertriglyceridemia with dyslipoproteinaemia may occur. High carbohydrate intakes may result in excessive carbondioxide production. Hepatic complications of PN include steatosis (fatty liver) and cholestasis. Special complications like metabolic bone disease wih bone demineralisation and osteoporosis may occur in patients receiving long-term PN. In all forms of PN there is an imminent risk of infectious complications. Finally, intestinal side-effects (mucosal atrophy, increased translocation of micro organisms and their toxins) may also occur.
Relevance of complications in short-term PN with respect to patient outcome
Compared to enteral or hypocaloric oral nutrition, total PN is not associated with increased mortality, overall frequency of complications, or longer length of hospital stay (LOS) (I).
Commentary
Benefits and side effects of PN have been the subject of extensive literature analyses and intensive scientific discussions. The most extensive meta analysis on this topic was presented by Koretz and co-workers in 2001 [3]. Eighty-two randomised controlled studies were evaluated in which parenterally nourished patients received intravenous fluids with a mixture of amino acids and at least 10 kcal/kg body weight per day of non-protein calories. Control patients were either not given intravenous nutrition at all (only spontaneous oral food intake) or received up to 5% glucose solutions intravenously as basal fluid intake. PN did not significantly change either mortality or morbidity (overall rate of complications) compared to the control group. There was, however, a significant increase in infectious complications (+5%) with PN, which caused one additional infection per 20 PN treated patients. A subgroup analysis revealed that this side-effect occurred mainly in tumour patients receiving oncologic therapy. In other subgroups, for example perioperative patients, the infection frequency was not increased. The overall LOS with PN was not increased despite the increased incidence of infection.
A further meta-analysis on the frequency of complications was published by Braunschweig and co-workers [4]. The inclusion criteria varied from those of Koretz's analysis in that only those studies were considered where the calorie intake with PN either met or exceeded energy requirements. Some 27 studies, with a total of 1829 patients, were identified comparing PN with appropriate enteral intake. This analysis showed that infectious complication incidence increased significantly when patients received PN relative to patients in the control group who received either isocaloric enteral nutrition or oral nutrition. Mortality was unaffected by PN. Malnourished patients receiving PN had a lower infection frequency and a significantly lower mortality than those receiving only oral food intake. The increased infectious morbidity is likely due to the relatively high amounts of carbohydrates administered parenterally in some of the older studies included in the analysis. The hyperglycaemic episodes that must be expected with high dosage of parenteral supply are a well-known complication of PN. The incidence of hyperglycaemia is evidently higher even with normocaloric PN intake as compared to enteral or oral food intake [4].
Persistent hyperglycaemia and associated immunosuppression are considered causal factors for a poor patient outcome [5], which would explain the better results of the meta-analysis by Koretz and co-workers who included in their meta-analysis studies with a minimum daily energy intake of approx. 15 kcal/kg body weight. The frequency of infectious complications is probably reduced with a low substrate intake and hence a low incidence of hyperglycaemia.
The known complications of PN caused a fierce controversy in 2003 on clinical benefits or damage [6]. A polemic point of view expressed (“Death by parenteral nutrition”, TPN = total poisonous nutrition) resulted in a numerous responses [7], [8], [9] criticising the extremely unbalanced evaluation and presentation of the literature, particularly data from animal studies. The known links between intravenous energy supply, frequency of hyperglycaemia and associated immune suppressive effects led experts to conclude that PN would likely be without adverse effects for patients if prescribed only when clinically indicated, handled correctly and administered in appropriate dosages [10], [11], [12]. Clear clinical benefits to justify PN are given clinical indications, particularly in malnourished patients with impaired gastrointestinal function.
Complication rates with short-term and long-term PN
Some complications (demineralisation of the bones, catheter infections) occur more frequently with long-term PN than with short-term PN because of metabolic alterations and venous access problems associated with long-term PN (III).
Commentary
In a meta-analysis of 37 studies evaluating long-term PN, the most frequent complication was catheter sepsis with 0.34 episodes per catheter and year [13]. The second most common complication was occlusion of the central venous catheter, with 0.071 episodes per catheter and year, and the third, central venous thrombosis with 0.027 episodes per catheter and year. Pathological hepatic changes with increased liver enzymes were found at 0.025 episodes per treatment year.
Metabolic bone changes are of significant clinical relevance, as they may result in severe illness. Slight changes in the bone metabolism probably occur frequently with PN. In some studies, bone pain and fractures occur in 29% or more of long-term PN patients [1]. A multitude of risk factors are associated with occurrence of these complications, such as long term systemic steroid therapy, short bowel syndrome, physical inactivity, menopause and a genetic disposition for specific bone disorders.
Refeeding syndrome
Initiation of refeeding in patients suffering from severe malnutrition may result in severe adverse effects, the so-called refeeding syndrome (III).
Commentary
Refeeding syndrome is especially prevalent in severely malnourished patients and usually occurs during the first few days after initiating refeeding [14]. The exact incidence is not known give a considerable heterogeneity of studies. Although some of the symptoms are typical of oral/enteral food intake (diarrhoea, nausea, vomiting), most symptoms are observed both with enteral and parenteral refeeding. Among the several complications are:
Vitamin B1 deficiency and acute beriberi,
Volume overload with oedema, cardiac insufficiency and lung oedema,
Electrolyte disorders including hypophosphataemia, hypokalemia and hypomagnesaemia,
Arrhythmia (bradycardia, ventricular tachyarrhythmia)
Glucose intolerance (hyperglycaemia, glucosuria, dehydration and hyperosmolar coma)
Intestinal complications
Altered intestinal function with total PN
In contrast to data from animal studies, clinically relevant changes in mucosal or intestinal function are usually not induced in patients by receiving long-term PN who are not critically ill. The extent to which this also applies to critically ill patients is not known (II).
Commentary
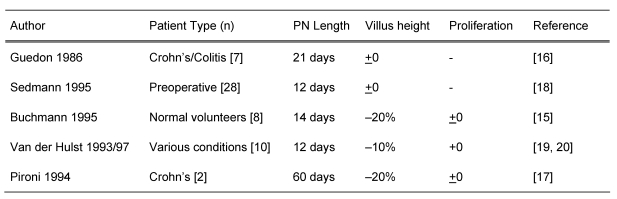
An overview of studies examining the influence of total PN on mucosal morphology or proliferation in patients without severe malnutrition or critically illness is shown in Table 1 (Tab. 1). All the studies available on this topic [15], [16], [17], [18], [19], [20] used glutamine-free amino acid solutions. Despite this potential selective amino acid deficit, total parenteral energy intake reduced the height of the villi only slightly or not at all, irrespective of the underlying illness. This was also the case in patients with mild malnutrition. The enterocyte proliferation was not affected by prolonged PN. It is remarkable that glutamine-free total parenteral nutrition over a 2-months period does not result in distinctive changes in mucosal morphology [17].
Table 1. Effects of total PN on mucosal function.
Parallel to these discrete histological or histochemical changes, protein homeostasis was unaltered in the small intestinal mucosa. Mucosal protein, DNA and RNA contents remain unchanged with glutamine-free total PN for over 2 weeks [15], and the concentration of free intercellular amino acids also remains unaltered [21]. However, selective functional changes are observed under glutamine-free PN despite complete maintenance of the mucosal structure. Enzyme activities on the brush border of the micro villi are reduced [16]. Furthermore, intestinal permeability assessed by the lactulose/mannitol ratio was increased after only 2 weeks of PN [15], [19]. The clinical relevance of this increased permeability is not clear, and translocation of (much larger) microorganisms from the intestinal lumen was not demonstrated under clinical conditions. Sedmann and co-workers [18] found no effect of preoperative total PN on bacterial growth in intestinal lymph nodes or on the intestinal serosa. These studies were carried out on surgical patients who had tissue samples surgically removed for microbiological examination after total PN. Preoperative total PN, over 12 days, resulted in microbial growth in 3 out of 28 patients, whereas in enterally nourished patients, 14 out of 175 demonstrated the presence of microorganisms in the lymph nodes of the serosa (not significant). It can be concluded that glutamine-free total PN does not result in a significant loss of structural integrity of the intestinal mucosa in patients who are not severely malnourished, which differs from findings in rodents. A discrete loss of function with reduced enzyme activity may be due to preferential utilisation of amino acids for the synthesis of structural protein, which might explain why the mucosal anatomy is largely maintained.
There is lack of data for critically ill patients and severely malnourished patients. Glutamine-free PN induced more distinctive impairment in mucosal function of intensive care patients. Duodenal protein content dropped significantly in critically ill patients receiving PN already within 4 days [22]. There is also a limited nutrient absorption and increased intestinal permeability in intensive care patients [23]. The extent to which these processes are influenced by PN, or only reflect an expression of an overall poor general condition, has not been clarified. Many findings in animal experiments so far have not been validated in patients [12].
Hepatic and metabolic complications
Metabolic and hepatic complications comprise hypertriglyceridemia, hyperglycaemia, fatty liver, non-alcoholic fatty liver disease, cholestasis, cholecystitis, cholelithiasis, osteomalacia and osteoporosis (III).
Commentary
Hypertriglyceridemia, hyperglycaemia, fatty liver, non-alcoholic fatty liver disease, cholestasis, cholecystitis, cholelithiasis, osteomalacia and osteoporosis occur after different durations of PN [2], [12], [24]. While hypertriglyceridemia and hyperglycaemia can also occur with short-term PN (intensive care), hepatic complications as well as changes in the bones usually manifest themselves after longer term PN for weeks, months or even years.
Hypertriglyceridemia
Hypertriglyceridemia is found in approximately 25–50% of PN patients. The extent of hypertriglyceridemia depends on the presence of accompanying hyperglycaemia, simultaneous renal insufficiency, steroid administration, extent of the illness and the amount of lipids infused (I).
Severe hypertriglyceridemia (>1000 mg/dL or 11.4 mmol/L and particularly >5000 mg/dL or 57.0 mmol/L) can induce acute pancreatitis, similar to patients with severe hypertriglyceridemia without PN, and it can affect micro circulation (I). It is not known whether long-term hypertriglyceridemia in PN patients is associated with increased cardiovascular risk.
One should aim for plasma triglyceride concentrations below 400 mg/dL (4.6 mmol/L) during PN infusion (C).
Commentary
A multicentre study demonstrated that not only the dosage of intravenous lipids, but also a variety of other factors (hyperglycaemia, renal insufficiency, steroid administration, disease severity, simultaneous administration of heparin) influence the extent of hypertriglyceridemia [25]. Severe PN-associated hypertriglyceridemia may present a similar risk of pancreatitis as severe hypertriglyceridemia of other origins. It is unclear whether PN-associated hypertriglyceridemia, even when long standing, presents a risk for atherosclerosis as it is not asscoaited with an increase in lipoproteins containing apolipoprotein B.
To manage hypertriglyceridemia the above-mentioned causative factors should be corrected. Hyperglycaemia plays an especially important role. Heparin activates lipoprotein lipase and hence can lower blood triglyceride levels but it increases non-esterified fatty acid concentrations.
Hyperglycaemia
Hyperglycaemia is found in up to 50% of PN patients. Important predictors are insulin resistance or diabetes mellitus, severity of the underlying illness, concomitant steroid therapy, and the amount of glucose provided (III).
Hyperglycaemia adversely affects morbidity and mortality in surgical and medical intensive care patients (I).
Normoglycaemia (approximately 80–145 mg/dL) should be aimed for in critically ill patients to improve outcome (A).
Commentary
Numerous clinical studies have associated hyperglycaemia with increased morbidity and mortality in surgical patients with sepsis, patients after bypass surgery, and patients with myocardial infarction or stroke [26], [27], [28], [29], [30], [31], [32], [33], [34]. Experimental data demonstrate a causal link between hyperglycaemia and complications.
It appears important to tightly control control blood sugar in intensive care patients irrespective of simultaneous PN administration. Hyperglycaemia is detected in up to 50% of intensive care patients who are not receiving PN, and the rate increases with added PN [35]. A study in surgical intensive care patients showed benefits of tight blood glucose control (glucose 80–110 mg/dL) with regards to mortality and morbidity when compared to conventional blood sugar control (glucose 80–200 mg/dL) [32]. A more recent study indicated a possible maximum threshold of 145 mg/dL beyond which there are no additional benefits [26]. Although a strict blood glucose control is linked to a more frequent rate of moderate hypoglycaemia, this does not seem to pose a significant clinical problem or have any influence on patient outcome [26], [32] as long as strict blood glucose monitoring is followed.
Acceptable blood glucose values for non-acutely ill patients receiving PN can not be determined based on controlled studies. Possible complications are similar to those seen in patients with diabetes mellitus. The risk of infectious complications is increased due of venous access for PN. The likelihood of hyperglycaemia induced complications may depend on concomitant diseases, duration of PN, and life expectancy. However, even in case of limited life expectancy, lasting blood glucose values over 200 mg/dL appear unacceptable because they interfere with quality of life by inducing e.g. dehydration and polyuria.
Hepatic complications
Hepatic complications of long-term PN are fatty liver, non-alcoholic fatty liver disease and intrahepatic cholestasis as well as cholecystitis and cholelithiasis. Hepatic complications may occur in 15–40% of patients (III).
Ursodeoxycholic acid may be tried in cholestatic liver disease (B). Phenobarbital or antibiotics have no demonstrated effect on hepatic complications (II).
Biliary complications occur frequently – after 6 weeks of therapy, up to 100% of patients have sludge in the gallbladder (III).
Establishing at least a minimal enteral food intake is recommended to lower the risks of biliary complications (C).
Commentary
Fatty liver, non-alcoholic fatty liver disease, intrahepatic cholestasis, cholecystitis and cholelithiasis may occur with PN [36], [37]. Fatty liver is the most common complication, whereas intrahepatic cholestasis or hepatitis are less frequent. Hepatic complications are particularly frequent in paediatric patients (cf. chapter “Neonatology/Paediatrics” http://www.egms.de/en/journals/gms/2009-7/000074.shtml). The etiology of the hepatic complications is unclear. Various factors such as unbalanced nutritional supply (excess or deficiency of specific amino acids), hormonal factors, excess calories, lipids and bacterial overgrowth of the small intestine are discussed [36], [37], [38], [39], [40]. Immaturity of the biliary secretory system and early infections seem to play an important role in paediatric patients. Potentially hepatotoxic substances (drugs) should be avoided whenever possible.
Most studies on the hepatic complications of PN either refer to paediatric patients or were carried out at a time when a much larger amount of energy was administered.
Ursodeoxycholic acid, phenobarbital and gentamycine have been evaluated for treatment therapy of PN induced cholestasis in clinical studies. Improvement of cholestasis was reported with ursodeoxycholic acid but not with phenobarbital or gentamycin [41], [42], [43].
Biliary complications are enhanced by a lack of enteral food intake, while a minimal enteral nutrient intake may largely prevent the occurrence of sludge [44]. Cholecystokinin and the rapid infusion of amino acid solution have been tested experimentally [36], [45], [46], [47] but not as part of clinical routine where they seem impractical to apply.
Metabolic bone diseases
An adequate intake of calcium, phosphate and vitamin D intake should be approached with PN to help prevent and treat osteoporosis and osteomalacia (C).
Bisphosphonates may be used for treating low bone density associated with PN (C).
Commentary
The prevalence and pathogenesis of osteomalacia and osteoporosis associated with PN is unclear, with the reported prevalence ranging from rare cases to up to 40% of patients with long-term PN. Bone changes are probably linked to supoptimal calcium, phosphate and vitamin D intakes, lack of physical activity, lack of light exposure with poor vitamin D status, and side-effects of other therapies (e.g. heparin, steroids) [48]. Therapeutic measures involve approaching adequate substrate intakes as well as preventing other risks. A small study show improved bone density in PN patients receiving pamidronate [49].
Monitoring
Monitoring of clinical efficiency
Regular monitoring is necessary in all types of PN. Efficient monitoring can result in reduced PN-associated complications and reduced costs (A).
Commentary
PN is associated with significant costs and sometimes serious complications. Regular clinical monitoring and diligent care of the patient are necessary to support a good outcome. Nutritional monitoring of patients receiving PN is needed to determine efficiency, to discover and prevent complications, and to document changes in the clinical course. Monitoring should preferably be carried out by a nutrition support team, which monitors efficiency and sufficiency of PN with regards to specific endpoints. The definition of these endpoints should depend on the underlying illness of the patient, his/her clinical state, the facilities available in the institution caring for the patient, and the requests of the individual patient [5], [14], [50], [51].
The aims of PN should be established according to specific markers and targets when the decision for PN is made. These goals can include the maintenance or regeneration of protein inventory in cells, a drop in morbidity and mortality, an improvement in quality of life or an improvement in clinical variables, e.g. a drop in LOS or treatment costs.
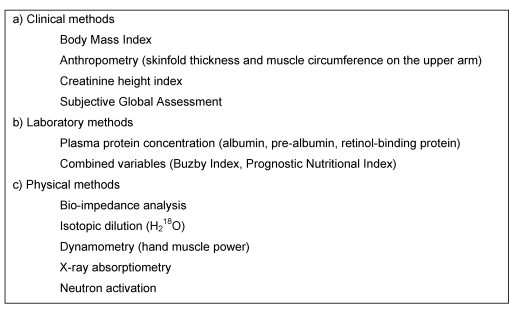
Most studies carried out on patients receiving artificial nutrition use surrogate variables of nutritional state as targets for outcome (Table 2 (Tab. 2)). These markers include energy balance, measures of body composition and body weight, determination of specific serum proteins, nitrogen balance, and functional outcomes [20]. Many of these parameters are influenced by the course of disease or injury and thus, do not only reflect nutritional supply. Nutritional state is an intermediate marker; while the aim of PN should ultimately be improvement in the clinical course and outcome. The monitoring of such clinical variables like quality of life, morbidity and mortality, as well as LOS and costs is clearly more relevant.
Table 2. Some approaches to monitoring PN effects.
Numerous clinical studies monitored PN patients, according to defined protocols, to evaluate the efficiency of a nutritional regime with certain markers. Such studies have been carried out in intensive care patients with polytrauma, and in perioperative patients. Most frequently nitrogen balance, concentrations of specific serum proteins and energy expenditure were used for the evaluation of the efficiency of a certain nutrition regime [52].
The extent to which such monitoring improves the patient’s clinical outcomes such as morbidity, mortality, and quality of life is unknown, but it is concluded that such monitoring is associated with an improved cost-benefit ratio. Three randomised prospective studies analysed the efficiency of nutritional monitoring on prognosis and costs [20], [25], [53]. The main outcome was a significant drop in complications and costs compared to studies in which such monitoring was not applied.
It is also important to perform target-oriented, consecutive re-evaluations of nutritional state. The parenteral substrate supply should be compared to the predicted energy and protein requirements. Changes in the clinical state and level of activity may require a periodic recalculation of energy requirements. Indirect calorimetry can be used, if the course appears to be unsatisfactory, to determine changes in the energy requirements and expenditure [54].
The requirement for total PN therapy should be regularly reevaluated, particularly to explore whether it might be complemented or replaced by enteral or oral feeding.
Metabolic complications
Measures to prevent the refeeding syndrome
Water and electrolyte balance, blood sugar, and cardiovascular function should be regularly monitored in patients receiving PN in order to detect a refeeding syndrome (C).
Commentary
Severely malnourished patients, in whom PN is provided after a long period of fasting, need to be strictly monitored. Water balance, cardiovascular function and serum electrolytes should be carefully monitored at the start and during PN. Serum electrolyte shifts, particularly hypokalemia and hypophosphataemia, should be corrected before starting PN. Sufficient thiamine intake should be established prior to PN. During the first week of PN, fluid intake should be limited to approximately 800 ml/day plus compensation for insensible losses thereby avoiding both fluid overload and dehydration. Daily monitoring of body weight can aid in determining the necessary fluid volume. A weight gain of >0.25 kg/day or 1.5 kg/week likely is typically indicative of fluid accumulation and not of an improved nutritional state. The amount of carbohydrates administered daily should not exceed 2–3 g/kg body weight/day and blood glucose concentration should be strictly monitored because of the high risk of hyperglycaemia. Patients with normal renal function should be generously substituted with phosphate, potassium and magnesium with appropriate laboratory checks. It is necessary to check also the fluid balance (fluid intake, urine production) on a daily basis [14].
Blood glucose monitoring
Depending on the patient's individual risk, blood glucose should be closely checked during PN in view of an inverse correlation between the extent or duration of hyperglycaemia and patient outcome (A).
Commentary
Hypo- and hyperglycaemia are the most severe metabolic complications occuring in patients receiving PN. Some 7% of patients on PN who receive a maximum of 5 mg/kg/min. glucose, develop hyperglycaemia (blood glucose >200 mg/dL). If patients receive more than 5 mg/kg/min glucose, the hyperglycaemia rate rises to almost 50% [51]. Patients with diabetes mellitus, systemic steroid therapy or organ failure were excluded in the latter study. In extreme cases, such hyperglycaemia can result in hyperosmolar hyperglycaemic coma, and mortality can increase up to 14% in patients over 50 years of age [50]. In addition to increased blood glucose concentrations, neurological symptoms like dementia, slowdown or lethargy often occur, preceding the actual coma. Thus, careful and regular examination of clinical-neurological symptoms is necessary for monitoring and diagnosis of hyperglycaemia-associated side effects. It is recommended that the maximum glucose intake should not exceed 3–4 mg/kg/min.
Hyperglycaemic complications are intensified by underlying disease such as diabetes mellitus or surgical trauma. Strict control and monitoring of blood glucose concentration (diurnal blood glucose profile with 3–4 values daily) is recommended particularly in these patients, because hyperglycaemia is associated with impaired immune function and increased rates of infection.
The frequency of severe hypoglycaemia whilst receiving insulin therapy (as an adjunct to PN) has greatly increased because of the wide use of intravenous insulin for lowering of blood glucose concentration below 150 mg/dL. The incidence ranges between 4 and 5% [5] and is accompanied by severe neurological function deficits. Therefore, it is of utmost importance to strictly control the blood glucose concentration especially with intravenous insulin therapy. Checks may be carried out every 2–3 hours in unstable patients and those with short-term insulin dosage changes [20].
Special attention is necessary if there is a sudden withdrawal of carbohydrate and insulin intake under insulin therapy. Severe hypoglycaemia can occur due to the longer biological effect of insulin (15–30 min). It may be necessary to prophylactically continue the carbohydrate supply beyond the end of insulin supply in case of low blood glucose levels to prevent rebound hypoglycaemia with unmeasurably low blood glucose concentrations.
Further monitoring measures
Regular checks of serum electrolytes and triglycerides are necessary with PN (A).
Enhanced monitoring is necessary in patients with altered renal function, electrolyte-free substrate intake, lipid infusions, and in intensive care patients (C).
The metabolic monitoring of patients under long-term PN should be carried out according to standardised procedures (C).
Commentary
Marked increases in plasma triglyceride concentrations may occur in patients receiving intravenous lipids. The frequency of such hypertriglyceridemia during a 7-day PN is up to 26% in unselected patients [25]. If left unnoticed and untreated, severe hypertriglyceridemia might induce pancreatitis and changes in lung function. Therefore, regular monitoring of plasma triglyceride concentrations whilst providing parenteral lipid intake is recommended.
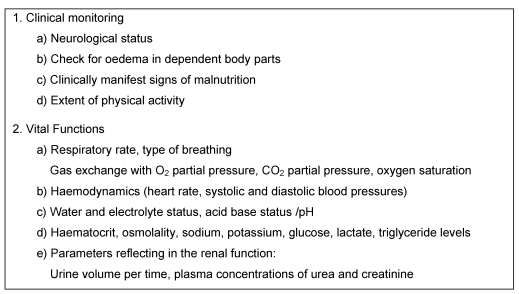
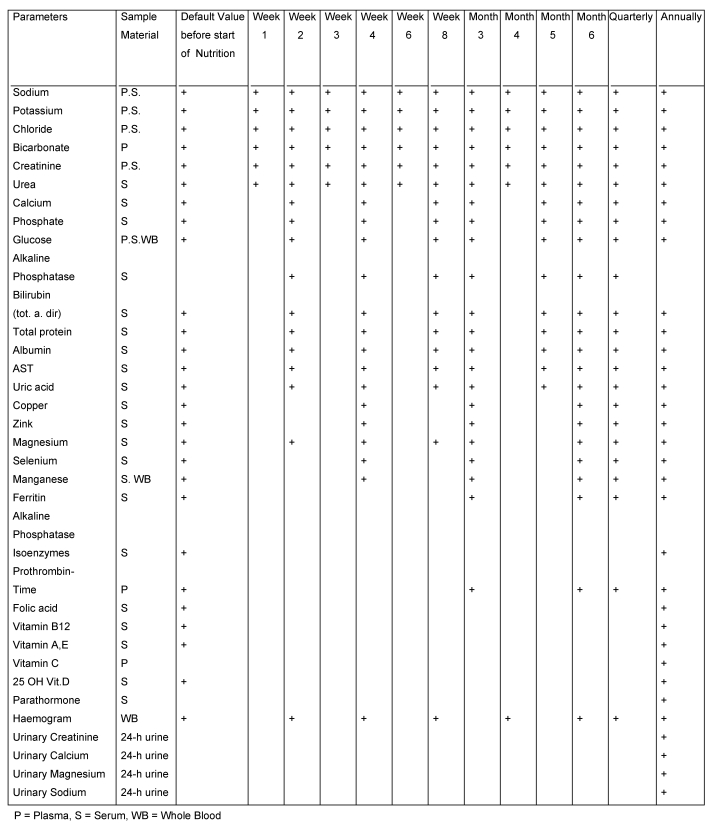
Pathological alterations of the acid-base status are often observed in PN patients. On average, pathological partial pressures of CO2 are present in 13% of cases, and abnormal values for serum chloride concentrations are found in 1–7% of cases [52]. These changes occur as a result of PN induced increases in glucose oxidation or associated CO2 production. It may be difficult to correct specific imbalances that develop with severe ileus or renal failure when using fixed, predetermined electrolyte concentrations in the PN solutions. As acid-base balance and electrolyte balance are closely linked, serum electrolytes as well as the acid-base status should be regularly monitored, particularly in intensive care patients and patients with altered renal function. The necessary targets for the monitoring of complications are shown in Table 3 (Tab. 3) and Table 4 (Tab. 4).
Table 3. Measures for the monitoring of patients on PN (according to [53]).
Table 4. Laboratory monitoring programme for patients with long-term PN (Mayo diagram [55]).
Special monitoring measures in long-term PN patients
The metabolic determinants of bone metabolism should be monitored in patients receiving long-term PN. Markers of intermediary, electrolyte and trace element metabolism require regular checks (C).
Commentary
Maintenance of physiological bone metabolism requires adequate serum concentrations of vitamin D, calcium, magnesium and phosphate. In long-term PN patients, severe disorders resulting in osteoporosis and fractures may occur. The parenterally administered substrates or cyclic infusion favour renal calcium excretion. This hypercalciuria is closely associated with the PN associated metabolic bone disease (PN-MBD) [54].
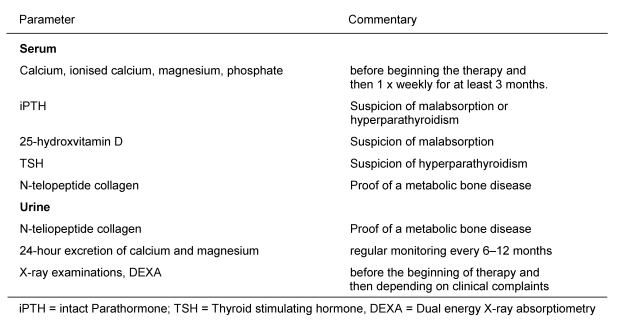
Monitoring of bone density by dual energy X-ray absorptiometry (DEXA) or peripheral quantitative computer tomography (pqCT) is recommended. Recommendations are available for diagnosing or monitoring at-risk patients or patients with bone-related complaints (Table 5 (Tab. 5)) [54].
Table 5. Monitoring and clarification of patients with metabolic bone diseases receiving PN (according to [54]).
Notes
This article is part of the publication of the Guidelines on Parenteral Nutrition from the German Society for Nutritional Medicine (overview and corresponding address under http://www.egms.de/en/journals/gms/2009-7/000086.shtml).
English version edited by Sabine Verwied-Jorky, Rashmi Mittal and Berthold Koletzko, Univ. of Munich Medical Centre, Munich, Germany.
References
- 1.ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(1 Suppl):1SA–138SA. [PubMed] [Google Scholar]
- 2.Maroulis J, Kalfarentzos F. Complications of parenteral nutrition at the end of the century. Clin Nutr. 2000;19:295–304. doi: 10.1054/clnu.1999.0089. Available from: http://dx.doi.org/10.1054/clnu.1999.0089. [DOI] [PubMed] [Google Scholar]
- 3.Koretz RL, Lipman TO, Klein S American Gastroenterological Association. AGA technical review on parenteral nutrition. Gastroenterology. 2001;121(4):970–1001. doi: 10.1016/S0016-5085(01)92000-1. Available from: http://dx.doi.org/10.1016/S0016-5085(01)92000-1. [DOI] [PubMed] [Google Scholar]
- 4.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74(4):534–542. doi: 10.1093/ajcn/74.4.534. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G. Insulin therapy for the critically ill patient. Clin Cornerstone. 2003;5(2):56–63. doi: 10.1016/S1098-3597(03)90018-4. Available from: http://dx.doi.org/10.1016/S1098-3597(03)90018-4. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Pinsky M. Death by parenteral nutrition. Intensive Care Med. 2003;29(6):867–869. doi: 10.1007/s00134-003-1995-5. Available from: http://dx.doi.org/10.1007/s00134-003-1995-5. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AD, Jain PK, MacFie J. Parenteral nutrition in the critically ill. Intensive Care Med. 2003;29(11):2103. doi: 10.1007/s00134-003-1996-4. Available from: http://dx.doi.org/10.1007/s00134-003-1996-4. [DOI] [PubMed] [Google Scholar]
- 8.Fürst P. Comment on "Death by parenteral nutrition" by Marik and Pinsky. Intensive Care Med. 2003;29(11):2102. doi: 10.1007/s00134-003-2023-5. Available from: http://dx.doi.org/10.1007/s00134-003-2023-5. [DOI] [PubMed] [Google Scholar]
- 9.Varga P, Griffiths R, Chiolero R, Nitenberg G, Leverve X, Pertkiewicz M, Roth E, Wernerman J, Pichard C, Preiser JC. Is parenteral nutrition guilty? Intensive Care Med. 2003;29(11):1861–1864. doi: 10.1007/s00134-003-2006-6. Available from: http://dx.doi.org/10.1007/s00134-003-2006-6. [DOI] [PubMed] [Google Scholar]
- 10.Alpers DH. Enteral feeding and gut atrophy. Curr Opin Clin Nutr Metab Care. 2002;5(6):679–683. doi: 10.1097/00075197-200211000-00011. Available from: http://dx.doi.org/10.1097/00075197-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Bistrian BR. Update on total parenteral nutrition. Am J Clin Nutr. 2001;74(2):153–154. doi: 10.1093/ajcn/74.2.153. [DOI] [PubMed] [Google Scholar]
- 12.Jeejeebhoy KN. Total parenteral nutrition: potion or poison? Am J Clin Nutr. 2001;74(2):160–163. doi: 10.1093/ajcn/74.2.160. [DOI] [PubMed] [Google Scholar]
- 13.Richards DM, Deeks JJ, Sheldon TA, Shaffer JL. Home parenteral nutrition: a systematic review. Health Technol Assess. 1997;1(1):i–iii, 1. [PubMed] [Google Scholar]
- 14.Klein S. A primer of nutritional support for gastroenterologists. Gastroenterology. 2002;122(6):1677–1687. doi: 10.1053/gast.2002.33574. Available from: http://dx.doi.org/10.1053/gast.2002.33574. [DOI] [PubMed] [Google Scholar]
- 15.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19(6):453–460. doi: 10.1177/0148607195019006453. Available from: http://dx.doi.org/10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 16.Guedon C, Schmitz J, Lerebours E, Metayer J, Audran E, Hemet J, Colin R. Decreased brush border hydrolase activities without gross morphologic changes in human intestinal mucosa after prolonged total parenteral nutrition of adults. Gastroenterology. 1986;90(2):373–378. doi: 10.1016/0016-5085(86)90935-2. [DOI] [PubMed] [Google Scholar]
- 17.Pironi L, Paganelli GM, Miglioli M, Biasco G, Santucci R, Ruggeri E, Di Febo G, Barbara L. Morphologic and cytoproliferative patterns of duodenal mucosa in two patients after long-term total parenteral nutrition: changes with oral refeeding and relation to intestinal resection. JPEN J Parenter Enteral Nutr. 1994;18(4):351–354. doi: 10.1177/0148607194018004351. Available from: http://dx.doi.org/10.1177/0148607194018004351. [DOI] [PubMed] [Google Scholar]
- 18.Sedman PC, MacFie J, Palmer MD, Mitchell CJ, Sagar PM. Preoperative total parenteral nutrition is not associated with mucosal atrophy or bacterial translocation in humans. Br J Surg. 1995;82(12):1663–1667. doi: 10.1002/bjs.1800821226. Available from: http://dx.doi.org/10.1002/bjs.1800821226. [DOI] [PubMed] [Google Scholar]
- 19.van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, Soeters PB. Glutamine and the preservation of gut integrity. Lancet. 1993;341(8857):1363–1365. doi: 10.1016/0140-6736(93)90939-E. Available from: http://dx.doi.org/10.1016/0140-6736(93)90939-E. [DOI] [PubMed] [Google Scholar]
- 20.van der Hulst RR, von Meyenfeldt MF, Tiebosch A, Buurman WA, Soeters PB. Glutamine and intestinal immune cells in humans. JPEN J Parenter Enteral Nutr. 1997;21(6):310–315. doi: 10.1177/0148607197021006310. Available from: http://dx.doi.org/10.1177/0148607197021006310. [DOI] [PubMed] [Google Scholar]
- 21.van der Hulst RR, von Meyenfeldt MF, Deutz NE, Stockbrügger RW, Soeters PB. The effect of glutamine administration on intestinal glutamine content. J Surg Res. 1996;61(1):30–34. doi: 10.1006/jsre.1996.0076. Available from: http://dx.doi.org/10.1006/jsre.1996.0076. [DOI] [PubMed] [Google Scholar]
- 22.Ahlman B, Ljungqvist O, Persson B, Bindslev L, Wernerman J. Intestinal amino acid content in critically ill patients. JPEN J Parenter Enteral Nutr. 1995;19(4):272–278. doi: 10.1177/0148607195019004272. Available from: http://dx.doi.org/10.1177/0148607195019004272. [DOI] [PubMed] [Google Scholar]
- 23.Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1545–1548. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- 24.Safe Practices for Parenteral Nutrition Formulations. National Advisory Group on Standards and Practice Guidelines for Parenteral Nutrition. JPEN J Parenter Enteral Nutr. 1998;22(2):49–66. doi: 10.1177/014860719802200249. Available from: http://dx.doi.org/10.1177/014860719802200249. [DOI] [PubMed] [Google Scholar]
- 25.Llop J, Sabin P, Garau M, Burgos R, Pérez M, Massó J, Cardona D, Sánchez Segura JM, Garriga R, Redondo S, Sagalés M, Ferrer D, Pons M, Vuelta M, Fàbregas X, Vitales M, Casasín T, Martínez J, Morató L, Soler M Hospital Pharmacy Artificial Nutrition Group of Catalonia. The importance of clinical factors in parenteral nutrition-associated hypertriglyceridemia. Clin Nutr. 2003;22(6):577–583. doi: 10.1016/S0261-5614(03)00082-7. Available from: http://dx.doi.org/10.1016/S0261-5614(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 26.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290(15):2041–2047. doi: 10.1001/jama.290.15.2041. Available from: http://dx.doi.org/10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 27.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314(7093):1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier JJ, Deifuss S, Gallwitz B, Klamann A, Schmiegel W, Nauck MA. Einfluss einer eingeschränkten Glukosetoleranz auf das Langzeitüberleben nach akutem Myokardinfarkt The LAngendreer Myocardial infarction and Blood glucose in Diabetic patients Assessment (LAMBDA) [Influence of impaired glucose tolerance on long-term survival after acute myocardial infarction]. Dtsch Med Wochenschr. 2002;127(21):1123–1129. doi: 10.1055/s-2002-31529. (Ger). Available from: http://dx.doi.org/10.1055/s-2002-31529. [DOI] [PubMed] [Google Scholar]
- 29.Norhammar AM, Rydén L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22(11):1827–1831. doi: 10.2337/diacare.22.11.1827. Available from: http://dx.doi.org/10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 30.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52(1):20–28. doi: 10.1002/ana.10241. Available from: http://dx.doi.org/10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 31.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jc.87.3.978. Available from: http://dx.doi.org/10.1210/jc.87.3.978. [DOI] [PubMed] [Google Scholar]
- 32.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. Available from: http://dx.doi.org/10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 33.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314(7090):1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zindrou D, Taylor KM, Bagger JP. Admission plasma glucose: an independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care. 2001;24(9):1634–1639. doi: 10.2337/diacare.24.9.1634. Available from: http://dx.doi.org/10.2337/diacare.24.9.1634. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Son E, Kim J, Choi K, Kim C, Shin W, Suh O. Association of hyperglycemia and markers of hepatic dysfunction with dextrose infusion rates in Korean patients receiving total parenteral nutrition. Am J Health Syst Pharm. 2003;60(17):1760–1766. doi: 10.1093/ajhp/60.17.1760. [DOI] [PubMed] [Google Scholar]
- 36.Angelico M, Della GP. Review article: hepatobiliary complications associated with total parenteral nutrition. Aliment Pharmacol Ther. 2000;14(2 Suppl):54–57. doi: 10.1046/j.1365-2036.2000.014s2054.x. Available from: http://dx.doi.org/10.1046/j.1365-2036.2000.014s2054.x. [DOI] [PubMed] [Google Scholar]
- 37.Chung C, Buchman AL. Postoperative jaundice and total parenteral nutrition-associated hepatic dysfunction. Clin Liver Dis. 2002;6(4):1067–1084. doi: 10.1016/S1089-3261(02)00057-0. Available from: http://dx.doi.org/10.1016/S1089-3261(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 38.Adamkin DH. Total parenteral nutrition-associated cholestasis: prematurity or amino acids? J Perinatol. 2003;23(6):437–438. doi: 10.1038/sj.jp.7210989. Available from: http://dx.doi.org/10.1038/sj.jp.7210989. [DOI] [PubMed] [Google Scholar]
- 39.Forchielli ML, Walker WA. Nutritional factors contributing to the development of cholestasis during total parenteral nutrition. Adv Pediatr. 2003;50:245–267. [PubMed] [Google Scholar]
- 40.Sandhu IS, Jarvis C, Everson GT. Total parenteral nutrition and cholestasis. Clin Liver Dis. 1999;3(3):489–508, viii. doi: 10.1016/S1089-3261(05)70082-9. Available from: http://dx.doi.org/10.1016/S1089-3261(05)70082-9. [DOI] [PubMed] [Google Scholar]
- 41.Gleghorn EE, Merritt RJ, Subramanian N, Ramos A. Phenobarbital does not prevent total parenteral nutrition-associated cholestasis in noninfected neonates. JPEN J Parenter Enteral Nutr. 1986;10(3):282–283. doi: 10.1177/0148607186010003282. Available from: http://dx.doi.org/10.1177/0148607186010003282. [DOI] [PubMed] [Google Scholar]
- 42.Spagnuolo MI, Iorio R, Vegnente A, Guarino A. Ursodeoxycholic acid for treatment of cholestasis in children on long-term total parenteral nutrition: a pilot study. Gastroenterology. 1996;111(3):716–719. doi: 10.1053/gast.1996.v111.pm8780577. Available from: http://dx.doi.org/10.1053/gast.1996.v111.pm8780577. [DOI] [PubMed] [Google Scholar]
- 43.Spurr SG, Grylack LJ, Mehta NR. Hyperalimentation-associated neonatal cholestasis: effect of oral gentamicin. JPEN J Parenter Enteral Nutr. 1989;13(6):633–636. doi: 10.1177/0148607189013006633. Available from: http://dx.doi.org/10.1177/0148607189013006633. [DOI] [PubMed] [Google Scholar]
- 44.Buchman AL. Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig Dis Sci. 2001;46(1):1–18. doi: 10.1023/A:1005628121546. Available from: http://dx.doi.org/10.1023/A:1005628121546. [DOI] [PubMed] [Google Scholar]
- 45.Doty JE, Pitt HA, Porter-Fink V, Denbesten L. Cholecystokinin prophylaxis of parenteral nutrition-induced gallbladder disease. Ann Surg. 1985;201(1):76–80. doi: 10.1097/00000658-198520110-00011. Available from: http://dx.doi.org/10.1097/00000658-198520110-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalfarentzos F, Vagenas C, Michail A, Vasilakos P, Markoy S, Kordossis T, Androulakis J. Gallbladder contraction after administration of intravenous amino acids and long-chain triacylglycerols in humans. Nutrition. 1991;7(5):347–349. [PubMed] [Google Scholar]
- 47.Sitzmann JV, Pitt HA, Steinborn PA, Pasha ZR, Sanders RC. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obstet. 1990;170(1):25–31. [PubMed] [Google Scholar]
- 48.Cohen-Solal M, Baudoin C, Joly F, Vahedi K, D'Aoust L, De Vernejoul MC, Messing B. Osteoporosis in patients on long-term home parenteral nutrition: a longitudinal study. J Bone Miner Res. 2003;18(11):1989–1994. doi: 10.1359/jbmr.2003.18.11.1989. Available from: http://dx.doi.org/10.1359/jbmr.2003.18.11.1989. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa RA. Intravenous pamidronate improves bone mineral density in home parenteral nutrition patients. Clin Nutr. 2003;22:88. doi: 10.1016/S0261-5614(03)80330-8. Available from: http://dx.doi.org/10.1016/S0261-5614(03)80330-8. [DOI] [Google Scholar]
- 50.Kaminski MV., Jr A review of hypersomolar hyperglycemic nonketotic dehydration (HHND): etiology, pathophysiology and prevention during intravenous hyperalimentation. JPEN J Parenter Enteral Nutr. 1978;2(5):690–698. doi: 10.1177/0148607178002005690. Available from: http://dx.doi.org/10.1177/0148607178002005690. [DOI] [PubMed] [Google Scholar]
- 51.Rosmarin DK, Wardlaw GM, Mirtallo J. Hyperglycemia associated with high, continuous infusion rates of total parenteral nutrition dextrose. Nutr Clin Pract. 1996;11(4):151–156. doi: 10.1177/0115426596011004151. Available from: http://dx.doi.org/10.1177/0115426596011004151. [DOI] [PubMed] [Google Scholar]
- 52.Owens JP, Geibig CB, Mirtallo JM. Concurrent quality assurance for a nutrition-support service. Am J Hosp Pharm. 1989;46(12):2469–2476. [PubMed] [Google Scholar]
- 53.Grünert A. Überwachung der Patienten mit Ernährungstherapie - Biophysikalische und biochemische Meßgrößen. [Monitoring patients on nutritional therapy--biophysical and biochemical measurement values]. Klin Anasthesiol Intensivther. 1990;40:193–195. (Ger). [PubMed] [Google Scholar]
- 54.Seidner DL. Parenteral nutrition-associated metabolic bone disease. JPEN J Parenter Enteral Nutr. 2002;26(5 Suppl):S37–S42. doi: 10.1177/014860710202600511. Available from: http://dx.doi.org/10.1177/014860710202600511. [DOI] [PubMed] [Google Scholar]
- 55.Kelly DG. Guidelines and available products for parenteral vitamins and trace elements. JPEN J Parenter Enteral Nutr. 2002;26(5 Suppl):S34–S36. doi: 10.1177/014860710202600510. Available from: http://dx.doi.org/10.1177/014860710202600510. [DOI] [PubMed] [Google Scholar]