Abstract
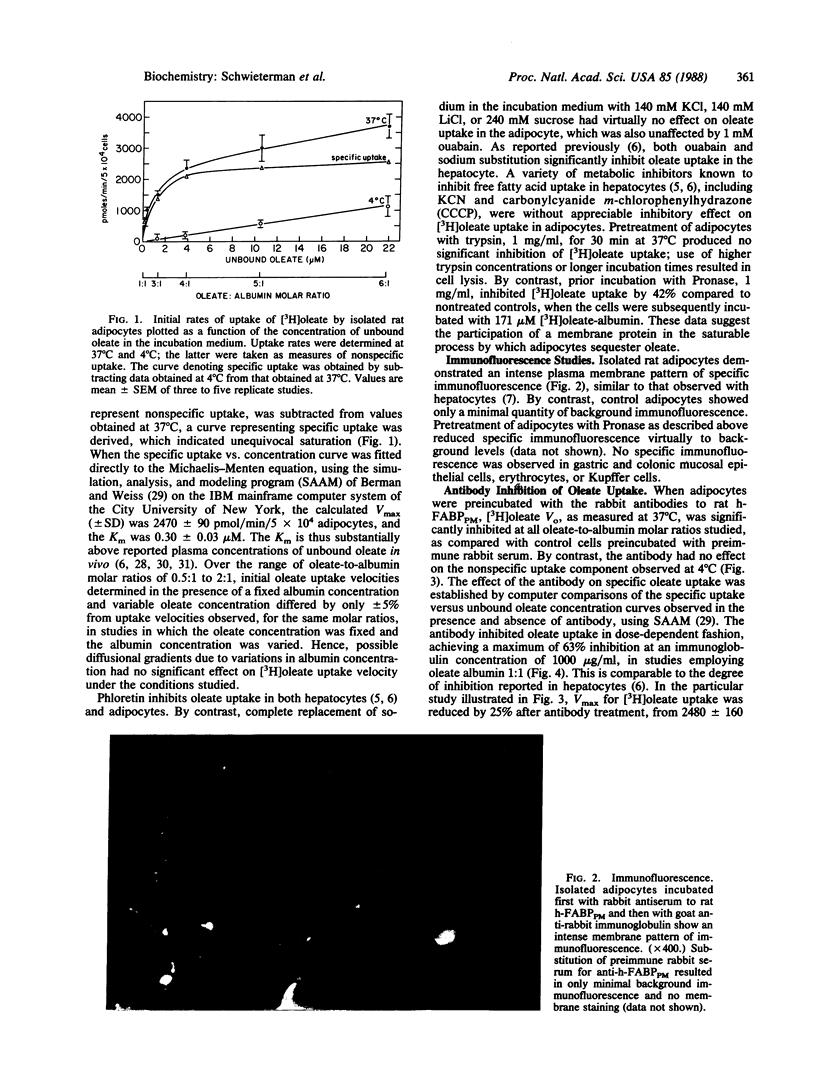
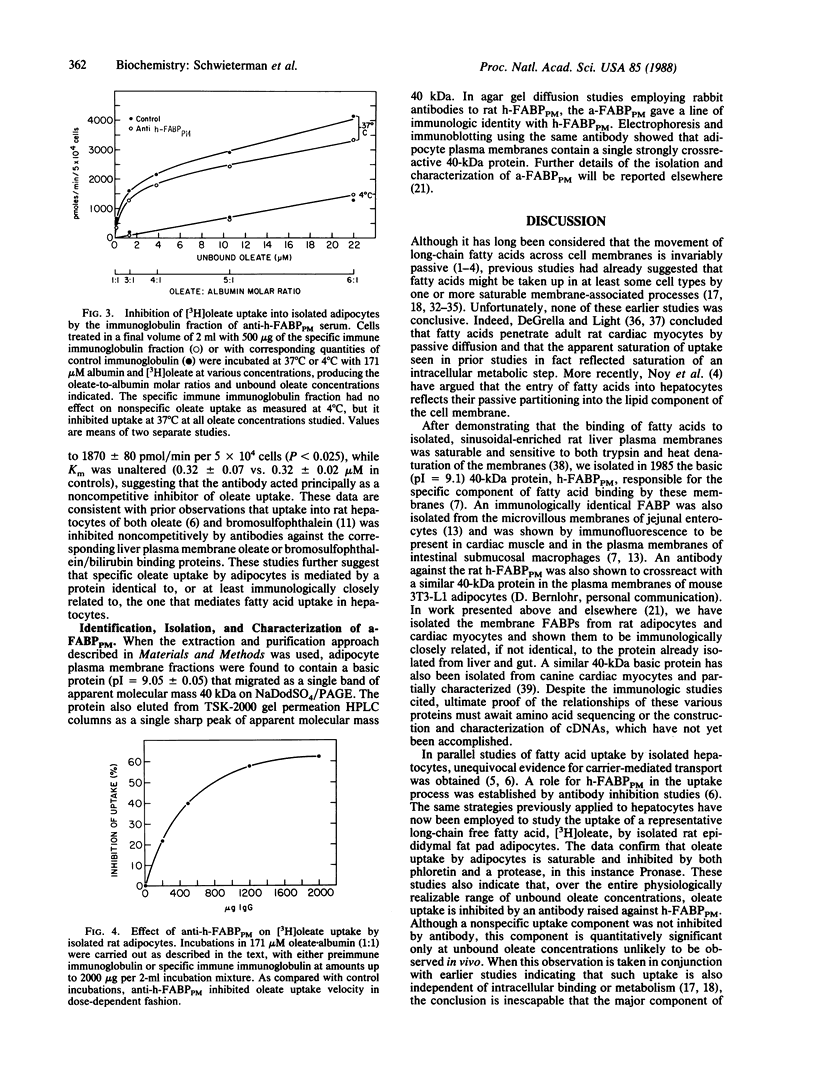
A portion of the hepatocellular uptake of nonesterified long-chain fatty acids is mediated by a specific 40-kDa plasma membrane fatty acid binding protein, which has also been isolated from the gut. To investigate whether a similar transport process exists in other tissues with high transmembrane fatty acid fluxes, initial rates (Vo) of [3H]oleate uptake into isolated rat adipocytes were studied as a function of the concentration of unbound [3H]oleate in the medium. Vo reached a maximum as the concentration of unbound oleate was increased (Km = 0.30 +/- 0.03 microM; Vmax = 2470 +/- 90 pmol/min per 5 X 10(4) adipocytes) and was significantly inhibited both by phloretin and by prior incubation of the cells with Pronase. A rabbit antibody to the rat liver plasma membrane fatty acid binding protein inhibited adipocyte fatty acid uptake by up to 63% in dose-dependent fashion. Inhibition was noncompetitive; at an immunoglobulin concentration of 250 micrograms/ml Vmax was reduced from 2480 +/- 160 to 1870 +/- 80 pmol/min per 5 X 10(4) adipocytes, with no change in Km. A basic (pI approximately equal to 9.1) 40-kDa adipocyte plasma membrane fatty acid binding protein, isolated from crude adipocyte plasma membrane fractions, reacted strongly in both agar gel diffusion and electrophoretic blots with the antibody raised against the corresponding hepatic plasma membrane protein. These data indicate that the uptake of oleate by rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Bass N. M. Function and regulation of hepatic and intestinal fatty acid binding proteins. Chem Phys Lipids. 1985 Aug 30;38(1-2):95–114. doi: 10.1016/0009-3084(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Berk P. D., Potter B. J., Stremmel W. Role of plasma membrane ligand-binding proteins in the hepatocellular uptake of albumin-bound organic anions. Hepatology. 1987 Jan-Feb;7(1):165–176. doi: 10.1002/hep.1840070131. [DOI] [PubMed] [Google Scholar]
- DeGrella R. F., Light R. J. Uptake and metabolism of fatty acids by dispersed adult rat heart myocytes. I. Kinetics of homologous fatty acids. J Biol Chem. 1980 Oct 25;255(20):9731–9738. [PubMed] [Google Scholar]
- DeGrella R. F., Light R. J. Uptake and metabolism of fatty acids by dispersed adult rat heart myocytes. II. Inhibition by albumin and fatty acid homologues, and the effect of temperature and metabolic reagents. J Biol Chem. 1980 Oct 25;255(20):9739–9745. [PubMed] [Google Scholar]
- Ikeda M., Shimada K., Sakaguchi T. High-performance liquid chromatographic determination of free fatty acids with 1-naphthylamine. J Chromatogr. 1983 Feb 11;272(2):251–259. doi: 10.1016/s0378-4347(00)86127-7. [DOI] [PubMed] [Google Scholar]
- Kuhl W. E., Spector A. A. Uptake of long-chain fatty acid methyl esters by mammalian cells. J Lipid Res. 1970 Sep;11(5):458–465. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S., Sauer F. Effect of trypsin, phospholipases, and membrane-impermeable reagents on the uptake of palmitic acid by isolated rat liver cells. Arch Biochem Biophys. 1974 Sep;164(1):185–193. doi: 10.1016/0003-9861(74)90021-6. [DOI] [PubMed] [Google Scholar]
- Moody A. J., Stan M. A., Stan M., Gliemann J. A simple free fat cell bioassay for insulin. Horm Metab Res. 1974 Jan;6(1):12–16. doi: 10.1055/s-0028-1093895. [DOI] [PubMed] [Google Scholar]
- Noy N., Donnelly T. M., Zakim D. Physical-chemical model for the entry of water-insoluble compounds into cells. Studies of fatty acid uptake by the liver. Biochemistry. 1986 Apr 22;25(8):2013–2021. doi: 10.1021/bi00356a027. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Colburn R. W., Black P. N. Transport of long-chain fatty acids in Escherichia coli. Evidence for role of fadL gene product as long-chain fatty acid receptor. J Biol Chem. 1986 Jan 5;261(1):167–171. [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974 Aug;54(2):326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S., Samuel D., Romey G., Ailhaud G. Uptake of fatty acids by cultured cardiac cells from chick embryo: evidence for a facilitation process without energy dependence. Biochimie. 1979;61(3):361–367. doi: 10.1016/s0300-9084(79)80129-7. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rand J. H., Gordon R. E., Sussman I. I., Chu S. V., Solomon V. Electron microscopic localization of factor-VIII-related antigen in adult human blood vessels. Blood. 1982 Sep;60(3):627–634. [PubMed] [Google Scholar]
- Reichen J., Blitzer B. L., Berk P. D. Binding of unconjugated and conjugated sulfobromophthalein to rat liver plasma membrane fractions in vitro. Biochim Biophys Acta. 1981 Jan 8;640(1):298–312. doi: 10.1016/0005-2736(81)90554-x. [DOI] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Kinetics of taurocholate uptake by the perfused rat liver. Gastroenterology. 1975 Jan;68(1):132–136. [PubMed] [Google Scholar]
- Samuel D., Paris S., Ailhaud G. Uptake and metabolism of fatty acids and analogues by cultured cardiac cells from chick embryo. Eur J Biochem. 1976 May 1;64(2):583–595. doi: 10.1111/j.1432-1033.1976.tb10338.x. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Stephens J. E. Transport of sodium, chloride, and taurocholate by cultured rat hepatocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):986–990. doi: 10.1073/pnas.78.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Spratt S. K., Black P. N., Ragozzino M. M., Nunn W. D. Cloning, mapping, and expression of genes involved in the fatty acid-degradative multienzyme complex of Escherichia coli. J Bacteriol. 1984 May;158(2):535–542. doi: 10.1128/jb.158.2.535-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci U S A. 1986 May;83(10):3086–3090. doi: 10.1073/pnas.83.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular uptake of sulfobromophthalein and bilirubin is selectively inhibited by an antibody to the liver plasma membrane sulfobromophthalein/bilirubin binding protein. J Clin Invest. 1986 Sep;78(3):822–826. doi: 10.1172/JCI112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Gerber M. A., Glezerov V., Thung S. N., Kochwa S., Berk P. D. Physicochemical and immunohistological studies of a sulfobromophthalein- and bilirubin-binding protein from rat liver plasma membranes. J Clin Invest. 1983 Jun;71(6):1796–1805. doi: 10.1172/JCI110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Kochwa S., Berk P. D. Studies of oleate binding to rat liver plasma membranes. Biochem Biophys Res Commun. 1983 Apr 15;112(1):88–95. doi: 10.1016/0006-291x(83)91801-6. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Lotz G., Strohmeyer G., Berk P. D. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985 Mar;75(3):1068–1076. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A. Dissociation from albumin: a potentially rate-limiting step in the clearance of substances by the liver. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1563–1567. doi: 10.1073/pnas.82.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell R. R., Abumrad N. A. Increased affinity predominates in insulin stimulation of glucose transport in the adipocyte. J Biol Chem. 1985 Mar 10;260(5):2894–2899. [PubMed] [Google Scholar]




