Abstract
In female mammals, reproduction is tightly regulated by energy status and food availability. Although acute energetic challenges inhibit female reproductive behavior and gonadotropin secretion, less attention has been given to the effects of short-term energetic challenge on pregnancy and gestation. Furthermore, species differences in pregnancy physiology necessitate more detailed analyses of specific pregnancy models. Here, we studied musk shrews, which display induced ovulation and obligate delayed implantation, and whose reproductive physiology is tightly linked to metabolic status. We tested whether acute energetic challenges of vary degrees given at specific pregnancy stages (including before and after delayed implantation) have different effects on gestational outcome and offspring postnatal development. We found that 48 h of either 40% or 50% food restriction, which reduced body weight and strongly inhibited sexual behavior, had minimal effects on pregnancy success and litter dynamics when administered early in gestation (pre-implantation). However, <30% of females experiencing short-term food restriction later in gestation successfully gave birth (versus ≥70% of ad-libitum fed controls), and the pups of these food-restricted females exhibited a 30% slower postnatal growth trajectory. Interestingly, although pregnancy success and litter dynamics were unaffected by food restriction before implantation, gestation length was increased by metabolic challenges experienced at this time, indicating that energy status may regulate the timing of implantation. We conclude that 1) there are critical periods of pregnancy, particularly after implantation, when short-term, mild energetic challenges have significant impacts on fertility and offspring postnatal development, and 2) delayed implantation may have evolved, in part, as a buffering mechanism to prevent pregnancy failure during impaired energy balance in early gestation.
Keywords: reproduction, pregnancy, fertility, gestation, delayed implantation, energy balance, food restriction, sexual behavior, development
1. Introduction
In the wild, energetic demands and opportunities for food acquisition can be erratic and often unpredictable, especially on short-term bases (hour-to-hour or day-to-day). Because energy intake and utilization can critically impact survival, animals have developed strategies to maintain energy balance homeostasis or to partition energy resources to select biological processes during times of low food availability. Thus, when food is scarce and energy balance is challenged, animals adjust their energetic priorities, diverting energy away from processes which are not essential in the short term to survival (such as growth and reproduction) [1, 2]. To this end, in most mammalian species, including humans, reproductive output is drastically reduced in the face of low energy availability, especially in females, for whom reproduction is significantly more energetically expensive than in males.
Energetic challenges in women and other female mammals can be due to a variety of factors, including severe dieting and weight loss (common in female athletes), different eating disorders (such as anorexia), or severe lack of environmental food availability (famine). In addition to the more extreme case of long-term famine, females may also experience more common short-term, transient reductions in food intake, such as diminished food availability within a single day or between days (which may critically impact species which rely on frequent feeding bouts). Impairments in female reproduction caused by metabolic deficiencies range from lack of sexual behavior/libido to delayed sexual maturation to acyclicity, amenorrhea, and anovulation [3–10]. In addition, several aspects of pregnancy and parturition can be affected by low food availability, including incidences of miscarriage, truncated durations of gestation (i.e., pre-term birth), fetal deaths (still births), and low birth weight of offspring [11–13]. Moreover, there is now accumulating evidence that nutritional deficits experienced in utero can have far-reaching developmental consequences on physiological and behavioral parameters later in adulthood (i.e., “metabolic programming”) [14–17]. Thus, energy balance and metabolic status during pregnancy can have pronounced effects on both maternal gestational parameters (gestation length, pregnancy success, etc.) and offspring growth and development.
Despite evidence indicating that energy status during gestation has critical effects on reproductive success and offspring development, there are a number of limitations and gaps within the published literature. First, there has been considerable inconsistency amongst all studies in both the selected gestational stage of energetic challenge and the final outcomes measured (pregnancy success, gestation length, litter size, etc). In general, most studies have assessed the effects of metabolic challenges during just one particular period of pregnancy (e.g., first week of gestation, or last 3 days of gestation, etc.), and have typically reported only 2 or 3 outcome measures. Unfortunately, the selected pregnancy stages and outcome measures often differ dramatically between studies, precluding meaningful comparisons between studies, species, or specific gestational stages. Second, not only has the magnitude of the energetic challenge varied considerably between studies, but there is a scarcity of studies using ecologically relevant manipulations of energy status. Most pregnancy studies have employed extreme perturbations in food availability, such that all food is completely removed for short periods of time [18] or some food is removed for very long periods during gestation (1–2 weeks in small species, or 1–2 months in bigger species) [19–26]. Although these studies mimic extreme conditions of ongoing famine, complementary studies using more modest degrees of food restriction, such as short-term, moderate challenges that would commonly be experienced in nature on a day-to-day basis, are lacking. Lastly, although there is accumulating data on this topic for laboratory rodents and sheep, there is a paucity of information for many other mammalian species, including those that exhibit different mechanisms of reproductive biology, such as induced estrous, delayed ovulation, or obligate embryonic diapause (i.e., delayed implantation). This lack of sufficient information in non-rodent, non-sheep species necessitates more detailed analyses of specific pregnancy models, and their regulation by energetic and metabolic cues, in a variety of mammalian species.
The early-evolved musk shrew (Suncus murinus), a small species with induced ovulation and delayed implantation, is an excellent animal model for examining the nutritional and energetic regulation of mammalian reproduction. Musk shrews have high metabolisms and little stored body fat, and their birth rates are higher in the rainy season when food is more prevalent [27]. Thus, similar to other species, musk shrew reproduction is dramatically regulated by energy status and food availability. Just 48 h of mild food restriction, at 60% of baseline food intake levels, significantly diminishes the occurrence of musk shrew female sexual behavior, reduces ovulation rates, and impairs the secretion of various reproductive neuropeptides, including variants of gonadotropin-releasing hormone (GnRH) [7, 10, 28, 29]. However, despite the evidence that energy status can severely impair female sexual behavior and neuronal GnRH secretion in musk shrews, no attention has been given to the effects of energetic challenges on shrew gestational dynamics or litter characteristics (including offspring postnatal growth).
In the present study, we examined how acute, short-term metabolic challenges impact fertility and litter parameters of pregnant female shrews. Specifically, we tested whether 48 h energetic challenges (as may commonly be experienced in nature) influence pregnancy outcome, gestation duration, litter size, and offspring development, and whether any of these parameters are influenced by the magnitude of short-term energetic challenge. Furthermore, given the differential energy demands associated with early and later gestational stages, as well as the presence of obligate delayed implantation in this species (normally occurring around gestational day 8 [30, 31], we tested whether the effects of short-term energy restriction are dependent on the stage of gestation, specifically before, during, and after embryo implantation. Lastly, we assessed whether mild, short-term energy restriction experienced right before mating (i.e., just prior to ovulation and conception) has detrimental effect on the ability of female shrews to become pregnant. Our findings identify critical periods of pregnancy when acute metabolic challenges have more severe impacts on reproductive success and litter characteristics. Moreover, the process of delayed implantation in shrews, and perhaps other mammals, may serve as a buffering mechanism to prevent pregnancy failure in the face of acute food restriction experienced early in gestation.
2. Methods and Materials
2.1 Animals
All studies used adult (5–12 months old), sexually-experienced musk shrews (Suncus murinus). The duration of pregnancy of musk shrews is 30 days, including a week-long period of delayed implantation (typically occurring on day 8) followed by approximately 3 weeks of gestation once implantation has occurred [30–32] (see Figure 1). All musk shrews were born in the breeding colony at the University of Virginia. Upon weaning, at 21 days of age, shrews were housed individually with food (Purina Cat Chow) and water available ad-libitum (unless noted differently in specific experiments). Except for periods of breeding or mating bouts, males and females were housed in separate rooms, each maintained on a 14L:10D photoperiod (lights off at 1900h) at a temperature of 24 ± 2°C. All experiments were performed in compliance with regulations of the Animal Care and Use Committee of University of Virginia.
Figure 1.
Schematic of the major physiological events and stages of gestation in female musk shrews (28). Double arrows above the horizontal bar depict 2 day periods when food restriction (40% or 50% decrease) was administered in the present experiments. P4, progesterone.
2.2 Food Restriction and Food Intake Measurements
For each experiment, daily food intake was measured (±0.1 g) over a pre-pregnancy baseline period (typically 3–5 days) as well as every several days throughout pregnancy, at approximately the same time each day (between 1000h and 1200h). Female musk shrews are induced ovulators and do not have ovarian or behavioral estrous cycles; thus, food intake in non-pregnant females is not influenced by daily or hourly variations in ovarian hormone secretion. The average daily food intake for each specific pre-pregnancy or pregnancy period was determined for each individual; animals undergoing subsequent food restriction then received either a 40% decrease (Experiments 1 and 2) or 50% decrease (Experiments 1 and 3) in their average daily food intake for two consecutive days. The % reduction in food intake was based on each animal’s own average food consumption for the several days preceding the food restriction period. After this 48 h restriction period, food-restricted females were returned to ad-libitum feeding for the remainder of the study. Control animals remained on ad-libitum food intake during these same 48 h food restriction periods.
2.3 Experiment 1: Assess the effects of pre-mating food restriction on fertility and litter parameters
We have previously shown that 48 h of moderate food restriction (40% restriction) reduces mating behavior of female, but not male, shrews [7]. However, whether or not such energetic challenges actually reduce fertility and fecundity in the few females that mate has not been assessed. To examine this issue, we determined whether females that were food restricted just prior to mating and ovulation could become pregnant and successfully produce offspring. Sexually-experienced females who had displayed prior reproductive success (i.e., had several previous litters) were either maintained on ad-libitum feeding or food restricted 40% or 50% for 48 h. At the end of the food restriction period, all females were immediately exposed to a sexually-experienced male for 4 hours. Female shrews, like many mammalian species, have induced estrous behavior, and sexual receptivity (i.e., mating) is induced with 20 minutes of direct exposure to a courting male. During the mating period, the presence (or absence) of tail-wagging (a female sexual behavior, indicative of receptivity) was confirmed, as was the verification of mounting attempts by the stud males. After the mating period, all females were returned to their individual cages and monitored for body weight and food intake over the next 4 weeks. In addition, several pregnancy outcomes were measured: successful parturition, day of parturition (i.e., duration of gestation), litter size, and average pup body weights on postnatal day 5. All groups were matched for average body weight and baseline food intake prior to food restriction; each group contained 8–10 females.
2.4 Experiment 2: Determine the effects of 40% food restriction on pregnancy success and litter characteristics
In this experiment we tested whether mild metabolic challenges imposed by short-term food restriction at different stages of pregnancy would impact the likelihood of successful gestation and parturition, as well as if such challenges would influence litter parameters or offspring development. Adult female musk shrews, all with prior mating and gestational experience, were either maintained on ad-libitum feeding or moderately food restricted (40% restricted) for just 48 h at various stages of gestation. Specifically, short-term 40% food restriction was imposed at one of the following time periods during gestation: embryonic days 2–4 (E2-4), E6-8, E11-13, and E19-21 (see Figure 1). These 2-day time periods were chosen to correspond with unique stages of pregnancy, including periods after ovulation but before implantation (when energy requirements are minimal), at the time of implantation, soon after implantation but before placental formation, and late in pregnancy after placental formation (when gestational progesterone levels and daily energy consumption are both at a maximum) (Figure 1). Body weights and food intake were measured at multiple time points for all animals over the course of gestation. In addition, successful parturition, day of parturition (i.e., duration of gestation), litter size, and average pup body weights on postnatal day 5 were determined. All treatment groups were matched for average preconception body weight and similar degree of prior mating/pregnancy experience, and each group contained 15–18 animals.
2.5 Experiment 3: Determine the effects of 50% food restriction on pregnancy success and litter characteristics
In this experiment we asked whether more dramatic metabolic challenges imposed by moderate short-term food restriction at varying stages of pregnancy would impact the gestational and litter parameters. Adult female musk shrews, all with prior mating and gestational experience, were either maintained on ad-libitum feeding or food restricted at 50% for 48 h at various stages of gestation: E2-4, E6-8, E11-13, and E19-21 of gestation (as in Experiment 2). Temple and Rissman [33] have previously shown that female shrews restricted at 50% for 2 days are far less likely to reinstate reproductive behavior following brief re-feeding versus females experiencing a milder 40% food restriction. Thus, there is precedence for this 10% difference in energy challenge being significant for the female’s reproductive physiology. Body weights and food intake were measured for all females over the course of gestation, and parturition and litter parameters were determined as in Experiment 2, with the additional measure of pup body weights also taken on post-natal day 2. All treatment groups were matched for average preconception body weight and similar degree of prior mating/pregnancy experience, and each group contained 11–14 animals.
2.6 Statistics
For each experiment, the % of animals in each group that successfully delivered litters was compared using chi-squared test. Group differences in mean maternal body weights, % change in body weight, mean food intake, gestational duration, and litter parameters were analyzed with one-way ANOVAs, with planned comparisons made between each food-restricted group and the ad-libitum fed control group using one-way ANOVAs and post-hoc Fisher’s PLSD test. For all statistical comparisons, significance level was set at p<0.05.
3. Results
3.1 Experiment 1: Pre-mating food restriction prevents successful pregnancy and decreases fecundity
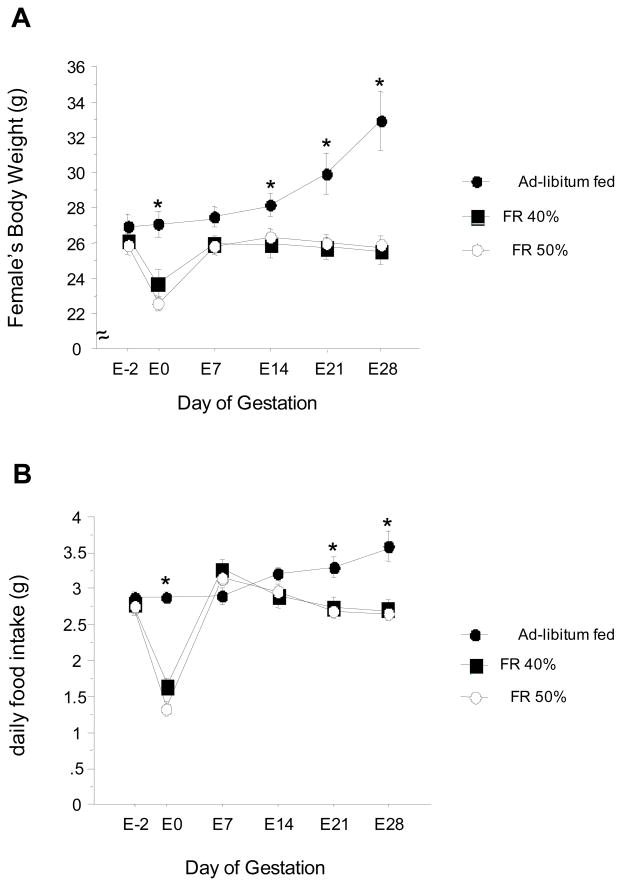
Sexually-experienced females were either maintained on ad-libitum feeding or food restricted at 40% or 50% for 48 h and then immediately mated to a sexually-experienced male. Ninety percent of the ad-libitum fed females engaged in mating behavior with the males, and 62% of these females gave birth to pups approximately 30 days later, with an average of 2.5 ± 0.5 pups per litter. In contrast, none of the females that were food-restricted at 50% displayed significant sexual behavior and none produced any litters. Likewise, the majority of females food-restricted at 40% did not display sexual behavior; the few females in this group that did mate with the male (~ 30%) failed to produce litters. Body weight and food intake data measured prior to mating and over the course of the gestational period indicated that, while the ad-libitum fed females gained significant weight and ate significantly more from pre-conception to the 4th week of gestation, the females in each of the food restricted groups did not gain any weight or show increased food intake (p<0.05 relative to ad-lib controls; Fig. 2); these data suggest that the absence of litters in the 40% food restricted group reflects impairment very early in (or before) pregnancy, rather than miscarriage midway through or late in gestation.
Figure 2.
Mean ± S.E.M. body weights (A) and daily food intake (B) of female musk shrews over the course of pregnancy. Shrews were fed ad-libitum throughout the experiment or food-restricted (FR) for 48 h (40% or 50% decrease) just prior to mating with a stud male. Day of mating is considered beginning of pregnancy (E0).
3.2 Experiment 2: 40% acute food restriction during late stages of gestation, but not early stages, reduces pregnancy success
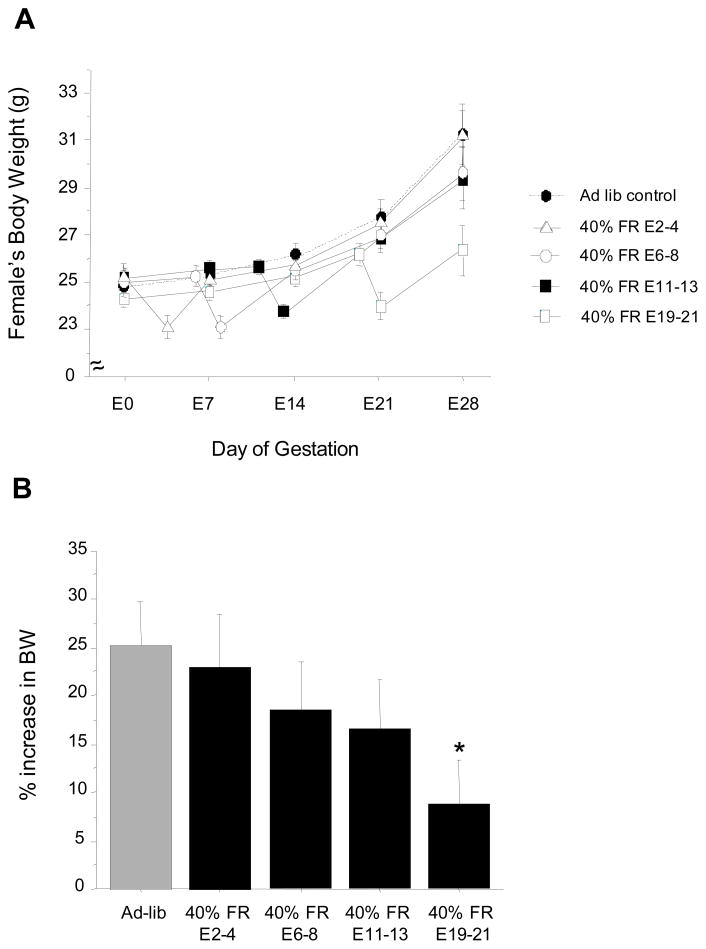
This experiment determined whether metabolic challenges imposed by moderate (40%) short-term food restriction, given at varying stages of pregnancy, would impact gestation, parturition, and/or litter parameters of female shrews. Body weight data indicated that ad-libitum females rapidly gained weight beginning around the second week of gestation, with body weight peaking at approximately 25% higher than that of pre-conception levels by the 3rd to 4th week of pregnancy (Fig 3). All 40% food restricted groups showed significant drops in body weight immediately following the 48 h food restriction period. However, all food restricted groups, except the E19-21 group, recovered body weight rapidly after return to ad-libitum feeding, and had significantly elevated body weights by the end of gestation, similar to ad-libitum fed controls (Fig. 3). Despite having elevated body weights before food restriction, the E19-21 group’s body weights rapidly returned to pre-mating levels immediately following the food restriction period and were significantly lower than the ad-libitum fed controls by the end of gestation (p<0.05; Fig 3). Food intake levels followed a similar pattern as body weight: food consumption increased over the course of gestation in ad-libitum fed controls and was ~ 20% higher than initial baseline levels by the end of pregnancy (data not shown). All of the food restricted groups showed a short-term spike in food intake immediately following the 48 h food-restriction period, after which food intake followed the pattern of the ad-libitum fed controls until the end of pregnancy (data not shown).
Figure 3.
(A) Mean ± S.E.M. body weights of female musk shrews over the course of pregnancy. Shrews were fed ad-libitum throughout gestation or moderately food-restricted for 48 h at various time points (40% decrease). Day of mating is considered beginning of pregnancy (E0). (B) Mean ± S.E.M. % increase in body weight (BW) from the beginning of pregnancy (E0) to last week of gestation (E28). * Significantly lower than ad-libitum fed controls.
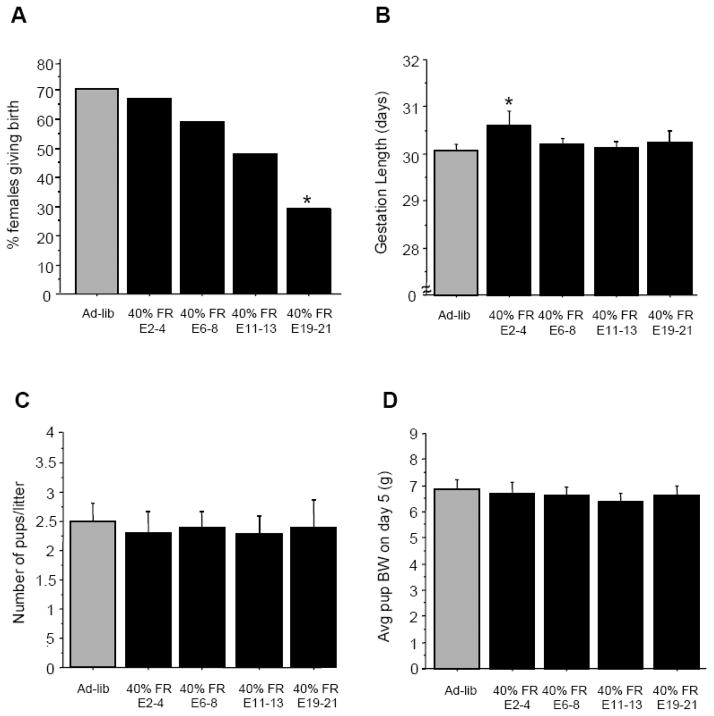
The majority (70%) of female shrews that were fed ad-libitum throughout the study successfully delivered litters, with parturition occurring around 30 days (Fig 4). A similar percentage of females that underwent 40% food restriction at early stages of pregnancy (E2-4, E6-8) successfully gave birth (Fig 4). In contrast, females that were 40% food restricted late in gestation (E19-21) showed a dramatic reduction in the likelihood to successfully deliver pups, with less than 30% of these females producing litters (p<0.05 relative to ad-lib controls; Fig 4). Females that were 40% food restricted on E11-13 showed a trend towards lower rates of pregnancy success than ad-libitum controls, but this was not statistically significant (p=0.12). Despite a lower incidence of litters in the E19-21 females, the day of parturition in this group and most other food-restricted groups was not significantly different from the ad-libitum fed group (Fig 4). However, gestational duration was slightly longer in the E2-4 food restricted group compared with ad-libitum fed animals (p<0.05; Fig 4). Litter characteristics, including litter size (i.e., pup number) and mean pup body weights on post-natal day 5 were not significantly different between any of the groups (Fig 4).
Figure 4.
(A) Percent of mated female shrews successfully delivering litters after moderate food restriction. Females were either maintained on an ad-libitum fed regimen for the entire duration of pregnancy or were food restricted 40% for 48 h at specific stages of gestation. (B–D) Parameters of gestation and litters of pregnant females exposed to 40% food restriction for 48 h during different stages of gestation. (B) Mean ± S.E.M. duration of gestation. (C) Mean ± S.E.M. litter size. (D). Mean ± S.E.M. individual pup body weight (BW) on postnatal day 5. For all panels: * Significantly different from ad-libitum fed controls.
3.3 Experiment 3: 50% food restriction during specific periods of gestation impairs pregnancy success and offspring growth and delays the timing of parturition
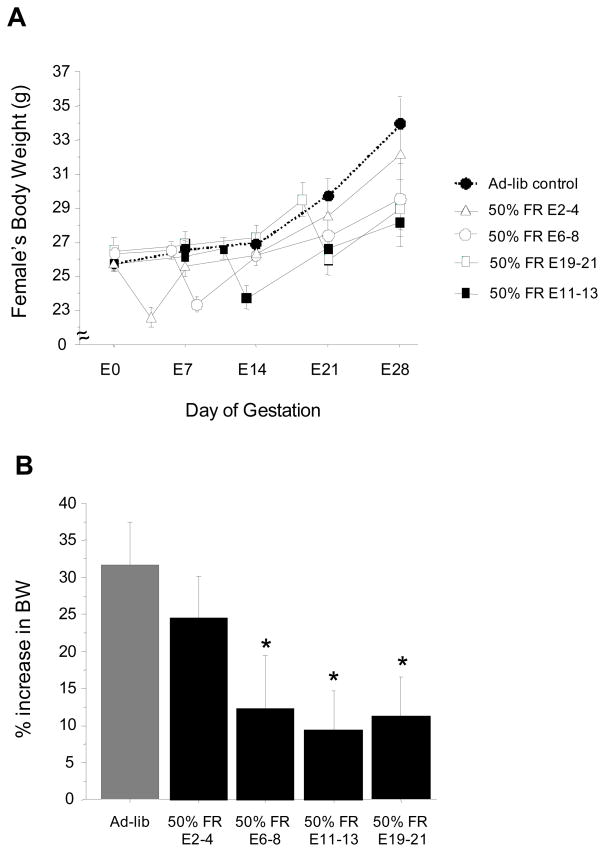
Use of a more dramatic short-term metabolic challenge imposed by 50% food restriction had a severe impact on gestational and litter parameters, not only at later stages of pregnancy, but also at earlier time points (including the period of implantation). As in Experiment 2, body weights of ad-libitum control females increased markedly after the second week of gestation, with body weight approximately 30% higher than that of pre-conception levels by the last week of pregnancy (Fig 5). Females in the 50% food restricted groups showed significant body weight loss immediately following the two-day 50% food restriction period (Fig 5). The E2-4 food-restricted group recovered body weight rapidly after return to ad-libitum feeding and had significantly elevated body weights by the last week of gestation that were not significantly different than ad-libitum fed females (Fig 5); in contrast, the E6-8, E11-13, and E19-21 food restricted groups had only mildly elevated mean body weights by the end of pregnancy, significantly lower than ad-lib controls (p<0.05 for each group relative to ad-libitum group; Fig 5). As in Experiment 2, patterns of food intake mirrored the body weight data, increasing significantly by almost 25% by the end of gestation in the ad-libitum fed and E2-4 groups, but not as much in the other food-restricted groups (data not shown).
Figure 5.
(A) Mean ± S.E.M. body weights of female shrews that were fed ad-libitum throughout gestation or severely food-restricted for 48 h at various time points (fed 50% of daily food intake). Day of mating is considered beginning of pregnancy (E0). (B) Mean ± S.E.M. % increase in body weight (BW) from the beginning of pregnancy (E0) to last week of gestation (E28). * Significantly lower than ad-libitum fed controls.
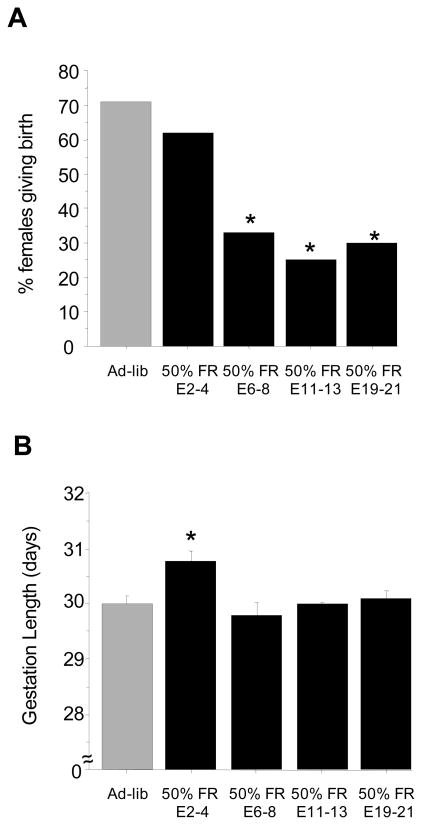
Similar to Experiments 1 and 2, the majority (70%) of female shrews that were fed ad-libitum throughout the study successfully delivered litters, with parturition occurring around 30 days (Fig 6). In contrast, females that were food restricted by 50% in mid-to-late gestation (on E6-8, E11-13 and E19-21) showed major reductions in the likelihood to deliver pups, with only 25–33% of these females producing litters (p<0.05 for each group versus ad-libitum fed controls; Fig 6). However, 50% food restriction did not impair pregnancy success when given early in gestation, prior to implantation (on E2-4); females in this food-restricted group were as likely to deliver litters as ad-libitum fed controls (Fig 6).
Figure 6.
(A) Percent of mated female shrews successfully delivering litters after severe food restriction. Females were either maintained on an ad-libitum fed regimen for the entire duration of pregnancy or were food restricted for 48 h (50% decrease) at specific stages of gestation. (B) Mean ± S.E.M. duration of gestation. For both panels: * Significantly different from ad-libitum fed controls.
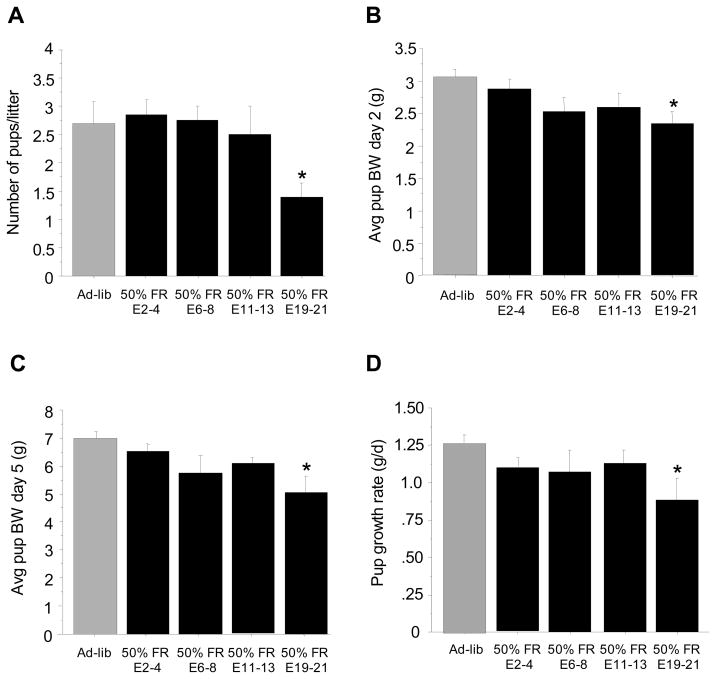
The length of the gestation for the majority of the 50% food-restricted groups was not significantly different from the ad-libitum fed control group, occurring around 30 days (Fig 6); however, as in Experiment 2, the E2-4 food restricted group had a significantly longer gestation (p<0.05 vs ad-libitum controls; Fig 6). Litter characteristics, including litter size and mean pup body weights on post-natal days 2 and 5 were not significantly different between the ad-libitum controls and most of the food-restricted groups, except the E19-21 group, which had significantly lower litter size and smaller average pup body weights on both postnatal d2 and d5 (p<0.05 versus ad-libitum fed for all measures; Fig 7). Moreover, the postnatal growth rate of pups from d2 to d5 was also ~ 30% slower in the E19-21 group versus ad-libitum controls (p<0.05; Fig 7), indicating a slower postnatal growth trajectory in these pups from E19-21 food-restricted mothers.
Figure 7.
Litter parameters and offspring development after pregnant females were exposed to 48 h of 50% food restriction at different stages of gestation. (A) Mean ± S.E.M. litter size. (B) Mean ± S.E.M. individual pup body weight (BW) on postnatal day 2. (C) Mean ± S.E.M. individual pup body weight (BW) on postnatal day 5. (D). Mean ± S.E.M. growth rate of pups (g/day) from postnatal day 2 to postnatal day 5. For all panels: * Significantly different from ad-libitum fed controls.
4. Discussion
This study examined the effects of acute energetic challenge of ecological relevance on pregnancy dynamics and identified critical periods of metabolic susceptibility during mammalian gestation. Our results illustrate a robust inverse relationship between short-term energy balance and pregnancy success, depending on the specific stage of pregnancy when the energetic challenge occurs. Specifically, later stages of pregnancy, such as the 3rd week of gestation in musk shrews, appear to be particularly vulnerable to short-term (48 h) decreases in energy availability (of both mild and moderate degrees), with both pregnancy success and pup growth affected. Thus, at mid and late stages of pregnancy, transient energetic challenges associated with acute underfeeding, as may often occur in nature, can significantly restrain shrew gestational success and offspring postnatal development; these effects are similar to those observed in rodent and ovine models of intra-uterine growth restriction which typically utilize more extreme paradigms of energy challenge (either greater absolute food reductions or longer periods of food restriction). In contrast, earlier stages of shrew pregnancy, particularly time periods prior to implantation, are more immune to acute fluctuations in energetic challenges, with parturition success and pup characteristics virtually unaffected. Even so, we found that acute pre-implantation food restriction lengthened gestation duration, suggesting that energetic and/or metabolic challenges experienced early in pregnancy delays the time of implantation. This conjecture suggests that the pre-implantation period may serve as an energetic buffer, delaying the onset of energetic investment towards pregnancy if sufficient energy or metabolic stores are unavailable soon after ovulation and conception.
Approximately 70% of ad-libitum fed female shrews successful maintained pregnancy and gave birth, corresponding well with observed pregnancy rates in our breeding colony, in which 70–75% of mated females typically give birth (Kauffman and Rissman, unpublished observations). Acute food restriction for just 48 h significantly impaired the likelihood of females to successfully give birth, depending on both the stage of gestation and the degree of energetic challenge. At both magnitudes of 48 h food restriction (40% and 50%), pregnancy success was dramatically impaired when the energetic challenge occurred during later stages of gestation (E19-21), with less than one third of these females delivering litters. Thus, unlike earlier gestational times, later stages of pregnancy appear to be extremely sensitive to rapid, short-term fluctuations in energy availability. Although the mid-stages of gestation (E6-8 and E11-13) were less susceptible to the effects of 40% food restriction, these stages were strongly affected by more severe food restriction (50%). In contrast, early pregnancy (before implantation) was effectively impervious to acute energetic challenges of either magnitude; food-restricted females in E2-4 groups were as likely to give birth as ad-libitum fed controls. Thus, the pre-implantation period is less susceptible, in terms of pregnancy success, to transient fluctuations in energy balance. This may be due to either lower energy requirements for mothers at this early gestational stage, and/or the plastic nature of the delayed implantation process, whose duration may be extended under times of energetic duress (discussed more below).
The sensitivity of pregnant shrews to acute food restriction appears to begin around E6-8 and becomes more salient as pregnancy advances. The mechanisms by which short-term energetic challenges inhibit pregnancy outcome at mid-to-late stages of gestation are unknown, although there are several possibilities. First, in the face of decreased energy intake, pregnant mothers may simply not have enough energetic and nutritional resources to concurrently support both fetal growth and their own survival. In support of this notion, in our study, the gestational stages of greatest susceptibility to metabolic inhibition (i.e., late pregnancy) correlate with the stages of highest female body weight and food intake, both of which significantly increased after the second week of gestation and peaked by the third week. A critical need for energy and/or metabolic resources may therefore be essential in the second half of pregnancy (but not earlier) to supply the growing fetuses with nutrients or energy. Second, it is also possible that food restriction in later stages alters the secretion of hormones which are critical to pregnancy maintenance. Progesterone (P4) levels in pregnant shrews begin to rise approximately halfway through gestation (~ E15) and peak by the third week of gestation [31, 34, 35], correlating well with the gestational stage of highest susceptibility to metabolic challenge observed in our study. Thus, food restriction may inhibit pregnancy outcome by decreasing P4 secretion and/or binding during later periods of gestation. Supporting this possibility, Furumura et al. [35] reported that only 44% of shrews that are ovariectomized (and hence, lacking P4) on E15 maintain pregnancy by E25. However, a similar study by Hasler et al. [31] found that 65% of both ovariectomized and control pregnant shrews give birth, indicating that additional studies are needed to resolve this issue.
The typical gestational period for ad-libitum fed female shrews was 30 days, similar to previous reports [31, 36]. For groups that were food restricted at the time of implantation or later (E6-8, E11-13, E19-21), neither 40% nor 50% food restriction had any impact on gestational duration, with birth occurring at a similar time as in ad-lib controls. In contrast, both 40% and 50% short-term food restriction occurring prior to implantation (days E2-4) resulted in slightly longer gestational periods and hence, a significant delay in parturition. In fact, one 50% food-restricted E2-4 female did not deliver pups until late on gestational day 33 (3 days later than controls). It is unclear whether even greater degrees of energetic challenge during this pre-implantation stage (or a longer duration of food restriction, such as 72 h) would result in even greater delays in parturition. The fact that food restriction at other times, including at implantation (E6-8), did not alter the gestational duration suggests that the longer gestation in E2-4 animals reflects a lengthening of the pre-implantation stage (i.e., a longer diapause and delay in the implantation process). Thus, the pre-implantation duration appears to be actively regulated in shrews, such that the timing of implantation is adjustable, contingent upon sufficient energetic and metabolic status. The pre-implantation period may therefore serve as an energetic buffer mechanism, allowing the postponement of implantation (and hence, later more costly stages of pregnancy) based on short-term reductions in environmental energy availability and internal metabolic stores. In support of this conjecture, some pregnant laboratory rodents can display facultative delayed implantation when they are already lactating a prior litter of substantial size during the pre-implantation period [37–40]. The physiological mechanism(s) by which available metabolic fuels subsequently regulate implantation status is currently unknown.
Under conditions of 40% food restriction, neither the average litter size nor postnatal pup body weight was significantly altered, regardless of stage that the energetic challenge occurred. However, 50% food restriction during later stages of pregnancy significantly reduced litter size and pup body weight, as well as pup developmental growth rates. Specifically, 50% food restriction late in gestation (E19-21) lowered litter size by almost 40% and reduced pup’s postnatal body weights by more than 20% relative to ad-libitum fed animals. Furthermore, pups’ postnatal growth rates between day 2 and day 5 were also 30% lower in the E19-21 group, indicating a significantly slower developmental growth trajectory. However, as with the likelihood to give birth, these offspring parameters were not affected in the pre-implantation group (E2-4), indicating that this early stage of pregnancy is less susceptible to energetic challenge in terms of ability to maintain pregnancy and maximally support offspring development.
Previous studies have shown that short-term (48 h) food restriction drastically reduces female sexual behavior in musk shrews and other mammals [6, 8, 10, 28, 41]. In shrews, typically only 10–40% of females display female sexual behavior when they are acutely food restricted prior to mating [10, 28, 41, 42], but it is unknown whether the few food-restricted females that actually mate are capable of getting pregnant. In the present study, most food-restricted females did not mate and the few that mated (~30%) failed to become pregnant (i.e., they did not gain weight or produce any litters). This suggests that food restriction just prior to mating interferes not only with female sexual behavior, but also some critical aspect(s) involved in the early initiation of pregnancy. While the exact mechanism is currently not known, energetic challenges during this pre-mating interval might interfere with such impending processes as ovulation, fertilization, or corpora lutea formation, all of which occur within the first 24 h after mating in shrews. Interestingly, our findings that pre-mating restriction impairs the ability to get pregnancy differ from those in rodents, particularly hamsters and mice, where short-term pre-mating caloric restriction does not as dramatically affect pregnancy outcomes [43, 44]; however, the degree of food restriction in these other studies was not as severe (ex.: 20–24% restriction in mice) and a larger magnitude of energetic challenge may support our results in shrews that were 40% restricted.
Recently, there has been increasing focus on in utero metabolic effects on subsequent offspring development and physiology later in adulthood. Such concepts as “intrauterine growth restriction” and “metabolic programming” have received considerable attention in disease etiology, both in humans and other specie (reviewed in [11, 15–17, 45]). Our present data indicate that shrew pup postnatal development, at least during the first week of life, is negatively impacted by energetic challenges experienced in utero. Moreover, unlike most other studies, we have shown that short-term (acute) impairments in in utero metabolic status (just 48 h rather than a week or month) can dramatically influence shrew offspring growth. Thus, while animal models for in utero effects have previously been limited primarily to rodents and sheep, our study suggests that musk shrews may be a good model for future studies on this topic. Like rodents, shrews are small and have rapid generational times, making them convenient to work with. In addition, like humans, musk shrews have small litters and thus intra-litter effects (such as intrauterine position) are less likely to influence the impact of in utero manipulations. While it remains to be determined whether fluctuations of energy availability experienced in utero result in metabolic, cardiovascular, or endocrine dysfunction in adult shrews, this species likely represents a useful animal model of metabolic programming, especially for cases of transient energy challenge rather than prolonged food restriction.
In summary, our findings delineate a critical relationship between short-term energy balance and reproductive success, and indicate that transient food cues regulate both female sexual behavior and pregnancy parameters, as well as offspring postnatal development. Unlike previous studies, we have utilized a food restriction paradigm which better mimics transient, day-to-day fluctuations in food availability, similar to what might commonly be experienced in nature. We conclude that mild, short-term food restriction, which impacts sexual behavior, has minimal effects on birth rates and litter parameters at earlier stages of pregnancy, but robustly impairs pregnancy during mid to late gestation. The effective immunity of the pre-implantation stage to energetic challenge may reflect the plastic nature of the pre-implantation period, such that the timing of implantation can be modified in the face of energetic challenges in order to optimize reproductive success and offspring development. To this end, the process of delayed implantation may have evolved, in part, as a mechanism to prevent pregnancy failure early in gestation.
Acknowledgments
The authors thank Aileen Wills for her expert technical assistance. The authors are supported by NIH grants R00 HD056157 (ASK) and R01 MH57759 (EFR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81(2):289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1277–96. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 3.Bronson FH. Effect of food manipulation on the GnRH-LH-estradiol axis of young female rats. Am J Physiol. 1988;254(4 Pt 2):R616–21. doi: 10.1152/ajpregu.1988.254.4.R616. [DOI] [PubMed] [Google Scholar]
- 4.Bronson FH. Puberty in female rats: relative effect of exercise and food restriction. Am J Physiol. 1987;252(1 Pt 2):R140–4. doi: 10.1152/ajpregu.1987.252.1.R140. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton GD, Bronson FH. Food restriction and reproductive development in wild house mice. Biol Reprod. 1985;32(4):773–8. doi: 10.1095/biolreprod32.4.773. [DOI] [PubMed] [Google Scholar]
- 6.Jones JE, Wade GN. Acute fasting decreases sexual receptivity and neural estrogen receptor-alpha in female rats. Physiol Behav. 2002;77(1):19–25. doi: 10.1016/s0031-9384(02)00780-1. [DOI] [PubMed] [Google Scholar]
- 7.Kauffman AS, et al. Gonadotropin-releasing hormone-II messenger ribonucleic acid and protein content in the mammalian brain are modulated by food intake. Endocrinology. 2006;147(11):5069–77. doi: 10.1210/en.2006-0615. [DOI] [PubMed] [Google Scholar]
- 8.Kauffman AS, Rissman EF. A critical role for the evolutionarily conserved gonadotropin-releasing hormone II: mediation of energy status and female sexual behavior. Endocrinology. 2004;145(8):3639–46. doi: 10.1210/en.2004-0148. [DOI] [PubMed] [Google Scholar]
- 9.Morgan CD, Wiederman MW, Pryor TL. Sexual functioning and attitudes of eating-disordered women: a follow-up study. J Sex Marital Ther. 1995;21(2):67–77. doi: 10.1080/00926239508404386. [DOI] [PubMed] [Google Scholar]
- 10.Temple JL, et al. Mating behavior is controlled by acute changes in metabolic fuels. Am J Physiol Regul Integr Comp Physiol. 2002;282(3):R782–90. doi: 10.1152/ajpregu.00383.2001. [DOI] [PubMed] [Google Scholar]
- 11.Coad J, Al-Rasasi B, Morgan J. Nutrient insult in early pregnancy. Proc Nutr Soc. 2002;61(1):51–9. doi: 10.1079/pns2001136. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer MS. Maternal nutrition and adverse pregnancy outcomes: lessons from epidemiology. Nestle Nutr Workshop Ser Pediatr Program. 2005;55:1–10. doi: 10.1159/000082589. discussion 11–5. [DOI] [PubMed] [Google Scholar]
- 14.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–21. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Newnham JP, et al. Nutrition and the early origins of adult disease. Asia Pac J Clin Nutr. 2002;11(Suppl 3):S537–42. doi: 10.1046/j.1440-6047.11.supp3.11.x. [DOI] [PubMed] [Google Scholar]
- 17.McMillan IC, et al. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin Pharmacol Toxically. 2008;102(2):82–9. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 18.Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846(2):236–42. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- 19.Desai M, et al. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007;196(6):555, e1–7. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyatt MA, et al. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J Endocrinol. 2007;192(1):87–97. doi: 10.1677/joe.1.06801. [DOI] [PubMed] [Google Scholar]
- 21.Muaku SM, et al. Maternal protein restriction early or late in rat pregnancy has differential effects on fetal growth, plasma insulin-like growth factor-I (IGF-I) and liver IGF-I gene expression. Growth Regul. 1995;5(3):125–32. [PubMed] [Google Scholar]
- 22.Rogers EH, et al. Lack of evidence for intergenerational reproductive effects due to prenatal and postnatal undernutrition in the female CD-1 mouse. Reprod Toxically. 2003;17(5):519–25. doi: 10.1016/s0890-6238(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Woodall SM, et al. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol. 1996;150(2):231–42. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- 24.Ford SP, et al. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85(5):1285–94. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- 25.Lesage J, et al. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142(5):1692–702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- 26.MacLaughlin SM, et al. Periconceptional nutrition and the relationship between maternal body weight changes in the periconceptional period and feto-placental growth in the sheep. J Physiol. 2005;565(Pt 1):111–24. doi: 10.1113/jphysiol.2005.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. Ilar J. 2004;45(1):25–34. doi: 10.1093/ilar.45.1.25. [DOI] [PubMed] [Google Scholar]
- 28.Gill CJ, Rissman EF. Female sexual behavior is inhibited by short- and long-term food restriction. Physiol Behav. 1997;61(3):387–94. doi: 10.1016/s0031-9384(96)00449-0. [DOI] [PubMed] [Google Scholar]
- 29.Temple JL, Rissman EF. Acute re-feeding reverses food restriction-induced hypothalamic-pituitary-gonad axis deficits. Biol Reprod. 2000;63(6):1721–6. doi: 10.1095/biolreprod63.6.1721. [DOI] [PubMed] [Google Scholar]
- 30.Dryden GL. Post-parturitional conception in captive musk shrews, Suncus murinus. J Reprod Fertil. 1970;23(3):493–5. doi: 10.1530/jrf.0.0230493. [DOI] [PubMed] [Google Scholar]
- 31.Hasler MJ, Nalbandov AV. Pregnancy maintenance and progesterone concentrations in the musk shrew, Suncus murinus (order: Insectivora) Biol Reprod. 1978;19(2):407–13. doi: 10.1095/biolreprod19.2.407. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa A, Tsubota Y, Namikawa T. Morphological and reproductive characteristics of musk shrews (Suncus murinus) collected in Bangladesh, and development of the laboratory line (BAN line) derived from them. Jikken Dobutsu. 1987;36(3):253–60. doi: 10.1538/expanim1978.36.3_253. [DOI] [PubMed] [Google Scholar]
- 33.Temple JL, Rissman EF. Brief refeeding restores reproductive readiness in food-restricted female musk shrews (Suncus murinus) Horm Behav. 2000;38(1):21–8. doi: 10.1006/hbeh.2000.1596. [DOI] [PubMed] [Google Scholar]
- 34.Hasler MJ, Nalbandov AV. Ovulation, ovum maturation and changes in plasma and adrenal progesterone concentrations in the musk shrew (Suncus murinus) Biol Reprod. 1980;22(2):377–81. doi: 10.1093/biolreprod/22.2.377. [DOI] [PubMed] [Google Scholar]
- 35.Furumura K, et al. Mammary growth and plasma progesterone level during pregnancy in the house musk shrew, Suncus murinus Linnaeus. Endocrinol Jpn. 1983;30(5):621–30. doi: 10.1507/endocrj1954.30.621. [DOI] [PubMed] [Google Scholar]
- 36.Dryden GL. Reproduction in Suncus murinus. J Reprod Fertil. 1969;6:377–396. [Google Scholar]
- 37.Norris ML, Adams CE. Mating post partum and length of gestation in the Mongolian gerbil (Meriones unguiculatus) Lab Anim. 1981;15(2):189–91. doi: 10.1258/002367781780958883. [DOI] [PubMed] [Google Scholar]
- 38.Norris ML, Adams CE. Mating post partum, concurrent lactation and reproduction in the laboratory rat. Lab Anim. 1979;13(2):167–70. doi: 10.1258/002367779780943530. [DOI] [PubMed] [Google Scholar]
- 39.Weichert C. The experimental shortening of delayed pregnancy in the albino rat. Anatomical Record. 1940;77:31–48. [Google Scholar]
- 40.Woodside B, Cohen LR, Jans JE. Effects of food restriction during concurrent lactation and pregnancy in the rat. Physiol Behav. 1987;40(5):613–5. doi: 10.1016/0031-9384(87)90106-5. [DOI] [PubMed] [Google Scholar]
- 41.Kauffman AS, et al. Evidence that the type-2 gonadotrophin-releasing hormone (GnRH) receptor mediates the behavioural effects of GnRH-II on feeding and reproduction in musk shrews. J Neuroendocrinol. 2005;17(8):489–97. doi: 10.1111/j.1365-2826.2005.01334.x. [DOI] [PubMed] [Google Scholar]
- 42.Temple JL, Millar RP, Rissman EF. An evolutionarily conserved form of gonadotropin-releasing hormone coordinates energy and reproductive behavior. Endocrinology. 2003;144(1):13–9. doi: 10.1210/en.2002-220883. [DOI] [PubMed] [Google Scholar]
- 43.Szymanski LA, Tabaac BJ, Schneider JE. Signals that link energy to reproduction: gastric fill, bulk intake, or caloric intake? Physiol Behav. 2009;96(4–5):540–7. doi: 10.1016/j.physbeh.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Marsteller FA, Lynch CB. Reproductive responses to variation in temperature and food supply by house mice. I. Mating and pregnancy. Biol Reprod. 1987;37(4):838–43. doi: 10.1095/biolreprod37.4.838. [DOI] [PubMed] [Google Scholar]
- 45.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]