Short abstract
A look at the temporal impact of advancements in therapeutic options in the last 10 years—from fluorouracil to irinotecan and oxaliplatin—on overall survival in a population-based cohort.
Abstract
Purpose:
In the past 10 years, the number of available therapeutic options for patients with metastatic colorectal cancer (MCRC) has expanded from fluorouracil (FU) -based therapy to include irinotecan and oxaliplatin. The temporal impact of these advances on the overall survival of a population-based cohort will be evaluated.
Patients and Methods:
Cohort A from years 1995 to 1996 was chosen to represent FU-based chemotherapy. In 2000 and in 2003 to 2004, irinotecan and oxaliplatin respectively became generally available to patients in British Columbia, Canada; cohorts B and C were chosen from these years, respectively. Included were 1,333 patients referred with MCRC (metastatic status, M1) in cohorts A (n = 357), B (n = 268), and C (n = 708). Survival was calculated from time of diagnosis of M1 disease to either death or date of last contact.
Results:
Cohorts were generally similar; more patients received chemotherapy in cohorts B (62%) and C (62%) compared with cohort A (49%; P < .001). In cohort C, 33% of patients received both irinotecan and oxaliplatin, and 10% of patients received biologic therapies. In cohorts A, B, and C, median overall survival was 9.4, 10.8, and 13.1 months (A v C, P = .002; B v C, P = .022) in all patients, respectively, and 12.6, 14.0, and 17.1 months (A v C, P = .004; B v C; P = .019) in patients treated with chemotherapy, respectively. Improvements between cohorts A and C achieved statistical significance, whereas those between A and B did not. Patients not treated with chemotherapy experienced poor outcomes; this remained unchanged.
Conclusion:
In this population-based study, a significant prolongation in overall survival was observed in patients with MCRC in the period in which both irinotecan and oxaliplatin were available. Outcomes parallelled those seen in phase III clinical trials.
Introduction
Colorectal cancer is the second most common malignancy worldwide. Currently, 25% of patients either present with metastatic disease or experience recurrence in this setting, and chemotherapy represents the predominant therapeutic modality with a demonstrated improvement in overall survival (OS).
There have been significant advances in chemotherapy for metastatic colorectal cancer (MCRC) in the past decade. Previously, therapy with combination fluorouracil (FU) and leucovorin (FL) resulted in median OS durations of approximately 12 months in phase III trials.1–3 The addition of irinotecan to FL resulted in an additional 3-month prolongation of OS.4,5 Trials of doublet therapy with oxaliplatin and FL resulted in additional increases in survival ranging from 16 to 20 months.6–8 Recent trials of first-line FL-based chemotherapy in combination with bevacizumab have demonstrated additional increases, with median OS of more than 20 months.9
In their analysis of the effect of chemotherapeutic agents, Grothey et al11 demonstrated that therapy with all three drugs (ie, FL, irinotecan, and oxaliplatin) was an important predictor of median OS in patients enrolled onto phase III trials. Our study was undertaken to estimate the impact of these agents on OS at a population-based level in patients diagnosed with MCRC in cohorts from three different time periods, who were referred to the British Columbia Cancer Agency (BCCA).
Patients and Methods
Patients with newly diagnosed or relapsed metastatic colorectal adenocarcinoma who were referred to the BCCA were included. The BCCA is a provincial care agency with four major centers to which more than 50% of patients with colorectal cancer diagnosed in the province of British Columbia, Canada, are referred. Patients residing outside of British Columbia at time of diagnosis were excluded. Distant metastatic disease or relapse was identified on imaging or biopsy. Patient, tumor, treatment, and outcome data were collected and obtained from the Colorectal Cancer Outcomes database. Time to relapse was determined from time of initial diagnosis to time of diagnosis of distant metastasis. Survival was calculated from time of diagnosis of M1 disease or of distant relapse to either death or date of last contact.
Definition of Cohorts
Three cohorts were chosen as being representative of different time periods in which the respective chemotherapy agents (ie, FL, irinotecan, and oxaliplatin) were available and in which all residents of British Columbia diagnosed with MCRC were reimbursed by a single-payer system. A 1995 to 1996 cohort (A) was chosen to represent the era of primary FU-based chemotherapy. In 2000 and in 2003 to 2004, irinotecan and oxaliplatin were respectively introduced and made accessible to patients; cohorts B and C were chosen to represent these periods, respectively. Patients who presented with either M1 or distant relapsed colorectal disease were included. Cohort A included 357 referred patients; cohort B, 268 patients; and cohort C, 708 patients.
Chemotherapy
Patients were considered to have received chemotherapy if at least one cycle of chemotherapy had been administered, according to the provincial pharmacy database. Treatment was administered according to evidence-based, standardized provincial treatment protocols that were developed by multidisciplinary committees, uniformly available in the internal information system, and—as of January 2000—posted on the BCCA Web site.12 Patients were assigned to mutually exclusive categories according to history of chemotherapy treatment. Bolus and infusional FL regimens as well as administration of oral capecitabine were classified as FU-based therapy. Irinotecan alone or in combination with infusional or bolus FL or capecitabine was classified as irinotecan-based therapy. Oxaliplatin was administered with FL or capecitabine, and this was classified as oxaliplatin-based therapy. Patients who received at least one cycle of both irinotecan- and oxaliplatin-based therapies were assigned to the category of irinotecan and oxaliplatin–based therapy. Patients were assigned to the category of other chemotherapy if they had received raltitrexed, levamisole, mitomycin C, or other therapy on a special-access, nonprotocol basis. Biologic therapies such as bevacizumab and cetuximab were typically administered with either oxaliplatin or irinotecan and were included in those respective groups.
Statistical Analysis
χ2 and analysis of variance were used to detect differences between the three cohorts. OS was calculated from time of distant metastasis to death or date of last contact. Kaplan-Meier curves were used to plot survival. The study was approved by the University of British Columbia Research Ethics Board.
Results
The study included a total of 1,333 patients. Patient demographics and clinical characteristics are summarized in Table 1. Median follow-up of patients still alive was 9.6, 6.0, and 2.1 years in cohorts A, B, and C, respectively. Median age did not significantly differ. Most prognostic characteristics were balanced among the three cohorts. In patients presenting with initial nonmetastatic disease, the time to relapse was shorter in cohort B than it was in cohorts A (P = .004) and C (P = .01). There was no difference among cohorts with respect to primary resection.
Table 1.
Demographics and Clinical Characteristics of Patients Referred in 1995 to 1996 (cohort A), 2000 (cohort B), and 2003 to 2004 (cohort C)
| Characteristic | Patients (N = 1,333) | P* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Between Cohorts | ||||||||||
| Cohort A (n = 357) | Cohort B (n = 268) | Cohort C (n = 708) | A v B | A v C | B v C | ||||||
| No. | % | No. | % | No. | % | ||||||
| Sex | .92 | ||||||||||
| Male, n = 796 | 216 | 60.5 | 158 | 59.0 | 422 | 59.6 | |||||
| Female, n = 537 | 141 | 39.5 | 110 | 41.0 | 286 | 40.4 | |||||
| Age at diagnosis of metastasis, years | .44† | ||||||||||
| Median | 68.3 | 66.4 | 67.7 | ||||||||
| < 50, n = 132 | 30 | 8.4 | 29 | 10.8 | 73 | 10.3 | .25‡ | ||||
| 50-70, n = 689 | 197 | 55.2 | 145 | 54.1 | 347 | 49.0 | |||||
| > 70, n = 512 | 130 | 36.4 | 94 | 35.1 | 288 | 40.7 | |||||
| Primary site of cancer | .73 | ||||||||||
| Colon, n = 996 | 262 | 73.4 | 204 | 76.1 | 530 | 74.9 | |||||
| Rectum, n = 337 | 95 | 26.6 | 64 | 23.9 | 178 | 25.1 | |||||
| Resection of primary | .26‡ | ||||||||||
| Yes, n = 1,023 | 284 | 79.6 | 206 | 76.9 | 533 | 75.3 | |||||
| No, n = 309 | 72 | 20.2 | 62 | 23.1 | 175 | 24.7 | |||||
| Unknown, n = 1 | 1 | 0.3 | 0 | 0 | |||||||
| Presentation of metastatic stage M1 | < .001 | < .001 | .008 | .03 | |||||||
| At diagnosis, n = 1,099 | 272 | 76.2 | 238 | 88.8 | 589 | 83.2 | |||||
| At relapse, n = 234 | 85 | 23.8 | 30 | 11.2 | 119 | 16.8 | |||||
| Site of metastasis at presentation | < .001 | < .001 | < .001 | .11 | |||||||
| None, n = 234 | 85 | 23.8 | 30 | 11.2 | 119 | 16.8 | |||||
| Liver only, n = 615 | 159 | 44.5 | 135 | 50.4 | 321 | 45.3 | |||||
| Lung only, n = 51 | 7 | 2.0 | 11 | 4.1 | 33 | 4.7 | |||||
| Distant nodal only, n = 29 | 9 | 2.5 | 2 | 0.7 | 18 | 2.5 | |||||
| Other solitary sites, n = 143 | 52 | 14.6 | 28 | 10.4 | 63 | 8.9 | |||||
| > 1 distant site, n = 261 | 45 | 12.6 | 62 | 23.1 | 154 | 21.8 | |||||
| Median follow-up, years | |||||||||||
| All patients | 9.2 | 6.0 | 2.1 | ||||||||
| Alive patients | 0.8 | 0.9 | 1.1 | ||||||||
| Adjuvant chemotherapy for metastatic stage M0/MX | .42‡ | ||||||||||
| Yes, n = 83 | 27 | 31.8 | 9 | 30.0 | 47 | 39.5 | |||||
| No, n = 151 | 58 | 68.2 | 21 | 70.0 | 72 | 60.5 | |||||
| Chemotherapy for metastasis | < .001‡ | ||||||||||
| Yes, n = 781 | 173 | 48.5 | 166 | 61.9 | 442 | 62.4 | |||||
| No, n = 552 | 184 | 51.5 | 102 | 38.1 | 266 | 37.6 | |||||
| Type of chemotherapy for metastasis | < .001‡ | < .001 | < .001 | < .001 | |||||||
| FU based alone, n = 297 | 141 | 81.5 | 50 | 30.1 | 106 | 24.0 | |||||
| Irinotecan based, n = 204 | 9 | 5.2 | 87 | 52.4 | 108 | 24.4 | |||||
| Oxaliplatin based, n = 70 | 0 | 0.0 | 2 | 1.2 | 59 | 13.3 | |||||
| Irinotecan and oxaliplatin based, n = 164 | 0 | 0.0 | 18 | 10.8 | 112 | 33.0 | |||||
| Bevacizumab/cetuximab, n = 46 | 0 | 0.0 | 1 | 0.6 | 45 | 10.2 | |||||
| Other chemotherapy, n = 43 | 23 | 13.3 | 8 | 4.8 | 12 | 2.7 | |||||
Abbreviation: FU, fluorouracil.
P calculated on known values only.
Calculated with analysis of variation.
Calculated with χ2.
More patients received chemotherapy for metastatic disease in cohorts B (61.9%) and C (62.4%) compared with those in cohort A (48.5%; P < .001). Irinotecan and oxaliplatin chemotherapy was administered to 10.8% and 33.0% of patients in cohorts B and C, respectively. The proportion of patients who received monoclonal antibody therapy was 0.6% in cohort B and 10.2% in cohort C.
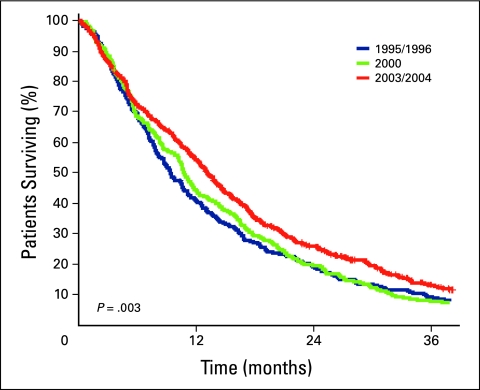
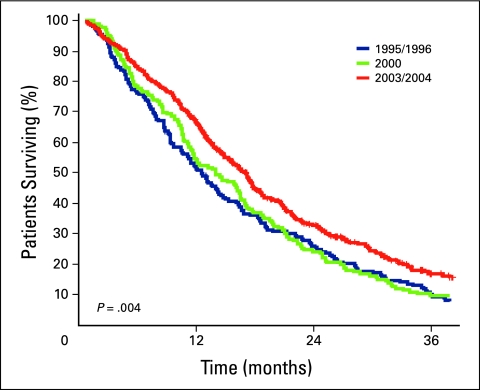
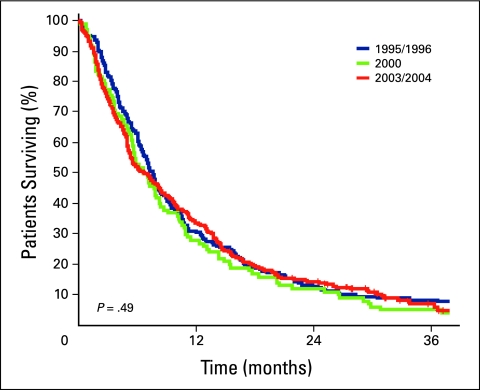
Survival estimates for all patients and for those who did and did not receive chemotherapy are listed in Tables 2 and 3, respectively. A 3.7-month improvement in median survival was observed in patients in cohort C—who were treated in the time period in which all three drugs were available—compared with those in cohort A, who were treated when FL only was available (P = .002). Median OS was longer in the subset of patients who received chemotherapy (Table 3), and the numeric differences between cohorts A (12.6 months) and C (17.1 months) were greater and remained statistically significant (P = .004). Outcomes in patients untreated with chemotherapy were poor and remained unchanged throughout the time periods. Kaplan-Meier survival curves are depicted in Figures 1 through 3.
Table 2.
Survival Estimates for Patients Referred in 1995 to 1996 (cohort A), 2000 (cohort B), and 2003 to 2004 (cohort C)
| Cohort | Survival Estimate | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | At 24 Months | At 36 Months | Overall | Between Cohorts | ||||||
| Months | 95% CI | Months | 95% CI | Months | 95% CI | A v B | A v C | B v C | ||
| A | 9.4 | 8.3 to 10.6 | 19.1 | 15.2 to 23.4 | 9.1 | 6.4 to 12.4 | .003 | .81 | .002 | .022 |
| B | 10.8 | 9.8 to 11.8 | 19.5 | 15.0 to 24.4 | 7.9 | 5.0 to 11.5 | ||||
| C | 13.1 | 12.1 to 14.1 | 25.8 | 22.5 to 29.2 | 13.2 | 10.3 to 16.5 | ||||
Table 3.
Survival Estimates for Patients Who Did and Did Not Receive Chemotherapy in 1995 to 1996 (cohort A), 2000 (cohort B), and 2003 to 2004 (cohort C)
| Cohort | Survival Estimate | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | At 24 Months | Overall | Between Cohorts | |||||
| Months | 95% CI | Months | 95% CI | A v B | A v C | B v C | ||
| Patients who did not receive chemotherapy | .49 | .24 | .61 | .52 | ||||
| A, n = 184 | 7.6 | 6.6 to 8.6 | 12.7 | 8.3 to 18.0 | ||||
| B, n = 102 | 6.7 | 5.0 to 8.3 | 11.9 | 6.5 to 19.1 | ||||
| C, n = 266 | 6.7 | 5.1 to 8.2 | 14.2 | 10.3 to 18.7 | ||||
| Patients who did receive chemotherapy | .004 | .74 | .004 | .019 | ||||
| A, n = 173 | 12.6 | 10.6 to 14.6 | 26.0 | 19.7 to 32.7 | ||||
| B, n = 166 | 14.0 | 11.0 to 17.1 | 24.1 | 17.9 to 30.8 | ||||
| C, n = 442 | 17.1 | 15.7 to 18.5 | 32.8 | 28.2 to 37.5 | ||||
Figure 1.
Kaplan-Meier survival curves of cohorts A (blue line), B (green line), and C (red line).
Figure 3.
Kaplan-Meier survival curves for patients who received chemotherapy in cohorts A (blue line), B (green line), and C (red line).
Figure 2.
Kaplan-Meier survival curves for patients who did not receive chemotherapy in cohorts A (blue line), B (green line), and C (red line).
Discussion
To our knowledge, this is the first population-based study to describe the impact of irinotecan and oxaliplatin on survival of patients with MCRC. Results indicate that significant survival gains occurred in the time period in which both agents were available. Improved survival was only seen in patients who received chemotherapy, the proportion of whom increased significantly across the three cohorts.
The population of British Columbia is similar to that of North America; there is a diverse ethnic mixture, but people are predominately of Western European backgrounds.13 The strength of this study lies in the fact that clinical trials have a selected population of patients who are deemed eligible to be enrolled. These patients tend to be fitter than the average population. This can lead to overestimation of the true magnitude of treatment. Although the median age of our group at 68 years was higher than that in most clinical trials, it was within the median age of diagnosis of colorectal cancer.14 It should also be noted that an increasing proportion of patients was treated over time. This is likely related to changes in practice; more patients were referred for consideration of treatment as newer therapies became available.15 However, it should be noted as well that the median survival for patients treated with best supportive care was not different among the three cohorts, suggesting that differences in baseline prognostic features and the impact of supportive interventions were likely not responsible for the survival gains appreciated over time.
This analysis is subject to the limitations of a retrospective study, in which differences observed among cohorts may be biased because no randomization has occurred. In an effort to overcome this bias, time cohorts rather than treatment cohorts were chosen. Results may also be subject to bias as a result of changes over time in a multitude of factors unrelated to chemotherapy, such as referral, diagnostic, and surgical treatment patterns. No information was available regarding the frequency of resection of metastatic disease in the three groups. Although metastatectomy impacts OS in patients diagnosed with MCRC, it is unlikely that a meaningful change in pattern or practice occurred in British Columbia between 2000 and 2003 or 2004, the time period in which significant improvements in survival were seen in this study.
Known prognostic factors16 were similar among cohorts A, B, and C, including initial stage, time to diagnosis of metastatic disease, history of adjuvant chemotherapy, and age. Of note, there were more patients with more than one site of metastatic disease. This may reflect the change in referral patterns, as more patients with greater burdens of disease were referred for consideration of systemic therapy. Time to diagnosis of metastatic disease was shorter in cohort B, which may explain in part the limited impact of irinotecan observed in this time period. Biologic agents such as bevacizumab and cetuximab, shown to improve OS in MCRC,9,13,17 likely contributed minimally to survival gains seen in cohort C, because only 10% of patients in this cohort received such therapy. A future analysis will be performed to assess the impact of these agents.
It is noteworthy that only 33% of patients in cohort C received both irinotecan and oxaliplatin. This rate is lower than treatment rates reported in phase III trials ongoing during the 2003 to 2004 time period. Although oxaliplatin was not approved in Canada during this era, it was available and fully reimbursed for both referred and nonreferred patients in British Columbia through a Health Canada Special Access Program. The diverse, population-based nature of the study groups may have contributed to the lower than expected rate of patients receiving all three drugs. All four of the BCCA clinics are in urban areas; however, all have large rural catchments that include areas with limited chemotherapy delivery facilities. Thus, some patients may have opted to forgo treatment and the associated travel time.
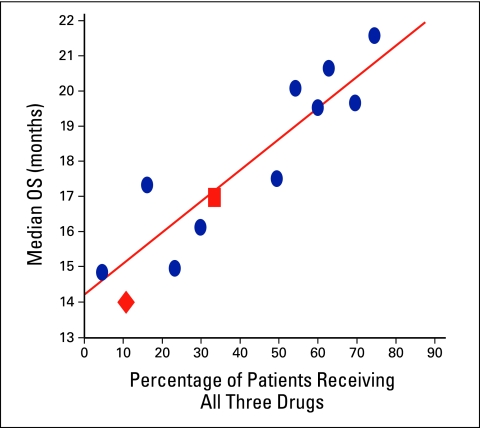
In Figure 4, the median survival of patients receiving chemotherapy in cohorts B and C is plotted on the graph developed by Grothey et al11 of median survival outcomes according to percentage of patients who received all three drugs. Outcomes of BCCA patients seemed similar to those who participated in international clinical trials, strengthening the observations of the current study. Median survival of patients who did not receive chemotherapy was short, likely attributable to significant differences in underlying characteristics (eg, performance status, comorbid illness, and age) among patients not offered or who elected not to receive palliative chemotherapy. The proportion of such patients was significantly lower in the more recent time periods.
Figure 4.
Plot of the Grothey et al11 analysis of overall survival in relation to receiving leucovorin, irinotecan, and oxaliplatin. •, data from published clinical trials included in the Grothey et al analysis; ▪, median survival of 17.1 months in the 33% of patients in cohort C who received all three drugs; ♦, median survival of 14 months in the 10.8% of patients in cohort B who received all three drugs. Adapted with permission from Grothey et al.
In conclusion, significant gains in survival in patients with MCRC were observed in the time period in which both irinotecan and oxaliplatin were available compared with the time periods in which only FU and irinotecan were available. Outcomes in patients who did not receive chemotherapy were unchanged and remained poor. Patients enrolled onto clinical trials are a select population with performance status and organ function higher than those of the general population. The patients presented in this study may well have had clinical factors that would have excluded them from clinical trial participation; however, the data show that a demonstrable benefit was still seen, comparable to that of clinical trials. For clinicians, patients, and policy makers, it is important to estimate the benefit of new therapies at the population-based level to understand the impact of an intervention when generalized to a real-world population. The demonstrated improvements documented in this study may be used in conjunction with clinical trial data to justify the resources used to fund costly yet efficacious therapies in the ongoing treatment of MCRC.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
Presented in part at the 2007 American Society of Clinical Oncology Gastrointestinal Cancers Symposium, January 26-28, 2007, Orlando, FL, and the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
References
- 1.Poon MA, O'Connell MJ, Moertel CG, et al: Biochemical modulation of fluorouracil: Evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 7:1407-1418, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Buroker TR, O'Connell MJ, Wieand HS, et al: Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 12:14-20, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Jäger E, Heike M, Bernhard H, et al: Weekly high-dose leucovorin versus low-dose leucovorin combined with fluorouracil in advanced colorectal cancer: Results of a randomized multicenter trial—Study Group for Palliative Treatment of Metastatic Colorectal Cancer Study Protocol 1. J Clin Oncol 14:2274-2279, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 355:1041-1047, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Cox JV, Blanke C, et al: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer: Irinotecan Study Group. N Engl J Med 343:905-914, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Grothey A, Deschler B, Kroening H, et al: Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24h 5-FU infusion/FA + oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC), Proc Am Soc Clin Oncol 21:129a, 2002. (abstr 512) [Google Scholar]
- 7.de Gramont A, Figer A, Seymour M, et al: Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938-2947, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Sargent DJ, Morton RF, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23-30, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Venook A, Niedzwicki D, Hollis D, et al: Phase III study of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX) ± cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results. J Clin Oncol 24:148s, 2006. ((suppl; abstr 3509)) [Google Scholar]
- 11.Grothey A, Sargent D, Goldberg, et al: Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209-1214, 2004 [DOI] [PubMed] [Google Scholar]
- 12.British Columbia Cancer Agency: Treatment protocols. http://www.bccancer.bc.ca/HPI/default.htm
- 13.Statistics Canada: 2006 Census. http://www12.statcan.gc.ca/census-recensement/index-eng.cfm
- 14.Canadian Cancer Society: Cancer statistics for 2008. http://www.cancer.ca/Canada-wide/About%20cancer/Cancer%20statistics.aspx?sc_lang=en
- 15.Renouf D, Kennecke H, Gill S: Trends in chemotherapy (CT) utilization for colorectal cancer: A provincial population-based analysis. Clin Colorectal Cancer 7:386-389, 2008 [DOI] [PubMed] [Google Scholar]
- 16.O'Connell MJ: Survival following recurrence in patients with adjuvant colon cancer: Findings from the 20,800-patient ACCENT dataset. J Clin Oncol 25:165s, 2007. ((suppl; abstr 4009)) [Google Scholar]
- 17.Saltz L, Clarke S, Diaz-Rubio E, et al: Bevacizumab (Bev) in combination with XELOX or FOLFOX4: Updated efficacy results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer. J Clin Oncol 25:170s, 2007. ((suppl; abstr 4028)) [Google Scholar]