The eukaryotic cell cycle comprises an ordered series of events that are controlled by the oscillating activity of cyclin-dependent kinases (Cdks). The unidirectionality of cell cycle transitions is fundamental for successful completion of this cycle. Increasing activity of Cdk1 in complex with its regulatory cyclin, CycB, drives entry into mitosis, whereas subsequent CycB proteolysis promotes exit from mitosis and return of the cell cycle to interphase (Morgan, 2007). It is a commonplace view that the thermodynamically irreversible nature of cyclin proteolysis underlies the unidirectionality of mitotic exit (Lodish et al, 2004). However, in a biological system, steady-state levels of proteins (including CycB) result from the relative rates of protein destruction and de novo synthesis—itself a thermodynamically irreversible reaction. We have, therefore, suggested that proteolysis is insufficient to explain the irreversibility of mitotic exit. Rather, irreversibiltiy requires systems-level feedback that locks the cell cycle machinery in a G1 state, with low Cdk1–CycB activity, after exiting from mitosis (Novak et al, 2007). Proof of principle for this idea has come from experiments in budding yeast (Lopez-Aviles et al, 2009). These show that mitotic exit is driven by cyclin proteolysis but becomes irreversible only when a double-negative feedback loop consisting of the stoichiometric Cdk1 inhibitor, Sic1, is engaged. Here we argue that systems-level feedback is likely to explain the irreversibility of mitotic exit in most, if not all, eukaryotes.
Bistability of mammalian mitotic exit
In two recent studies, Gorbsky and coworkers have investigated the irreversibility of mitotic exit in mammalian cells (Potapova et al, 2006, 2009). Cells were collected in metaphase stage after treatment with the spindle poison nocodazole. The spindle assembly checkpoint (SAC) inactivates the Cdc20–APC ubiquitin ligase in this arrest, thereby preventing the degradation of CycB and further cell cycle progression. In addition, the proteasome inhibitor, MG132, was added to prevent ubiquitin-dependent CycB degradation by Cdh1/APC, which becomes active when Cdk1 activity is low. In these arrested cells, chemical inhibition of Cdk1 using flavopiridol induced mitotic exit as indicated by chromosomal decondensation, nuclear envelope formation and early stages of cytokinesis. Mitotic exit was also accompanied by dephosphorylation of Cdk1 tyrosine-modifying enzymes and consequent inhibitory phosphorylation of Cdk1. When the Cdk inhibitor, flavopiridol, was removed after 30 min, Cdk1 activity recovered, Cdk1-modifying enzymes were re-phosphorylated, and the majority of cells returned to mitosis, that is, mitotic exit was reversible. However, after a longer period of Cdk inhibition (60 min), the mitotic state could not be reestablished after flavopiridol removal. Re-activation of Cdk1 was blocked and cells exited irreversibly into G1 phase. Importantly, the transition to irreversibility after 60 min depended on the activity of the Cdk1 inhibitory kinase Wee1. These experiments demonstrate the bistable characteristic of mammalian mitotic exit. After transient Cdk inhibition, metaphase-arrested cells either return to a mitotic state, or proceed to a stable G1-like state, representing two alternative steady states. Preference for the G1 over the mitotic state depends on Cdk1 inhibitory phosphorylation.
The Cdk1 phosphorylation bistable switch
Remarkably, the Cdk inhibitory kinase Wee1, and the counteracting Cdk-activating phosphatase, Cdc25, are themselves regulated by Cdk1–CycB-mediated phosphorylation. Thus, Cdk1–CycB inactivates Wee1, but activates Cdc25, thereby defining a Cdk1–Wee1 double-negative feedback motif interlinked with a Cdk1–Cdc25 positive feedback loop (Figure 1A). Bistability is an emergent property of both of these network motifs (Tyson et al, 2003), which act synergistically to control Cdk1 activity (Figure 1B). Although the identity of a phosphatase that would dephosphorylate Wee1 and Cdc25 remains to be ascertained, experimental evidence regarding candidate phosphatases suggests that they might themselves be regulated by Cdk1 (Mochida and Hunt, 2007; Wu et al, 2009), which further adds to the bistability.
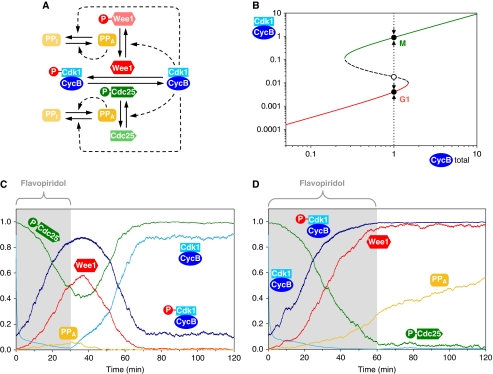
Figure 1.
Systems-level feedback makes mitotic exit irreversible in mammalian cells. (A) The molecular network controlling Cdk1–CycB inhibitory phosphorylation. Unphosphorylated, active Wee1 kinase inactivates Cdk1–CycB complex, which is reversed by phosphorylated, active Cdc25 phosphatase. Cdk1–CycB promotes the phosphorylation of both Wee1 and Cdc25 directly and indirectly (through inhibition of the Cdk-counteracting phosphatase). We assume that the Cdk-counteracting phosphatase is autoactivated by dephosphorylation. (B) The dependence of active Cdk1–CycB on total CycB levels follows an S-shaped curve characteristic for bistable systems. At intermediate CycB levels, there are two stable steady states (filled circles) along the high (green) and low (red) branches of the S-shaped curve, corresponding to M and G1 states. The unstable steady state (open circle) along the middle branch (dashed) of the curve divides the trajectories of chemical kinase inhibition at constant CycB levels (dotted arrows). (C, D) Numerical simulations of the experiments by Potapova et al. (2009) for (C) reversible and (D) irreversible mitotic exit. The active forms of the enzymes (Wee1, Cdc25 and PPase) are plotted. The Cdk inhibitor is added at time point 0 at a level 10-fold higher than its IC50 value and is thought to bind reversibly to both phosphorylated and unphosphorylated Cdk1–CycB dimers.
Using a stochastic model of this bistable switch (see Supplementary information), we simulated mitotic exit of mammalian cells. Simulations were started from a metaphase stage, that is, high Cdk1 activity, Cdc25 active, Wee1 and the Cdk-counteracting phosphatase inactive stage. We estimated that the addition of Cdk inhibitor at time point 0 causes an abrupt 10-fold drop in Cdk1–CycB activity. This leads to the dephosphorylation of Wee1 and Cdc25 by the Cdk-counteracting phosphatase. The resulting activation of Wee1 and inactivation of Cdc25 causes rapid phosphorylation and, therefore, inhibition of Cdk1. When flavopiridol was removed at 30 min, all these changes were observed to be still reversible and cells returned to the M-phase steady state (Figure 1C). Low levels of active Cdk1–CycB are able to re-phosphorylate Wee1 and Cdc25, and thereby reverse mitotic exit. When the inhibitor is removed at 60 min, the inhibitory phosphorylation of Cdk1 was observed to have progressed far enough to maintain Cdk1–CycB inactive even in the absence of Cdk inhibitor. Cells had reached the G1 steady state (Figure 1D). Our simulations predict that the percentage of cells that complete irreversible mitotic exit gradually increases between 30 and 70 min of Cdk inhibition (data not shown), consistent with the experiments (Potapova et al, 2009). During this transition period, the choice between the M or G1 phase is stochastic in nature as cells recover from Cdk inhibition in the neighborhood of the unstable steady state depicted in Figure 1B.
Conclusions
Recent experimental data (Potapova et al, 2006, 2009), together with our analysis, show that systems-level double-negative and positive feedbacks render mammalian mitotic exit irreversible, even in the absence of cyclin degradation. The mammalian feedback mechanism largely depends on Cdk phosphorylation rather than a stoichiometric Cdk inhibitor as observed in budding yeast (Lopez-Aviles et al, 2009). We note that in both mammalian and yeast cells, the activation of Cdh1/APC and inactivation of CycB transcription at low Cdk1 activity form additional feedback loops, contributing to the irreversibility of mitotic exit. Although the molecular details may differ, the underlying principles seem to be conserved from yeast to human: regulatory motifs confer bistability to Cdk1–CycB inactivation during mitotic exit. In mammalian cells, the phosphatase that counteracts Cdk1–CycB is yet to be identified, but is likely to impact mitotic exit regulation (Skoufias et al, 2007), and elucidation of the underlying systems-level mechanisms will be a challenge for future research.
Supplementary Material
Stochastic model for mammalian mitotic exit
Footnotes
The authors declare that they have no conflict of interest.
References
- Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipusky L, Darnel J (2004) Molecular Cell Biology, 5th edn, New York: Freeman [Google Scholar]
- Lopez-Aviles S, Kapuy O, Novak B, Uhlmann F (2009) Irreversibility of mitotic exit is the consequence of systems-level feedback. Nature 459: 592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Hunt T (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340 [DOI] [PubMed] [Google Scholar]
- Morgan DO (2007) The Cell Cycle: Principles of Control. London: New Science Press [Google Scholar]
- Novak B, Tyson JJ, Gyorffy B, Csikasz-Nagy A (2007) Irreversible cell-cycle transitions are due to systems-level feedback. Nat Cell Biol 9: 724–728 [DOI] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Byrd KS, Gorbsky GJ (2009) Fine tuning the cell cycle: activation of the Cdk1 inhibitory phosphorylation pathway during mitotic exit. Mol Biol Cell 20: 1737–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, Gorbsky GJ (2006) The reversibility of mitotic exit in vertebrate cells. Nature 440: 954–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias DA, Indorato RL, Lacroix F, Panopoulos A, Margolis RL (2007) Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J Cell Biol 179: 671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JJ, Chen KC, Novak B (2003) Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15: 221–231 [DOI] [PubMed] [Google Scholar]
- Wu JQ, Guo JY, Tang W, Yang CS, Freel CD, Chen C, Nairn AC, Kornbluth S (2009) PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol 11: 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stochastic model for mammalian mitotic exit