Abstract
Technologic advances, medical specialization, novel payment structures, and an increased scientific knowledge base have resulted in a health care system requiring trained experts to deliver guidance as patients complete care plans: Enter the concept of patient navigation.
The word navigate comes from two Latin root words—Navis (ship) and agree (to drive). The definition of navigate is to travel over or through safely. Over the past 20 years, the concept of patient navigation through a complex and fragmented health care system has evolved. During that time, the proliferation of technologic advances, medical specialization, novel payment structures, and a massively increased scientific knowledge base has resulted in a system that requires someone from within the health care system to deliver guidance to consumers: Enter the concept of patient navigation.
From the Streets of Harlem: Focus on Access to Care and Disparities
Dr Harold Freeman, a surgeon whom many consider to be the founding father of patient navigation, initially developed an approach to address the heavier burden of disease borne by the low-income population that he served in Harlem, New York City, New York. The Harlem Patient Navigation Program was designed as a system to reduce disparities in access to health care. The program was composed of navigators who were from the community or who were culturally similar to the population served. These navigators were trained experts in the course of clinical care that the patient must traverse in order to complete care plans. This system was able to demonstrate a reduction in racial, ethnic, and poverty-driven disparities in care.1
Dr Freeman's work gained national recognition, ultimately resulting in the passage of the Patient Navigator Act of 2005. This bill was meant to “ensure that everyone will have an advocate at their side, helping them navigate through today's complicated health care system.”2 The bill also designated funds to create an outreach program with a focus on prevention, access to care, and screening in disparate communities. The patient navigator was defined as an individual who could educate and empower patients, serving as their advocate in navigating the health care system.2
Pilot programs such as Dr Freeman's provided early evidence of benefit, but were limited in broad reproducibility. Their main limitations were that they were single-site interventions and had varying definitions of navigation. Stimulated by the Patient Navigation Act, the National Cancer Institute funded a nine-site Patient Navigation Research Program (PNRP). The PNRP was charged with designing, implementing, and evaluating a reproducible patient navigation program targeting vulnerable populations. The PNRP defined patient navigation as “support and guidance offered to vulnerable persons with abnormal cancer screening or a cancer diagnosis, with the goal of overcoming barriers to timely, quality care.”3 The primary outcomes were time to diagnostic resolution, time to initiation of cancer treatment, patient satisfaction with care, and cost effectiveness. The PNRP focused on four malignancies—breast, colorectal, cervical, and prostate cancer. The first work reported by this group defines the metrics that will assess the processes and outcomes of patient navigation. These metrics are currently being evaluated by the program sites for validity and reliability.3
Despite the lack of consensus as to how patient navigation is to be defined, implemented, and measured, these interventions have been increasingly adopted throughout the United States and Canada. A recent review of the literature found 16 of 45 published studies provided data on the efficacy of patient navigation services in improving timeliness and receipt of cancer screening, diagnostic follow-up care, and treatment. The reported increases in screening ranged from 10.8% to 17.1%, and increases in adherence to diagnostic follow-up care ranged from 21% to 29.2% relative to control groups. Most of the published trials had significant methodological limitations, including lack of a control group, small sample sizes, and contamination with other interventions. Wells et al4 conclude that further rigorous research is necessary to evaluate the efficacy and cost effectiveness of patient navigation in improving cancer care.
Enter the Business Model: Efficiency and Patient Retention
The practice of conventional oncology has arguably seen the most significant changes of any medical specialty over the past 15 years. Advances in imaging, surgical techniques, chemotherapeutic agents, and radiation oncology technologies have made the delivery of care more effective, but increasingly complex and expensive. This complex care requires significant coordination. Health care providers have increasingly looked to patient navigators to develop a patient centered approach to integrated care. Cost savings from the care coordination provided by patient navigation can include decreased emergency department visits, reduction in inappropriate admissions and readmissions, reduction in unnecessary diagnostic testing, standardized treatment protocols, effective patient management throughout the continuum of care, and increased appropriate use of hospice care.
On the positive revenue side, as early disease detection and better implementation of improved therapeutic options continue to increase cancer survival rates, cancer becomes more of a chronic condition. In this scenario, health care providers have an opportunity to ensure that patients have a positive experience of care and to foster patient loyalty, which may lead to new and repeat service utilization over a longer life span. From the perspective of a business model, patient navigation can therefore improve outcomes and efficiency for patients, physicians, and administrators. There is also the real potential of delivering a return on investment in actual dollars through increased utilization and cost savings.5
Cancer Care for the Whole Patient: The Standard of Care
The Institute of Medicine published a report in 2007 that called for a new standard of care that addresses psychosocial problems associated with cancer. The report states that “all patients with cancer and their families should expect and receive cancer care that ensures the provision of appropriate psychosocial health services.”6 These services include “those interventions that enable patients, their families and health care providers to optimize biomedical health care and to manage the psychological/behavioral and social aspects of illness and its consequences so as to promote better health.” The report goes on to emphasize the need to evaluate how to deliver this level of care at no cost to the patient, while evaluating how to best implement this in different settings through a work force with a variety of clinical and nonclinical competencies. Research priorities include several areas that are a perfect fit for the hypothesized advantages of patient navigation: improved patient-provider communication; screening tool utilization to identify vulnerable populations with increased needs; approaches for linking patients with services and coordinating care; and evaluation of and referral to appropriate illness and wellness management interventions.6
Hierarchy of Patient Needs
The recognition of a huge variety of patient, family, and provider needs can be overwhelming. It is helpful to develop a system that takes into account the patients disease trajectory—from screening through diagnosis, treatment, survivorship, and end-of-life care—and then to match interventions appropriate for each phase. There has been some work toward this end matching psychosocial needs and suggested interventions (Table 1).7,8 It is interesting to note that the patients' levels of involvement is low to medium during diagnosis and treatment when procedures and psychoeducational support are being done or delivered to them, whereas their levels of involvement becomes higher during recovery, recurrence, and end-of-life phases, when their responsibility for managing life outside of cancer becomes more self driven and less system driven.
Table 1.
Hierarchy of Psychosocial Needs, Suggested Interventions, and Patient Level of Involvement Across Care Continuum
| Stage of Care | Predominant Psychosocial Picture | Suggested Psychosocial Intervention | Patient Level of Involvement in Driving Process |
|---|---|---|---|
| Diagnosis | Anxiety, information seeking, depression | Psychoeducation, information provision, emotional support | Low to low medium |
| Treatment | Anxiety, treatment adverse effects | Coping skills training, emotional support | Medium |
| Recovery | Reintegration, depression | Emotional support, psychotherapy | Medium high |
| Recurrence | Depression, death and dying | Psychotherapy, spiritual/existential therapy | High |
NOTE. Data adapted.8
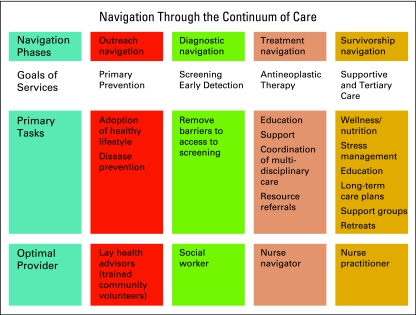
From a patient navigation perspective, there are multiple variables that must be taken into account at all of these points of contact. The general categories of patient services can be divided into prevention, supportive care, and antineoplastic therapies. Navigation phases can be divided into outreach, diagnostic, treatment, and survivorship. Tasks will vary across that continuum, as will the level of expertise of the individual helping to provide navigation services. Figure 1 offers a general guide to how this could look across the spectrum of care.
Figure 1.
Navigation through the continuum of care.
Future Directions
The field of patient navigation touches all levels of health care and has the potential to bring an integrated, health-focused approach to a fragmented system of disease care. An integrative approach to oncology can include all participants, at all levels of their being—their experience of body, mind, soul, and spirit within the self, their specific culture, and the natural world.9 In order to accomplish these lofty goals, significant research will need to be performed concerning best practices, optimal processes, reproducibility of interventions, resource allocation, and a variety of health-related outcomes. Until the time that this research is available, it seems logical to provide services that address obvious prevention, supportive, and antineoplastic gaps in the system through interventions with evidence base for both safety and efficacy.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Vargas RB, Ryan GW, Jackson CA, et al. Characteristics of the original patient navigation program to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008;133:426–433. doi: 10.1002/cncr.23547. [DOI] [PubMed] [Google Scholar]
- 2.Democratic Caucus of the US House of Representatives: The Patient Navigator Act of 2005 Passes in the House of Representatives. www.dems.gov/index.asp?Type=B_PR&SEC={14574552-CA30-4FDB-985E-0EF376B2D1A7}&DE={4104C04C-6A87-4CD9-A852-98C7CC98661B}
- 3.Freund KM, Battaglia TA, Calhoun E, et al. National Cancer Institute Patient Navigation Research Program: Methods, protocol, and measures. Cancer. 2008;113:3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: State of the art or is it science? Cancer. 2008;133:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SG2 Health Care Intelligence: Clinical Intelligence: Cancer Care Coordination with Nurse Navigators. 2006. http://www.sg2.com.
- 6.Institute of Medicine. Cancer Care for the Whole Patient. Washington, DC: National Academies Press; 2008. [Google Scholar]
- 7.Cunningham AJ. Group psychological therapy for cancer patients: A brief discussion of indications for its use, and the range of interventions available. Support Care Cancer. 1995;3:244–247. [PubMed] [Google Scholar]
- 8.Carlson L. Mind body interventions. In: Mumber MP MP, editor. Integrative Oncology: Principles and Practice. London, United Kingdom: Taylor and Francis; 2006. p. 324. [Google Scholar]
- 9.Mumber MP MP. Principles of integrative oncology. In: Mumber MP MP, editor. Integrative Oncology: Principles and Practice. London, United Kingdom: Taylor and Francis; 2006. p. 4. [Google Scholar]