Abstract
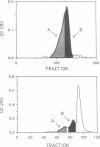
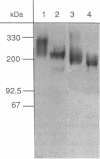
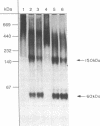
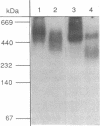
The heat-labile sexual agglutinin from Saccharomyces kluyveri strain 17 (alpha-agglutinin) has been isolated in an apparently intact form from wild-type and mnn1 (glycosylation-defective) cells. The wild-type agglutinin is polydisperse, due to variable degrees of glycosylation, and migrates on native gels with an apparent mass of greater than 400 kDa, whereas under denaturing conditions it appears somewhat smaller. The mnn1 agglutinin is also heterogeneous but has a lower molecular mass due to the presence of shorter N- and O-linked polymannose chains. Both agglutinins are converted sequentially by proteolysis to active fragments of approximately equal to 150 and 60 kDa, but the rate of proteolysis of the more highly glycosylated wild-type agglutinin is much slower than that of the mnn1 agglutinin. The 60-kDa fragment was isolated by HPLC and found to contain 46% carbohydrate as mannose, all of which was linked to serine and threonine. Thus, the N-linked oligosaccharides are restricted to that part of the agglutinin molecule that presumably anchors the agglutinin in the cell wall.
Full text
PDF
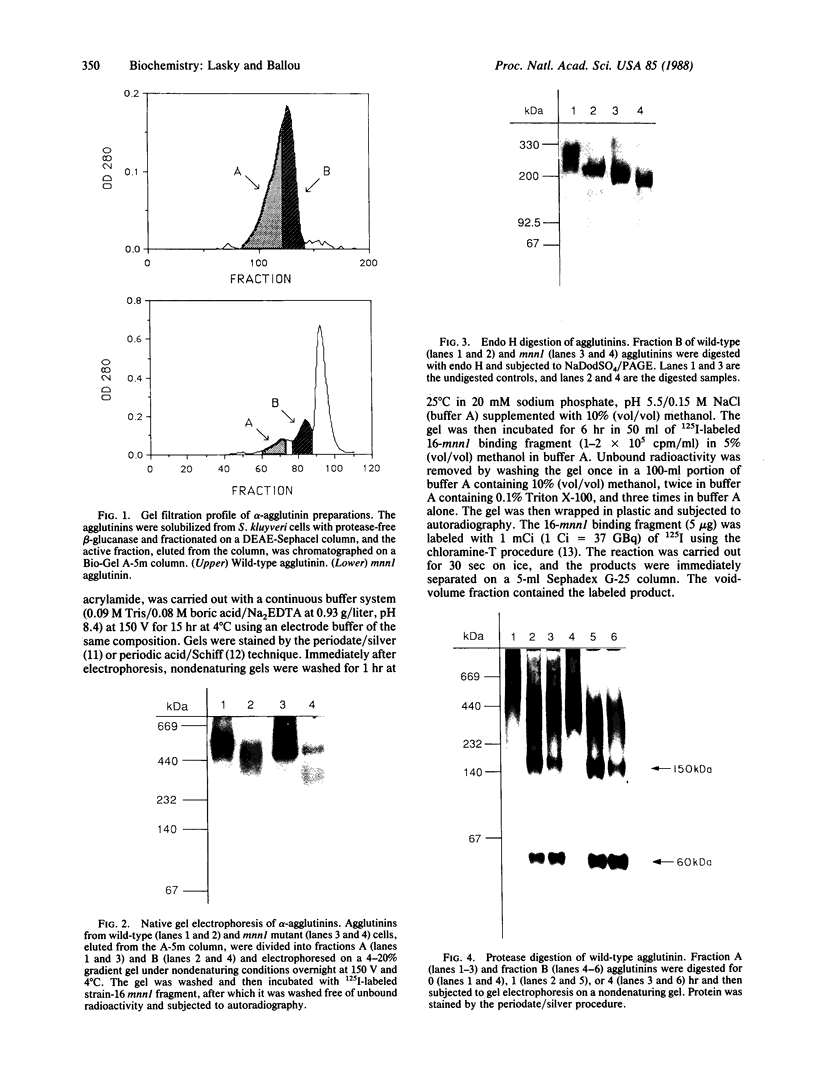
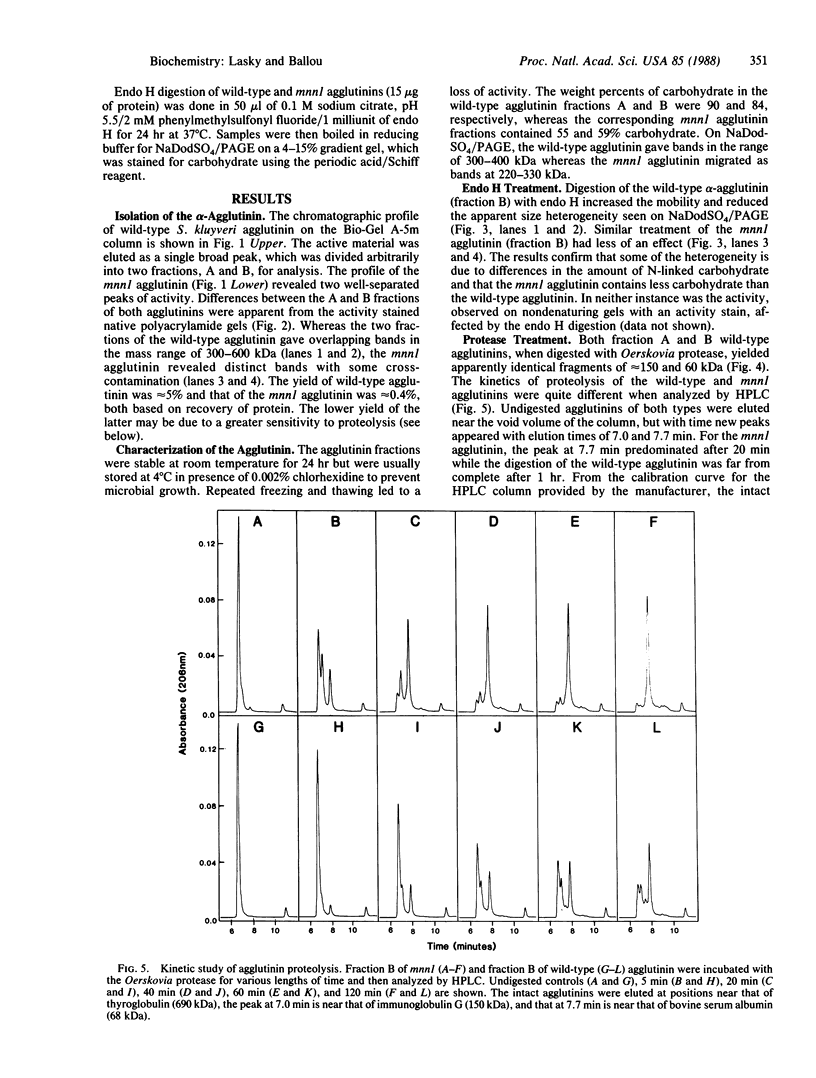
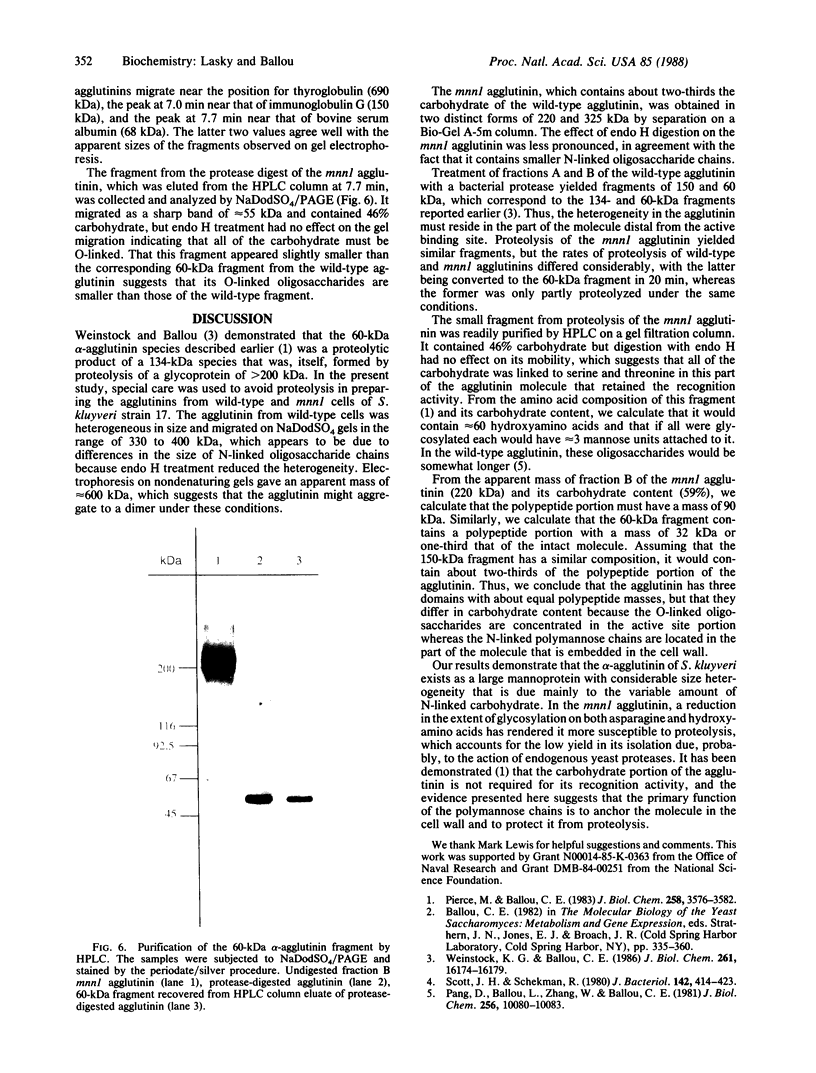

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pang D., Ballou L., Zhang W. J., Ballou C. E. Saccharomyces kluyveri mannoprotein mutants. J Biol Chem. 1981 Oct 10;256(19):10080–10083. [PubMed] [Google Scholar]
- Pierce M., Ballou C. E. Cell-cell recognition in yeast. Characterization of the sexual agglutination factors from Saccharomyces kluyveri. J Biol Chem. 1983 Mar 25;258(6):3576–3582. [PubMed] [Google Scholar]
- Scott J. H., Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980 May;142(2):414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Weinstock K., Ballou C. E. Cell-cell recognition in yeast. Molecular nature of the sexual agglutinin from Saccharomyces kluyveri 17-cells. J Biol Chem. 1986 Dec 5;261(34):16174–16179. [PubMed] [Google Scholar]