Abstract
Over the past few decades, behavioral, neuroimaging and molecular studies of neurogenetic conditions, such as Williams, fragile X, Turner and velocardiofacial (22q11.2 deletion) syndromes, have led to important insights regarding brain development. These investigations allow researchers to examine “experiments of nature” in which the deletion or alteration of one gene or a contiguous set of genes can be linked to aberrant brain structure or function. Converging evidence across multiple imaging modalities has now begun to highlight the abnormal neural circuitry characterizing many individual neurogenetic syndromes. Furthermore, there has been renewed interest in combining analyses across neurogenetic conditions in order to search for common organizing principles in development. In this review, we highlight converging evidence across syndromes from multiple neuroimaging modalities, with a particular emphasis on functional imaging. In addition, we discuss the commonalities and differences pertaining to selective deficits in visuospatial processing that occur across four neurogenetic syndromes. We suggest avenues for future exploration, with the goal of achieving a deeper understanding of the neural abnormalities in these affected populations.
Keywords: monogenic, neurogenetic, Williams syndrome, fragile X syndrome, Turner syndrome, velocardiofacial syndrome, 22q11.2 deletion syndrome, neuroimaging, fMRI, MRI, DTI, visuospatial
1. INTRODUCTION
During the past 25 years, structural and functional neuroimaging researchers have made enormous progress in characterizing the typically developing human brain. Recent investigations have sought to understand the interactions between genes, brains, and behavior by probing the molecular and genetic influences on the typical neurodevelopmental trajectory (Thompson, Cannon, Narr, van Erp, Poutanen, Huttunen, Lönnqvist, Standerskjöld-Nordenstam, Kaprio, Khaledy, Dail, Zoumalan, and Toga, 2001; Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz, Kolachana, Egan, Mattay, Hariri, and Weinberger, 2005; Fan, Fossella, Sommer, Wu and Posner, 2003). However, certain atypical populations provide a unique opportunity to observe the effects of only a few genes on brain development. In particular, developmental disorders arising from discrete, known differences in one gene or a contiguous group of genes present an invaluable opportunity to tie brain structure and function to the disruption of a specific gene or gene product. This approach, termed “behavioral neurogenetics” (Reiss and Dant, 2003), has been enthusiastically pursued by a few labs over the past two decades, leading to a wealth of information linking genes, brain morphology and function, and behavior. In this paper we refer to a group of “neurogenetic syndromes,” a designation that refers to conditions resulting from the alteration or deletion of one (monogenic) or more (polygenic) discrete genes.
Previous researchers have made a strong case for investigating specific genetic disorders, such as fragile X syndrome (Reiss and Dant, 2003), and Williams syndrome (Meyer-Lindenberg, Mervis, Berman, 2006; Bellugi, Lichtenberger, Mills, Galaburda, Korenberg, 1999) in order to understand gene-brain-behavior interactions. Individuals with discrete neurogenetic disorders are particularly useful for imaging studies because it can be generally assumed that the perturbations caused by atypical genes result in similar structural and functional abnormalities within the affected population (Reiss, Eliez, Schmitt, Patwardhan and Haberecht, 2000). That is, each group is relatively homogeneous with respect to sharing a common risk factor for atypical brain development and function. Unlike behaviorally defined psychiatric disorders such as autism or schizophrenia that may encompass a spectrum of sub-populations, the ability to detect differences between healthy and disordered groups is relatively increased when studying neurogenetic disorders. One goal of this review is to highlight recent investigations into four neurogenetic syndromes: fragile X, Williams, Turner and velocardiofacial syndromes, with a particular focus on functional neuroimaging, as well as converging evidence across functional, structural, and connectivity neuroimaging.
Another dimension of neurogenetic neuroimaging research stems from a comparison of commonalities and differences across a number of syndromes that have overlapping cognitive/behavioral profiles. A number of excellent reviews have recently described links between behavior, genetics and structural imaging findings across multiple neurogenetic disorders (e.g. Bearden, Glahn, Lee, Chiang, van Erp, Cannon, Reiss, Toga and Thompson, 2008; Reiss et al, 2000; Kaufman and Moser, 2000; Cornish, Scerif, and Karmiloff-Smith, 2007). A second goal of this review is to highlight behavioral and neurobiological commonalities and differences seen across the disorders, particularly in the domain of visuospatial processing.
This neuroimaging research is useful from a basic-science perspective, as an understanding of gene-brain links within a neurodevelopmentally disordered population may lead to a more thorough understanding of the gene’s effects in the typically developing brain. In these populations, changes in gene function can be directly linked to changes in brain morphology, function and organization. Indeed, one of the ultimate objectives of this neuroimaging is to inform and improve treatments for the populations under consideration. By discovering dysfunctional systems using structural and functional imaging techniques, interventions can be addressed to many levels of brain function – from behavioral training and environmental change to real time fMRI (rtfMRI) and pharmacological manipulations. It may also be possible to correlate an intervention with a change in brain activation, adding evidence for the direct involvement of a brain region with a particular behavior. In this manner, future experiments may point towards a cause-effect relationship between brain and behavior, in addition to typically measured correlations, and support both basic science and therapeutic interventions.
2. NEUROGENETIC SYNDROMES: GENETICS, BEHAVIOR AND IMAGING
This section reviews the genetics, behavior, and neuroimaging results within each of four well-characterized neurogenetic conditions: Williams, fragile X, Turner and velocardiofacial (22q11.2 deletion) syndromes. A brief summary of the underlying genetic disruption and resulting behavioral and neural differences observed in each syndrome is discussed below, with an emphasis on recent results from functional imaging and converging evidence from multiple imaging modalities.
2.1 Williams syndrome
Williams syndrome (WS; also called Williams-Beuren syndrome) is associated with a hemizygous deletion of approximately 28 genes on chromosome 7q11.23, and occurs with a frequency of approximately 1:7500 live births (Strømme, Bjørnstad, and Ramstad, 2002). In general, individuals with WS exhibit distinctive facial characteristics (e.g., an upturned nose, wide mouth and small chin) and show hypersociability in addition to developmental delay and particular difficulty with visuospatial skills. Though the most common deletion comprises 28 genes, some individuals with WS have atypical deletions comprising only a subset of these genes, which has allowed for microdeletion mapping (Meyer-Lindenberg et al, 2006). A number of genes in the deleted region have been linked to the specific physical or cognitive features associated with this syndrome. For example, elastin (ELN) has been implicated in the cardiovascular and facial feature abnormalities (Ewart, Morris, Ensing, Loker, Moore, Leppert and Keating, 1993), while there is other evidence that the deletion of LIMK1, GTF2I, and GTF2IRD1 may be responsible for impaired visuospatial constructive skills (Frangiskakis, Ewart, Morris, Mervis, Bertrand, Robinson, Klein, Enging, Evertt, Green, Pröschel, Gutowski, Noble, Atkinson, Odelberg and Keating, 1996; Hirota, Matsuoka, Chen, Salandanan, Lincoln, Rose, Sunahara, Osawa, Bellugi, and Korenberg, 2003; though see Gray, Karmiloff-Smith, Funnell and Tassabehji, 2006). Other deleted genes, such as CYLN2, appear to regulate cytoskeletal structure (Hoogenraad, Koekkoek, Akhmanova, Krugers, Dortland, Miedema, van Alpern, Kistler, Jaegle, Koutsourakis, Van Camp, Verhoye, van der Linden, Kaverina, Grosveld, De Zeeuw and Galjart, 2002).
The cognitive profile observed in WS is rather unusual. Individuals typically have mild to moderate intellectual impairment, with IQs in the high 50s to low 70s reported in a number of studies (reviewed in Meyer-Lindenberg et al, 2006). While language and a predilection for music are relatively spared as compared with overall intellectual impairment (Mervis and Becerra, 2007), visuospatial and visuomotor tasks are particularly affected. WS individuals exhibit a number of low-level visual deficits, including strabismus (Winter, Pankau, Amm, Gosch and Wessel, 1996; Kapp, von Noorden, Jenkins, 1995), reduced visual acuity (Atkinson, Anker, Braddick, Nokes, Mason and Braddick, 2001), reduced stereopsis (van der Geest, Lagers-van Haselen, van Hagen, Brenner, Govaerts, de Coo and Frens, 2005), as well as impairment in making simple saccades (van der Geest, Lagers-van Haselen, van Hagen, Govaerts, de Coo, de Zeeuw and Frens, 2004). Other studies have found evidence for impairment in visuospatial long-term memory (LTM) tasks, with typical LTM for objects (Vicari, Bellucci and Carlesimo, 2005). Though visuospatial processing is aberrant, WS individuals are able to perform as well as typically developing individuals in face processing tasks (Wang and Bellugi, 1994). In addition, although a hallmark of WS is an excessive drive to socialize, and individuals with WS appear to be particularly drawn to faces (Gagliardi, Figerio, Burt, Cazzaniga, Perrett, and Borgatti, 2003), their socialization is often “shallow,” with conversations focusing on their own interests rather than engaging in a more typical give-and-take exchange (Einfeld, Tonge and Florio, 1997). Finally, those with WS typically have a strong predilection for auditory stimuli, most notably music and rhythm (Levitin and Bellugi, 1998).
In general, the overall brain size of individuals with WS tends to be much smaller than those of typically developing individuals, though the temporal-limbic structures (Jernigan, Bellugi, Sowell, Doherty and Hesselink, 1993) and the cerebellum (Bellugi, Bihrle, Jernigan, Trauner and Doherty, 1990; Jernigan et al, 1993) are relatively preserved in size. In general, parietal and occipital lobes appear to be reduced to a greater degree than are frontal and temporal regions (Reiss et al, 2000). More recent structural studies have suggested that brain tissue volume is specifically reduced in the occipital lobe and brainstem, while the superior temporal gyrus (STG) is relatively preserved (Reiss, Eliez, Schmitt, Straus, Lai, Jones and Bellugi, 2000). The parietal and occipital lobes have also been found to exhibit increased gyrification (Schmitt, Eliez, Bellugi and Reiss, 2001; Schmitt, Watts, Eliez, Bellugi, Galaburda, and Reiss, 2002). Kippenhan and colleagues found reduced sulcal depth bilaterally in the intraparietal/occipitoparietal sulcus, with particularly dramatic reductions in the left hemisphere (Kippenhan, Olsen, Mervis, Morris, Kohn, Meyer-Lindenberg and Berman, 2005). These sulcal reductions correlated with reduced grey matter volume found in similar regions using voxel-based morphometry. White matter tracts appear to be aberrant in WS as well, with white matter volumes reduced to a greater extent than grey matter volumes, when compared to a typically developing group (Reiss et al, 2000). Previous work also found a decrease in the volume of the corpus callosum in WS (Wang, Doherty, Hesselink and Bellugi, 1992). A preliminary study of five high-functioning individuals with WS indicated underlying aberrant white matter tracts in a number of regions of interest highlighted by previous multimodal imaging studies. In particular white matter was found to be disrupted in the in the collateral sulcus, orbitofrontal sulcus, and inferior parietal sulcus (Marenco, Siuta, Kippenhan, Grodofsky, Chang, Kohn, Mervis, Morris, Weinberger, Meyer-Lindenberg, Pierpaoli and Berman, 2007).
Functional studies have explored behavioral inhibition in WS, finding reduced activity in the striatum, dorsolateral prefrontal cortex (DLPFC) and dorsal anterior cingulate cortex in WS as compared to control participants in a modified Go/NoGo task (Mobbs, Eckert, Mills, Korenberg, Bellugi, Galabura and Resis, 2007). Briefly, the Go/NoGo task is a measure of response inhibition. The task indexes a participant’s ability to withhold a prepotent response, by requiring a rapid button-press during the majority of the trials when a stimulus is detected (“Go” events), while requiring the participant to withhold a response in a small fraction of the trials (“NoGo” events). Other functional studies of participants with WS have pointed to anomalies in amygdala activation when viewing threatening faces (Meyer-Lindenberg, Hariri, Munoz, Mervis, Mattay, Morris and Berman, 2005), positive and negative affect faces (Haas, Mills, Yam, Hoeft, Bellugi, and Reiss, 2009), as well as increased activation in a frontal-subcortical pathway when determining gaze direction (Mobbs, Garrett, Menon, Rose, Bellugi, and Reiss, 2004).
WS participants are specifically impaired in visuospatial processing, and both structural and functional neuroimaging studies have uncovered anomalies in the parietal cortex, a key brain region for processing visuospatial information. For example, in a multi-modal imaging study of high-functioning WS adults, Meyer-Lindenberg and colleagues (2004) observed a symmetrical grey matter volume reduction in the intraparietal sulcus (IPS) using voxel-based morphometry, which dovetailed with deficits in visuospatial processing observed in a functional MRI study using the same participants. This functional study used identical visual stimuli for two different tasks, asking participants to make either an object-based decision (“Do the two objects match?”) or to make a visuospatial decision (“Do the two objects combine to create a square?”). They found that WS participants had similar behavioral accuracies and response times, and activated similar ventral areas as controls when performing the object-based decision. However, during the visuospatial task, WS participants had significantly worse accuracy and shorter reaction times than controls, and they did not activate superior parietal lobe, although the typically developing participants did. A follow-up task involving attention to either the identity or the location of two objects on the screen yielded similar results: WS activated ventral areas for objects, similar to typically developing participants, while attention to location resulted in reduced activity in bilateral parietal areas for WS as compared to control participants. Eckert and colleagues (2005) later found that bilateral superior parietal (SPL) volumes were smaller in individuals with WS. In addition, a DTI study found increased fractional anisotropy (FA) in the superior longitudinal fasciculus (SLF) in WS relative to control participants (Hoeft, Barnea-Goraly, Haas, Golari, Ng, Mills, Korenberg, Bellugi, Galaburda and Reiss, 2007). This DTI finding suggests that the underlying white matter tracts subserving the dorsal stream of visual processing may also be aberrant in WS. In short, the weight of evidence in gray matter, white matter and functional activations strongly suggests that the genes deleted in WS may play a role in the proper development and functioning of the parietal cortex.
2.2 Fragile X syndrome
Fragile X syndrome (FXS) is the most common cause of inherited mental impairment (Crawford, Acuna and Sherman, 2001; Freund, Reiss and Abrams, 1993), as well as the most common known genetic cause of autism, and is thought to affect approximately 1:4000 males and 1:8000 females, (Reiss and Dant, 2003; Turner, Webb, Wake, and Robinson, 1996; Crawford et al, 1999). Individuals with FXS often demonstrate intellectual impairment and tend to exhibit a number of characteristic physical features (such as elongated face, large or protruding ears, flat feet and macroorchidism in males). In keeping with the X-linked nature of the disorder, males tend to be severely affected (typical IQ 35–55), while females show a range from severely affected to only subtle learning difficulties (Cornish, Turk, Wilding, Sudhalter, Munir, Kooy and Hagerman, 2004). The degree to which an individual with FXS exhibits these symptoms is highly variable; many of those with FXS do not show typical facial features, and, if female, may have average overall intellectual ability. Both males and females with FXS typically exhibit difficulties with many social and cognitive tasks, and have deficits processing visuospatial information, including mental manipulation of the spatial relationships between objects, as well as difficulties with visuomotor coordination and arithmetic (Reiss and Dant, 2003). Males tend to have particular difficulty with visuomotor coordination, spatial memory and arithmetic (Freund and Reiss, 1991) while females tend to have specific problems with visuoconstructive (e.g. Block Design) tasks and drawing (Cornish, Munir, and Cross, 1998), as well as with math (Mazzocco, 2001). There is currently no cure for FXS, though a number of behavioral and occupational therapies as well as medications are in use to improve behavioral and cognitive outcomes for individuals with FXS (Reiss and Hall, 2007; Berry-Kravis, Krause, Block, Guter, Wuu, et al, 2006; Berry-Kravis and Potanos, 2004; Hagerman and Hagerman, 2002).
FXS itself is caused by an expansion of CGG repeats in the promoter region upstream of the FMR1 gene on the long arm of the X chromosome (locus Xq27.3) and is usually verified by Southern blot analysis. Typical adults have between 0–40 CGG repeats in this region. Adults with 60–200 repeats are said to be “premutation carriers” and it is likely that the repeats in this region will undergo further expansion when passed to offspring from the mother. When the number of CGG repeats surpasses 200, individuals are said to have the full FXS mutation, which usually results in hypermethylation of the promoter region (Oberle, Rousseau, Heitz, Kretz, Devys, Hanauer, Boue, Bertheas and Mandel, 1991), ultimately leading to reduced transcription of the downstream FMR1 gene (discovered by Verkerk, Pieretti, Sutcliffe, Fu, Kuhl, et al, 1991) and lower levels of FMRP expression (Fragile X Mental Retardation Protein; Garber, Smith, Reines and Warren, 2006; Bardoni, Mandel and Fisch, 2000). FXS is related to two other syndromes resulting from premutations at the CGG repeat site, namely fragile X-associated tremor/ataxia syndrome (FXTAS), seen in older males, and fragile X-associated primary ovarian insufficiency (FXPOI) in females.
A number of well-replicated structural imaging studies, thoroughly reviewed in a recent publication (Bearden et al, 2008), have confirmed morphological changes associated with FXS. Bilateral enlargement of the caudate, a structure that has many cortical connections, primarily to the frontal lobes, has been well-replicated (Reiss et al, 1995; Gothelf, Furfaro, Hoeft, Eckert, Hall, et al, 2008). The role of the caudate as an important regulator, organizer and filter of incoming information makes this a particularly exciting finding (Hessl, Rivera, and Reiss, 2004). In addition, those with FXS show a decrease in the size of the cerebellar vermis (the tissue connecting the right and left hemispheres of the cerebellum; Reiss, Aylward, Freund, Joshi, and Bryan, 1991; Reiss, Freund, Tseng and Joshi, 1991) that is correlated with the extent of FMR1 gene methylation (Mostofsky, Mazzocco, Aakalu, Warsofsky, Denckla, and Reiss, 1998). Enlargement of the thalamus has been found in females with FXS (Reiss, Abrams, Greenlaw, Freund and Denckla, 1995). In addition, Reiss and colleagues (Reiss, Lee, and Freund, 1994) found that the superior temporal gyrus decreased in volume with age in FXS males and females relative to IQ-matched controls. DTI results indicate that, relative to controls, very young males with FXS have increased fiber density in the left ventral frontostriatal pathway (Haas, Barnea-Goraly, Lightbody, Patnaik, Hoeft, Hazlett, Piven, and Reiss, 2009). In addition, females with FXS have decreased white matter connectivity in frontostriatal and parietal sensory-motor pathways (Barnea-Goraly, Eliez, Hedeus, Menon, White, Moseley, and Reiss, 2003).
Though FXS is defined by the number of repeats found in the genetic code, these repeats can have varying effects on the resultant levels of FMRP, possibly due to differences in methylation patterns (reviewed in Bardoni et al, 2000). Some researchers have proposed measuring FMRP levels (Kaufmann, Abrams, Chen and Reiss, 1999; Tassone, Hagerman, Taylor, Mills, Harris, Gane, and Hagerman, 2000), and indeed, levels of FMRP may turn out to be a more helpful measure to correlate with brain function (e.g. Reiss, Abrams, et al, 1995; Mostofsky et al, 1998; Menon, Leroux, White and Reiss, 2004; Kwon, Menon, Eliez, Warsofsky, White, Dyer-Friedman, Taylor, Glover, and Reiss, 2001; Rivera, Menon, White, Glaser, and Reiss, 2002). For example, Kwon and colleagues (2001) found that females with FXS had difficulty with a difficult (2-back) visuospatial working memory task, but not with the 1-back (easier) version of the task. During the more difficult version of the task, control participants performed at high levels and showed increased brain activity in prefrontal and parietal cortex. However, the FXS group performed at a lower level and did not show any activity change in this network as task difficulty increased. Interestingly, FMRP levels measured in the FXS participants correlated with activity in right inferior and bilateral middle frontal gyri as well as with bilateral supramarginal gyri in the difficult (2-back) task, suggesting that the degree of processing abnormality in FXS depends on the relative deficit of available FMRP. In a separate study, Rivera and colleagues (2002) conducted an fMRI task investigating arithmetic processing by females with FXS. During this task, participants made a judgment of accuracy for arithmetic operations comprised of two or three operands. Though the participants with FXS were able to perform the easier (two-operand) task as well as controls, they were impaired at processing the three-operand stimuli. While typically developing participants performed well on both tasks, and increased activity in the fronto-parietal network during the more difficult task, participants with FXS had lower performance on the more difficult task and did not show increases in this network. Again, in this task, FMRP levels were significantly correlated with activity in bilateral prefrontal and motor/premotor cortex as well as left angular and supramarginal gyrus (in inferior parietal cortex) during the three-operand conditions. These experiments demonstrate that the gene product of FMR1 (FMRP) may be particularly important for development of brain circuits underlying processing of visual spatial and mathematical information.
Two behavioral hallmarks of FXS are an avoidance of eye-gaze (Cohen, Fisch, Sudhalter, Wolf-Schein, Hanson, Hagerman, Jenkins, and Brown, 1988; Teisl, Reiss and Mazzocco, 1999), and an overall lack of inhibitory control. With respect to eye gaze, fMRI studies have found abnormalities in the functional circuits underlying gaze processing in both females (Garrett, Menon, MacKenzie, and Reiss, 2004) and males (Watson, Hoeft, Garrett, Hall and Reiss, 2008) with FXS. Inhibitory control is known to involve the frontal-striatal network, connecting the right caudate and right ventrolateral prefrontal cortex (VLPFC), and converging results from multiple modes of neuroimaging have found many anomalies in these neural circuits. For example, caudate volume is significantly larger in FXS populations, and the magnitude of this abnormality is correlated with FMRP levels (Reiss, Abrams, et al, 1995). In addition, the white matter of the left and right frontostriatal networks was found be disrupted in FXS (Barnea-Goraly, et al, 2003). Furthermore, functional imaging studies have found that activations in the right VLPFC and basal ganglia were reduced in both female participants with FXS (Menon et al, 2004), as well as in males with FXS (Hoeft, et al, 2007) relative to controls. Menon (2004) found that activity in right VLPFC and bilateral activation in the basal ganglia were correlated with levels of FMRP, while Hoeft and colleagues (2007) found that left VLFPC activation in males with FXS correlated with performance in a response inhibition task. The combination of these results suggest that right fronto-striatal dysfunction is a neurophenotypic feature of FXS, and further suggests that the left VLPFC may be part of a compensatory network (Hoeft et al, 2007).
Finally, an intriguing fMRI study suggested a possible intervention for the learning and behavioral problems associated with FXS. In a study of visual memory encoding in females with FXS, Greicius and colleagues (2004) found that the FXS group showed less activation in the hippocampus and basal forebrain when encoding novel photographs of outdoor scenes as compared to typically developing individuals. Since cholinergic pathways in the basal forebrain and hippocampus are known to subserve executive attention, learning, and memory (Sarter, Bruno and Givens, 2003), the reduced basal forebrain activation in FXS suggested that reduced acetylcholine (ACh) production might be a factor in FXS learning deficits. In this case, available ACh might be increased by use of an acetylcholinesterase (AChE) inhibitor. In a pilot study administering an AChE inhibitor (donepezil) to eight individuals, preliminary results indicate an overall reduction in problem behaviors, as well as improved performance in executive function skills over the course of the trial (Kesler, Lightbody and Reiss, 2009). While these results are preliminary, they illustrate the potential for neuroimaging studies of neurogenetic syndromes to inform interventions for these populations.
2.3 Turner syndrome
Turner syndrome (TS) refers to any condition resulting from the complete loss or partial deletion of one of the two female X chromosomes, with an occurrence of approximately 1:2000 live births (females only; Jacobs, 1992; Jones, 1997). In the most typical case, an individual with Turner syndrome has only one X chromosome (monosomy); less commonly a partial second X chromosome is present but is not structurally intact (e.g., ring chromosome or deleted chromosome). And, in a minority of cases, some of the cells in the body have one X chromosome while others have two or more (referred to as “mosaicism”). TS is diagnosed with a karyotype (from a blood test) identifying all of an individuals’ chromosomes. Women with TS are typically of short stature and tend to exhibit a number of hallmark physical characteristics such as webbed neck, low-set ears, and shield chest (Romans, Stefanatos, Roeltgen, Kushner, and Ross, 1998). TS is associated with a number of medical issues, such as cardiovascular malformations, thyroid disorder, and kidney abnormalities, and generally results in delay or lack of the development of secondary sexual characteristics and infertility (due to nonfunctional ovaries). Though there is no cure, treatment for TS generally includes growth hormone (to increase final adult height) as well as estrogen therapy (to induce normal sexual maturation). Individuals with TS are often less socially active and have difficulties establishing or maintaining social interaction with their peers, and may use less adaptive coping strategies in difficult situations (reviewed in Kesler, Blasey, Brown, Yankowitz, Zeng, Bender and Reiss, 2003). However, studies of adult women with TS indicate they are generally well-educated and productively employed (Ross, Zinn, McCauley, 2000).
Globally impaired cognition is not a hallmark of TS (most demonstrate typical IQs; Van Dyke, Wiktor, Roberson and Weiss, 1991), though women with this condition tend to have specific difficulties with mathematics cognition and visuospatial tasks (Reiss, Mazzocco, Greenlaw, Freund and Ross, 1995; Romans et al, 1998), as well as with visuomotor control (Romans et al, 1998). More recently, executive function problems have also been described as commonly occurring in individuals with TS (Romans, Roeltgen, Kushner and Ross, 1997). The visual-spatial weaknesses observed in TS are thought to be genetically influenced (that is, women with TS are at increased risk because of reduced expression of one or more X chromosome genes), in part, because these problems are not corrected with exogenous hormonal therapy. In contrast, Ross and colleagues (2000) have suggested that the memory and reaction time difficulties seen in TS are the result of lowered estrogen occurring because of nonfunctional ovaries (as these deficits are somewhat reversible with estrogen therapy).
The cognitive deficits observed in TS participants are more severe when there is complete X monosomy rather than partial X monosomy (Jones, 1997). In addition, some variability in cognition may result from genomic imprinting, in which the amount of gene expression depends on its parental origin (Skuse, James, Bishop, Coppin, Dalton, Aamodt-Leeper, Bacarese-Hamilton, Creswell, McGurk and Jacobs, 1997). Kesler and colleagues (Kesler et al, 2003) found the superior temporal gyrus (STG) was larger in TS participants than in controls, and furthermore, that the sizes were more aberrant for those with maternally derived X chromosome than those with a paternally derived chromosome (Kesler et al, 2003). In addition, a recent study using a mouse model of TS indicated that imprinting specifically affects spatial working memory ability (Davies, Isles, Smith, Karunadasa, Burrmann, Humby, Ojarikre, Biggin, Skuse, Burgoyne, and Wilkinson, 2005).
The most affected brain areas in TS include decreases in volume in the occipital and parietal cortices, specifically in superior parietal lobe (SPL) and postcentral gyri (Murphy, DeCarli, Daly, Haxby, Allen, White, McIntosh, Powell, Horwitz, Rapoport, et al, 1993; Brown, Kesler, Eliez, Warsofsky, Haberecht and Reiss, 2004), suggesting that parietal cortex abnormalities may be related to the visuospatial deficits seen in TS. Other brain differences include increases in the volume of the superior temporal gyrus (Kesler et al, 2003), enlarged amygdala (Good, Lawrence, Thomas, Price, Ashburner, Friston, Frackowiak, Oreland and Skuse, 2003; Kesler, Garrett, Bender, Yankowitz, Zeng and Reiss, 2004), and reduced hippocampal volume (Kesler et al, 2004). Interestingly, full scale IQ is positively correlated with postcentral gyrus tissue volume in persons with TS.
Functional imaging studies of working memory hint at differences in the frontal-parietal network in participants with TS. A study of inhibition using a modified Go/NoGo task found that individuals with TS recruited more prefrontal regions than did typically developing control participants (Tamm, Menon and Reiss, 2002), although both groups had the same behavioral performance. This result suggests that additional brain regions were recruited by the TS participants in order to achieve the same level of performance. Hart and colleagues (2006) asked participants with TS to perform a visuospatial working memory task, and found that visuospatial working memory accuracy was worse and reaction times were longer than for controls. In addition, accuracy was more impaired with increasing task demands (distractors) than for controls. Though frontoparietal areas showed sustained activation in typically developing participants during visuospatial working memory tasks, this sustained activity was reduced in TS. A related study (Haberecht, Menon, Warsofsky, White, Dyer-Friedman, Glover, Neely, and Reiss, 2001) found that TS individuals had significantly longer reaction times in a 1-back and 2-back visuospatial working memory task. In addition, participants with TS exhibited decreased activity in the supramarginal gyrus (in the inferior parietal lobe; IPL) during the 2-back task, likely reflecting deficits in visuospatial encoding and working memory that have been described behaviorally in TS. This study also noted decreased activity in dorsolateral prefrontal cortex (DLPFC) and caudate, suggesting abnormalities in both fronto-parietal and fronto-striatal networks in women with TS.
The fronto-parietal network is also directly implicated in another visuospatial task, the “judgment of line orientation” (JLO) task, in which participants assess the angular orientations of pairs of lines. Kesler and colleagues (2004) performed a functional imaging task of JLO in participants with TS. The results suggested that participants with TS were impaired at the task behaviorally and they did not activate parietal-occipital regions to the same extent that typically developing participants did, nor did they recruit frontal regions for the more demanding tasks as was the case for the control participants. Finally, a recent DTI study (Holzapful, Barnea-Goraly, Eckert, Kesler and Reiss, 2006) provides converging evidence of parietal dysfunction, finding reduced fractional anisotropy (FA) in the deep WM of left parietal-occipital region (anterior along the superior lateral fasciculus) that connects to the frontal lobe. Decreased FA was also found bilaterally along the internal capsule into the globus pallidus in right PFC, and an increase in FA in the language areas of IPL and temporal lobe. Previous DTI imaging studies (e.g. Molko, Cachia, Riviere, Mangin, Bruandet, LeBihan, Cohen and Dehaene, 2004) had found that individuals with TS show morphometric abnormalities in right intraparietal sulcus. These white matter findings are consistent with the abnormal fronto-parietal network observed in functional imaging studies, as well as normal language function observed in the TS population.
2.4 Velocardiofacial (22q11.2 deletion) syndrome
Individuals with velocardiofacial syndrome (VCFS; also known as 22q11.2 deletion syndrome) are missing a portion of the long arm of chromosome 22 (near the middle, at location 22q11.2), a deletion that encompasses approximately 30 genes (Scambler, Kelly, Lindsay, Williamson, Goldberg, Shprintzen, Wilson, Goodship, Cross and Burn, 1992; Driscoll, Salvin, Sellinger, Budarf, McDonald-McGinn, Zackai and Emanuel, 1993; Carlson, Sirotkin, Pandita, Goldberg, McKie, Wadey, Patanjali, Weissman, Anyane-Yeboa, Warburton, 1997; Dunham, Hunt, Collins, Bruskiewich, Beare, et al, 1999). VCFS has a prevalence estimated at 1:4000 individuals (Óskarsdóttir, Vujic, and Fasth, 2004; Tezenas Du Montcel et al, 1996), and individuals with this syndrome exhibit characteristic physiological malformations of the heart, mouth (often including a submucosal or actual cleft palate) and face, and are at greater risk for immune deficiencies (Ryan et al, 1997). In general, persons with VCFS have been found to have mild cognitive deficits (IQ in the mild range of intellectual disability, with scores in the 70s to 80s; Wang, Woodin, Kreps-Falk and Moss, 2000), and show relative strengths in language tasks and impairments in math and spatial processing tasks (Wang et al, 2000). One particular gene within the deleted region, TBX1, is thought to play a role in the cardiac malformations (Merscher, Funke, Epstein, Heyer, Puech, Lu, Xavier, Demay, Russell and Factor, 2001), and other typically deleted genes, such as UFD1L, appear to be involved in neurodevelopment of the heart and brain (Yamagishi, Garg, Matsuoka, Thomas and Srivastava, 1999) and may be responsible, in part, for the learning deficits observed. VCFS has been studied in part because it may be a genetic risk factor for schizophrenia (Bassett and Chow, 1999).
Individuals with VCFS, similarly to the three other syndromes detailed above, tend to exhibit higher verbal IQ (VIQ) than performance IQ (PIQ), even though children with VCFS typically experience speech-language delays (Wang, et al, 2000). In addition to difficulties with visuospatial skills, individuals with VCFS have difficulty with arithmetic. VCFS is comorbid with many other psychiatric problems. For example, children with VCFS have bipolar mood disorders more frequently than individuals without this condition, and approximately 30% of individuals with VCFS will develop a schizophrenia-like psychosis during adulthood (Murphy, Jones and Owen, 1999). High rates of depression, ADHD, oppositional defiant disorder and specific phobias are also observed in affected individuals. (Michaelovsky, Gothelf, Korostishevsky, Frisch, Burg, Carmel, Steinberg, Inbar, Apter and Weizman, 2007; Antshel, Fremont, Roizen, Shprintzen, Higgins, Dhamoon and Kates, 2006; Wang et al, 2000; Ryan, Goodship, Wilson, Phillip, Levy et al, 1997). Bearden and colleagues (2002) described selective deficits in visual spatial working memory in a group of children and adolescents with VCFS, and in another study (Bearden, Woodin, Wang, Moss, McDonald-McGinn, Zackai, Emannuel, and Cannon, 2001), deficits in visuospatial memory, visuospatial cognition and arithmetic were observed in participants with VCFS relative to controls. In contrast to these spatial deficits, both studies found that object-based memory was found to be a relative strength in the participants with VCFS.
The overall brain size of individuals with VCFS tends to be smaller than those of typically developing individuals (Eliez, Schmitt, White and Reiss, 2000). In addition, brain areas that appear to be most affected by VCFS include a decreased size of the cerebellar vermis (Mitnick, Bello and Shprintzen, 1994), larger amygdala (Kates, Miller, Abdulsabur, Antshel, Concheos, Fremont and Roizen, 2006) and increased incidence of cavum septum pellucidum (Chow, Mikulis, Zipursky, Scutt, Weksberg and Bassett, 1999). Other studies have found a smaller left temporal lobe with accompanying asymmetry of ventricles (van Amelsvoort, Daly, Robertson, Suckling, Ng, Critchley, Owen, Henry, Murphy and Murphy, 2001). Eliez and colleagues (2000) found that the frontal lobe was relatively enlarged, and that left parietal cortex was significantly reduced as compared to right parietal cortex. Kates and colleagues (2001) found significantly decreased white matter volumes in parietal and temporal areas. Others have found enlargements within the sylvian fissure that might explain the often observed delays in language ability (Bingham, Zimmerman, McDonald-McGinn, Driscoll, Emanuell, and Zackal 1997).
Structural imaging studies have found that individuals with VCFS have a smaller cerebellar vermis and smaller posterior fossa (Mitnick, et al, 1994) than typically developing individuals. Barnea-Goraly and colleagues (2005) noted that fractional anisotropy (FA) values in the white matter of left supramarginal gyrus and angular gyri (in IPL) as well as near the intraparietal sulcus (IPS) were positively correlated with scores on the arithmetic subtest of the WISC/WAIS, and suggest that the differences in white matter in these areas may contribute to reduced arithmetic ability in VCFS. One of the genes deleted in the typical VCFS deletion, COMT, is known to regulate dopamine metabolism, and is particularly important for regulation of dopamine in the prefrontal cortex. Gothelf and colleagues found that individuals with VCFS who carry the lower-activity COMT allele (who thus experience unusually large amounts of dopamine in the prefrontal cortex) also show reduced grey matter volume in the prefrontal cortex, lowered cognitive ability, and more severe psychotic symptoms than did those with the high-activity COMT allele (Gothelf, Eliez, Thompson, Hinard, Penniman, Feinstein, Kwon, Jin, Jo, Antonarakis, Morris and Reiss, 2005).
Relatively few functional imaging studies have been conducted with VCFS participants. Van Amelsvoort and colleagues (van Amelsvoort, Schmitz, Daly, Deeley, Critchley, Henry, Robertson, Owen, Murphy, and Murphy, 2006) performed an fMRI study of facial emotion processing in a small sample of participants with VCFS. These researchers found that, as compared with typically developing participants, those with VCFS showed less activation in insula and frontal cortex and relatively more activation in bilateral occipital cortex. The authors interpret this as evidence that the neural pathways between ‘face perception’ areas and the rest of the ventral visual processing pathway may be dysfunctional. In another study, Eliez and colleagues (Eliez, Blasey, Menon, White, Schmitt and Reiss, 2001), asked participants with VCFS to perform an arithmetic computation task (similar to that performed in participants with FXS by Rivera et al, 2002). Participants had to determine whether equations were correct when they included either two-operands or three-operands. Control participants performed well on both easy and difficult tasks (99% and 95% correct), and showed similar activations on both tasks. However, in the more difficult condition, participants with VCFS had reduced performance (dropping from 90% to 75.8% correct) and showed increased activation in the left supramarginal gyrus (SMG) above that seen in the easier task. This difference from controls is a potential indicator of parietal lobe functional abnormality in VCFS.
3. CONVERGING EVIDENCE ACROSS SYNDROMES
Though the genetic alterations or deletions underlying these four disorders involve genes on three different chromosomes (the X-chromosome in FXS and TS, chromosome 7 in WS and chromosome 22 in VCFS), it is interesting that visuospatial skills, and especially visuoconstructive abilities, are relatively more impaired in all syndromes. That is, across the disorders, Verbal IQ typically exceeds Performance IQ in most individuals, suggesting that the primary cognitive deficits are nonverbal. However, other researchers (e.g. Cornish, Turk, Wilding, Sudhalter, Munir, Kooy and Hagerman, 2004) have argued that the deficits may be due to an underlying difference in executive function ability and may reflect adaptation to widespread underlying dynamic-process weaknesses, such as impaired inhibition, rather than single, static higher-level deficits (e.g., spatial cognition). Clearly, it is necessary to obtain a more detailed understanding of the underlying neural circuitry to fully answer this question. In the following sections, we explore some of the behavioral, structural, and neurobiological similarities and differences across these syndromes, particularly for visuospatial processing.
3.1 Visuospatial processing disruptions
From a neural standpoint, visual processing can be divided into two general “streams” which solve two basic visual problems: “what am I looking at?” and “where is it going?” (Ungerleider and Mishkin, 1982). Neural circuits along a swath of cortex running anteriorly and ventrally from the occipital to the temporal lobes process “what” is being looked at, and include modules dedicated to processing form and identity. In contrast, the “where” stream of processing runs dorsally from occipital cortex to parietal cortex and is optimized for interacting with things in the world, including modules dedicated to ascertaining motion and guiding limb movements. Visuospatial processing is thought to arise primarily from the dorsal stream of visual processing, including many modules in and around the intraparietal sulcus (IPS). However, both visual processing systems are essentially always in use, and must share results so that the visuospatial system guides actions to the intended objects in the world.
Visuospatial experiments generally refer to any task that requires an analysis of the spatial location or arrangement of objects or parts of objects in order for successful completion. Some visuospatial tasks require only a perception of the spatial features, such as during the perception of visuospatial stimulus (e.g. during the judgment of line orientation task, JLO; Kesler et al, 2004), or during a visuospatial working memory task, where the spatial arrangement of one stimulus must be held in mind and compared with the spatial arrangement of another stimulus. Visuoconstructive and visuomotor tasks require spatial manipulation (or mental rearrangement) of parts in order to create a prespecified spatial arrangement or form (e.g. Block Design Task). To accurately perform visuoconstructive tasks, one must visualize an object (or picture) as a set of parts, and be able to construct a replica from those parts (Frangiskakis et al, 1996).
Individuals diagnosed with the four syndromes discussed throughout this review tend to have difficulty performing visuospatial tasks, though they exhibit relatively intact object processing abilities. As a result, a number of researchers have proposed that a neural hallmark of these syndromes is a specific deficit in the dorsal, “where” stream of visual processing (e.g. Meyer-Lindenberg et al, 2004; Reiss, Eckert, Rose, Karchemskiy, Kesler, Chang, Reynolds, Kwon and Galaburda, 2004; Kogan, Bertone, Cornish, Boutet, Der Kaloustian, Anderman, Faubert and Chaudhuri, 2004; Kogan, Boutet, Cornish, Zangenehpour, Mullen, Holden, Der Kaloustian, Andermann and Chaudhuri, 2004). In further support of this hypothesis is the finding of structural and connectivity (WS: Hoeft, Barnea-Goraly, et al, 2007; Eckert et al, 2005; Meyer-Lindenberg et al, 2004; FXS: Barnea-Goraly et al, 2003; TS: Murphy et al, 1993; Brown et al, 2004; Molko et al, 2004; Holzapful et al, 2006; VCFS: Barnea-Goraly et al, 2005) as well as functional (WS: Meyer-Lindenberg et al, 2004; Atkinson, Braddick, Anker, Curran, Andrew, Wattern-Bell and Braddick, 2003; Atkinson, King, Braddick, Nokese, Anker, and Braddick, 1997; FXS: Kwon et al, 2001; Rivera et al, 2002; TS: Kesler et al, 2004; Hart et al, 2004; VCFS: Eliez et al, 2001) abnormalities in the parietal lobe and superior longitudinal fasciculus (the primary white matter pathway running from occipital towards parietal cortex) across all four syndromes (see Table 1).
Table 1.
Table of functional, structural and connectivity abnormalities found in sub-regions of the parietal cortex in four syndromes: Williams syndrome (WS), fragile X syndrome (FXS), Turner syndrome (TS) and velocardiofacial (VCFS) syndrome. Arrows indicate amount of BOLD activation in region for patients relative to controls.
| Superior Parietal Lobe (SPL) | Inferior Parietal Lobe (IPL) |
Intraparietal sulcus (IPS) | Superior Longitudinal Fasciculus (SLF) | Postcentral Gyrus (PCG) | ||
|---|---|---|---|---|---|---|
| Supramarginal Gyrus (SMG) | Angular Gyrus (AG) | |||||
| Williams syndrome (WS) | SPL size ↓ (Eckert et al, 2005); ↓ activation in SPL during visuospatial fMRI task (Meyer- Lindenberg et al, 2004) |
GM ↓ in IPS (Meyer- Lindenberg et al, 2004) | ↑ FA in SLF (Hoeft et al, 2007) | |||
| Fragile X syndrome (FXS) | ↓ activation in SPL and SMG during visuospatial working memory task (Kwon et al, 2001) | ↓ modulation in SPL and SMG during visuospatial working memory task (Kwon et al, 2001); FMRP levels correlate with activity in left SMG and AG (Rivera et al, 2002) |
↓ modulation in during difficult arithmetic task (Rivera et al, 2002); FMRP levels correlate with activity in left SMG and AG (Rivera et al, 2002) |
↓ WM connectivity in parietal sensory- motor pathways (Barnea-Goral et al, 2003) | ||
| Turner syndrome (TS) | SPL size ↓ (Murphy et al, 1993; Brown et al, 2004); ↓ activation during judgment of line orientation task (Kesler et al, 2004) |
↓ activation in IPL during judgment of line orientation task (Kesler et al, 2004) ↓ activation in SMG and IPL during visuospatial working memory (Haberecht et al, 2001) |
↓ activation in IPL during judgment of line orientation task (Kesler et al, 2004) | morphometric anomalies in right IPS (Molko et al, 2004); ↓ lack of sustained activity in IPS during visuospatial working memory task (Hart et al, 2006) |
↓ FA along SLF (Holzapful et al, 2006) | ↓ size of postcentral gyrus (Murphy et al, 1993; Brown et al, 2004) |
| Velocardiofacial syndrome (VCFS; 22q11.2 deletion syndrome) | ↑ activation in SMG during difficult arithmetic task (Eliez et al, 2001) | FA values in IPL as well as IPS correlated with arithmetic ability (Barnea- Goraly et al, 2005) | ||||
Though evidence suggests that “dorsal stream” processing is aberrant in these syndromes, it should be noted that assessment of visuospatial ability is extremely difficult. Visuospatial tasks tend to be complex, requiring veridical low-level visual inputs, intact integration of these inputs in early visual areas, a well-functioning dorsal “where” pathway, and accurate executive control of the inputs processed by that path. Low-level visual processing abnormalities may impact an individual’s ability to manipulate visuospatial information. For example, in WS, a number of basic visual processing differences (such as reduced stereopsis, van der Geest et al, 2005; strabismus, Winter et al, 1996; Kapp et al, 1995; and impairment in making simple saccades, van der Geest et al, 2004) are present from a very early age, and may result in an individual who has been processing atypical images throughout development. Previous researchers have maintained that the low-level deficits seen in WS are dissociable from visuospatial deficits, when tested in a group of young children with WS (e.g. Atkinson et al, 2001). However, it is possible that the low correlation values observed were a result of dichotomous outcomes (passed/failed) for many of the tests, which may have masked a gradation in ability. It is also possible that the overall developmental delay and/or attention difficulties overshadowed any contribution that the low-level deficits may have made to visuospatial difficulties.) For example, a number of labs have noted that saccade behavior is aberrant in FXS and TS participants (Cornish et al, 2004; Lasker, Mazzocco, and Zee, 2007), and Van der Geest and colleagues (2004) have reported impairments in horizontal and vertical saccades in WS. It is possible that impaired eye-movements (or poor executive control of subsequent eye-movements) could result in difficulty with complex visuospatial, and especially visuoconstructive tasks.
The visuospatial studies highlighted above show that although most individuals with these syndromes exhibit visual-spatial deficits, there are also differences between the syndromes in terms of low-level visual acuity, the precise locations of structural abnormalities, as well the extent of executive function difficulties that may impact task performance. Future studies may find that the deficits seen across these four neurogenetic syndromes are the result of disruption at one or more different stages of visual or executive processing. A more thorough characterization of the abnormal neural processing and cognitive differences in these patient populations may provide crucial links for understanding the neural systems that contribute to successful visuospatial processing in a typically developing population.
3.2 Potential neurobiological links across syndromes
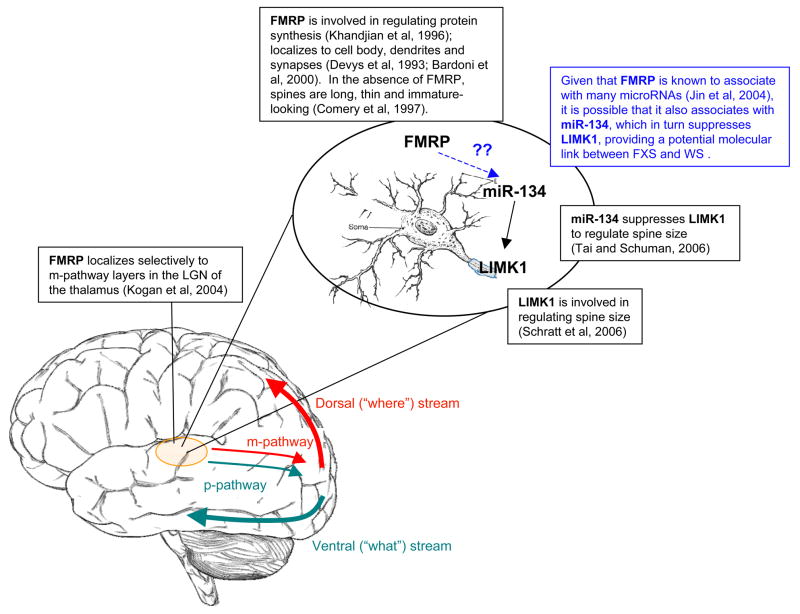
Although the visuospatial deficits demonstrated across these syndromes are not identical, it remains curious that visuospatial processing is generally disrupted while object-oriented processing remains relatively intact (Bearden, 2002). At the level of the lateral geniculate nucleus (LGN) of the thalamus, the visual system is broadly divided into two main processing pathways, the magnocellular and parvocellular paths. Neurons from the magnocelluar layers of the LGN (the m-pathway) funnel into the layers of primary visual cortex which, in turn, project dorsally to the “where” stream of processing to the parietal cortex. In contrast, the parvocellular layers of the LGN (p-pathway) become the “what” stream of visual processing, projecting ventrally from occipital cortex to the temporal lobe. The m-pathway neurons are relatively larger in size, and have relatively larger dendritic fields than do the p-pathway neurons (Dacey and Petersen, 1992). Notably, dendritic abnormalities have been found in many genetic disorders that result in mental retardation, including FXS and WS as well as Rett, Down and Rubinstein-Taybi syndromes (Kaufman and Moser, 2000). In the case of FXS, Koukoui and Chaudhuri (2007) have proposed that the m-pathway, which feeds into the dorsal stream of visual processing, may be more susceptible to alteration because of its reliance on relatively larger dendritic fields than is the p-pathway, which feeds into the ventral stream of visual processing. In this section we speculate on the hypotheses that there may be a molecular link between three of these neurogenetic syndromes, FXS, WS, and VCFS such that they affect dendritic organization and tend to preferentially disrupt the m-pathway that feeds into the dorsal visual processing stream.
FMRP, the protein product disrupted when the FMR1 gene is silenced in fragile X syndrome, has been found in the cell body, dendrites and in synapses (Devys, Lutz, Rouyer, Bellocq and Mandel, 1993; Bardoni et al, 2000). A particularly relevant result, given the behavioral findings of difficulty with visuospatial tasks, comes from Kogan and colleagues (2004) who performed immunohistochemical staining of the LGN of the thalamus (an important relay station for visual information) in a typically developing human male. They found that FMRP localized selectively to the magnocellular layers. In addition, similar staining of the thalamus of a male with FXS found morphological differences in the LGN, and behavioral studies have shown that males with FXS show reduced sensitivity for tasks that tap into the m-pathway (e.g. global motion), but typical sensitivity for the parvocellular (object-based; p-pathway) probes such as form perception and chromatic sensitivity. This further suggests that the dorsal stream of visual processing is specifically altered in FXS at a relatively early stage of processing.
The FMRP family of proteins is generally thought to be involved in typical neuronal development and is active in ribosome translation during protein synthesis (Khandjian, Corbin, Woerly, and Rousseau, 1996). Thus, it is likely that reduction in FMRP levels could alter activity of a number of other proteins involved in synaptic formation and axon development (Brown, Jin, Ceman, Darnell, O’Donnell, Tenenbaum, Jin, Feng, Wilkinson, Keene, Darnell and Warren, 2001). In particular, FMRP is thought to be involved in regulating protein translation at postsynaptic regions necessary for proper development of learning and memory (Jin, Alisch and Warren, 2004; Worley, 1998). Animal and human data also suggests that the reduction in levels of FMRP causes a corresponding disruption in the normal maturation and pruning of dendritic spines, leaving immature, elongated spines (Irwin, Galvez and Greenough, 2000). In animal studies, FMRP has also been found to be involved in experience-dependent plasticity (Gabel, Won, Kawai, McKinney, Tartakoff and Fallon, 2004).
Turning now to WS, one of the genes knocked out in the typical WS deletion, LIMK1, is thought to be involved in regulating dendritic spine size (Schratt, Tuebing, Nigh, Kane, Sabatini, Kiebler and Greenberg, 2006). LIMK1 encodes a cytoplasmic protein kinase, which is expressed throughout the developing brain (Proschel, Blouin, Gutowski, Ludwig and Noble, 1995) and is thought to be involved in controlling growth cone motility in cultured neurons (Hoogenraad, Akhmanova, Galjart and DeZeeuw, 2004). Meng and colleagues (2002) found that knockout mice lacking the LIMK1 pathway showed abnormal dendritic morphology. Interestingly, this gene, LIMK1, is thought to be specifically involved in the visuospatial deficit seen in WS (Frangiskakis et al, 1996). Recently, Gray and colleagues (Gray, Karmiloff-Smith, Funnell, Tassabehji, 2006) reported that the loss of one copy of LIMK1 was not enough to result in robust visuospatial deficits in people, and suggest that LIMK1 might contribute to visuospatial deficits when it is deleted in combination with other genes commonly missing within the most common WS deletion. Even so, the fact that genes from both FXS and WS contribute to dendritic formation leaves open the possibility that these genes are involved in interacting or overlapping molecular pathways.
Though no link has yet been proven, an interesting potential connection between FMRP (downregulated in FXS), and LIMK1 (typically deleted in WS) is illustrated in Figure 1. FMRP is thought to associate with many RNAs, including microRNAs, with which it acts synergistically to regulate synaptogenesis (Jin, Zarnescu, Ceman, Nakamoto, Mowrey, Jongens, Nelson, Moses and Warren, 2004; Garber, Smith, Reines and Warren, 2006). The first microRNA to be known to localize in dendrites, miR-134, is involved in repressing the translation of LIMK1 in order to regulate spine size (Tai, Schuman, 2006). Given that FMRP is known to associate with microRNAs, it is possible that FMRP might associate with miR-134, which in turn regulates LIMK1. An association between these two gene products would suggest that some of the overlapping cognitive deficits in FXS and WS may arise because of alterations to molecules that shape the dendritic pathways.
Figure 1.
Potential molecular mechanism for m-pathway (dorsal visual stream) disruption in fragile X syndrome and Williams syndrome. FMRP is downregulated in fragile X syndrome; LIMK1 is deleted in the typical Williams syndrome deletion.
A similar biochemical pathway may also be disrupted in VCFS. In particular, miR-134 appears to be down-regulated in a mouse model of VCFS (22q11.2 deletion syndrome; Stark, Xu, Bagchi, Lai, Liu, Hsu, Wan, Pavlidis, Mills, Karayiorgou and Gogos, 2008). These authors suggest that one of the genes deleted in the 22q11.2 deletion, Dgcr8, is responsible for mediating a number of microRNAs, including miR-134. As a result of Dgcr8 deficiency, a “bottleneck” is created in miRNA processing, which has been shown in mice (heterozygous for the knockout) to affect dendritic spine density and morphology, as well as to cause problems in spatial working memory tasks (Stark et al, 2008). Thus, there is some evidence across FXS, WS, and VCFS suggesting that a common molecular mechanism affecting dendritic spine growth may be involved in these disorders. Though speculative, this mechanism may provide one possible explanation for the observed visuospatial deficits across three neurogenetic syndromes.
3.3 Functional neuroimaging across neurogenetic syndromes
Functional imaging studies of neurogenetic populations are relatively few in number, due to difficulties with excessive head motion and inferior behavioral performance during task-oriented scans. Nevertheless, it is useful to compare results from visuospatial fMRI studies, as this domain is commonly impaired across the four disorders. A comparison of parietal cortex activations across fMRI studies indicates that activations were generally reduced in the affected populations, relative to controls, for all visuospatial and math tasks (Table 1). Reduced parietal activations occurred in conjunction with worse behavioral performance across all syndromes, suggesting that overlapping neural circuits were affected by different neurogenetic conditions. It should be noted that Table 1 shows only the differences between populations, and that the corresponding fMRI studies also reported many similarities (i.e. no significant differences) between the healthy and affected populations for other tasks in similar experiments.
The lone exception to the pattern of reduced activation is in the difficult math condition, comparing VCFS to controls (Eliez et al, 2001). The experimental design and the reported behavioral performance of the affected population (Eliez et al, 2001) were both very similar to the math experiment performed with FXS participants (Rivera et al, 2002). While it is possible that the VCFS participants engaged an unusual brain network during the difficult math condition, no difference was found between the normal and VCFS groups for the easy math condition in this experiment. It is also possible that the results may be due to the limited number of participants in the sample (VCFS: n = 8). In fact, all of the fMRI studies shown in Table 1 were performed with relatively limited sample sizes ranging from 8 to 16 affected participants.
One additional factor to consider when interpreting functional imaging studies is the potential limitation in accuracy resulting from large head movements during the scan session. Lying still is particularly challenging for younger participants, and behaviorally, children with FXS, WS, TS, and VCFS have all been described as being at increased risk for hyperactivity and impulsivity (reviewed in Reiss et al, 2000). Successful functional imaging requires that participant head motion be minimal (e.g. less than 3 mm) across a scan session, and it is known that large head movements may depress true activations or cause false statistical activations (Bullmore, Brammer, Rabe-Hesketh, Curtis, Morris, Williams, Sharma and McGuire, 1999). Because physical motion constraints (e.g. bite-bars) cannot always be used for these participants, one method to help reduce participant motion is to desensitize the participants to the noise of the scanner environment, and to practice lying very still in a mock scanner before the actual scan (e.g. Hoeft et al, 2007). In addition, new data analysis methods can be applied to mitigate the statistical effects of large and rapid head motions (Lemieux, Salek-Haddadi, Lund, Laufs and Carmichael, 2007), spontaneous deep breaths (Birn, Smith, Jones and Bandettini, 2008), and artifacts in the data (Mazaika, Whitfield-Gabrieli and Reiss, 2007). These recent analysis techniques were not available for previous fMRI studies, and one cannot rule out the possibility that the use of a more current analysis method could affect the results. One suggestion for future fMRI analyses with clinical populations is to report the number of participants who show more than 3 mm of motion, as an indicator of the risk that motion confounds may have affected the fMRI results. Despite the difficulty of performing fMRI analyses in high motion populations, the unique view offered by functional MRI continues to prove invaluable to advance our understanding of cognition in neurogenetic disorders.
4. CONCLUSIONS
Human behavior and development arise from a complex interplay between genes, biology and the environment. Neurogenetic disorders provide a unique opportunity to link known genetic alterations with specific cognitive characteristics and aberrant neural development. From a basic science perspective, these analyses provide a window for localizing and investigating the neural circuits underlying behavior, and allow researchers to explicitly link genes and gene expression to the development of these neural circuits. This review highlighted some of the behavioral, neuroimaging and genetic insights obtained from the study of visuospatial processing ability across four neurogenetic syndromes.
Additional behavioral neurogenetics research will further elucidate the interconnections between genes, brains, and behavior. Developmental studies are particularly informative, as they allow the researcher to understand how a change in the trajectory of brain development may lead to a cascade of cognitive, behavioral, and emotional problems (Reiss and Dant, 2003). These findings can be invaluable from a clinical perspective if they lead to more effective interventions that can nudge an atypically-developing brain back towards a typically-developing path, and potentially reduce the need for more extensive interventions later in life.
Though the task of linking genes to brains and behavior is somewhat easier with neurogenetic disorders in which the genetic difference is known, this general approach may serve as a model for how most brain disorders will be treated in the future. That is, individuals with “autism” or “schizophrenia” will not be treated as members of a monolithic group, but rather as individuals with specific biological and environmental risk factors that led to, and are now maintaining, suboptimal brain development and function (Meyer-Lindenberg and Weinberger, 2006). Furthermore, as noted by Bearden and colleagues (2008), these imaging methods may allow for the identification of subgroups of behaviorally defined disorders, based on neural endophenotypes, who may respond differently to certain treatments than other subgroups. Thus, further progress in behavioral neurogenetics holds the potential to advance our understanding of the brain and to develop individualized interventions in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antshel K, Fremont W, Roizen N, Shprintzen R, Higgins A, Dhamoon A, Kates W. ADHD, Major Depressive Disorder, and Simple Phobias are Prevalent Psychiatric Conditions in Youth with Velocardiofacial Syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45 (5):596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Anker S, Braddick O, Nokes L, Mason A, Braddick F. Visual and visuospatial development in young children with Williams syndrome. Developmental Medicine and Child Neurology. 2001;43:330–337. doi: 10.1017/s0012162201000615. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Anker S, Curran W, Andrew R, Wattern-Bell J, Braddick F. Neurobiological models of visuospatial cognition in children with Williams syndrome: Measures of dorsal-stream and frontal function. Developmental Neuropsychology. 2003;23(1):139–172. doi: 10.1080/87565641.2003.9651890. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. NeuroReport. 1997;8(8):1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Bardoni B, Mandel JL, Fisch J. FMR1 gene and fragile X syndrome. American Journal of Medical Genetics. 2000;97:153–163. doi: 10.1002/1096-8628(200022)97:2<153::aid-ajmg7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. White matter tract alterations in fragile X syndrome: Preliminary evidence from diffusion tensory imaging. American Journal of Medical Genetics Part B. 2003;118B:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Menon V, Bammer R, Reiss AL. Arithmetic ability and parietal alterations: A diffusion tensor imaging study in Velocardiofacial syndrome. Cognitive Brain Researchs. 2005:735–740. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biological Psychiatry. 1999;46(7):882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden C. Williams syndrome cognitive profile also characterizes velocardiofacial/DiGeorge syndrome. American Journal of Medical Genetics. 2002;114:689–692. doi: 10.1002/ajmg.10539. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Lee AD, Chiang MC, van Erp TGM, Cannon TD, Reiss AL, Toga AW, Thompson PW. Neural phenotypes of common and rare genetic variants. Biological Psychology. 2008;79(1):43–57. doi: 10.1016/j.biopsycho.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Wang PP, Simon TJ. Williams syndrome cognitive profile also characterizes Velocardiofacial/DiGeorge syndrome. American Journal of Medical Genetics. 2002;114(6):689–692. doi: 10.1002/ajmg.10539. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Emannuel B, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: Selective deficit in visual-spatial memory. Journal of Clinical and Experimental Neuropsychology. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Bihrle A, Jernigan T, Trauner D, Doherty S. Neuropsychological, neurological and neuroanatomical profile of Williams syndrome. American Journal of Medical Genetics Supplement. 1990;6:115–125. doi: 10.1002/ajmg.1320370621. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR. Bridging cognition, the brain and molecular genetics: Evidence from Williams syndrome. Trends in Neurosciences. 1999;22(5):197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Krause SE, Block SS, Guter S, Wuu J, Leurgans S, Decle P, Potanos K, Cook E, Salt J, Maino D, Weinberg D, Lara R, Jardini T, Cogswell J, Johnson SA, Hagerman R. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: A controlled trial. Journal of Child and Adolescent Psychopharmacology. 2006;16(5):525–540. doi: 10.1089/cap.2006.16.525. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome–Present and future. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:42–48. doi: 10.1002/mrdd.20007. [DOI] [PubMed] [Google Scholar]
- Bingham PM, Zimmerman RA, McDonald-McGinn D, Driscoll D, Emanuel BS, Zackal E. Enlarged sylvian fissures in infants with interstitial deletion of chomosome 22q11. American Journal of Medical Genetics. 1997;74:538–543. [PubMed] [Google Scholar]
- Birn RA, Smith MA, Jones TB, Bandettini PA. The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:47–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Reiss AL. A volumetric study of parietal lobe subregions in Turner syndrome. Developmental Medicine and Child Neurology. 2004;46(9):607–609. doi: 10.1017/s0012162204001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SCR, Sharma T, McGuire PK. Methods for Diagnosis and Treatment of Stimulus-Corrrelated Motion in Generic Brain Activation Studies Using fMRI. Human Brain Mapping. 1999;7:38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C, Sirotkin H, Pandita R, Goldberg R, McKie J, Wadey R, Patanjali S, Weissman S, Anyane-Yeboa K, Warburton D. Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. American Journal of Human Genetics. 1997;61:620–629. doi: 10.1086/515508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EWC, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biological Psychiatry. 1999;46(10):1436–1442. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, Brown WT. Social gaze, social avoidance, and repetitive behavior in fragile X males: A controlled study. American Journal of Mental Retardation. 1988;92(5):436–446. [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proceedings of the National Academy of Sciences. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Munir F, Cross G. The nature of the spatial deficit in young females with Fragile-X syndrome: A neuropsychological and molecular perspective. Neuropsychologia. 1998;36(11):1239–1246. doi: 10.1016/s0028-3932(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Cornish K, Scerif G, Karmiloff-Smith A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex. 2007;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, Hagerman R. Annotation: Deconstructing attention deficit in fragile X syndrome: a developmental neuropsychological approach. Journal of Child Psychology and Psychiatry. 2004;45(6):1042–1053. doi: 10.1111/j.1469-7610.2004.t01-1-00297.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. New England Journal of Medicine. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Acta Geneticae Medicae. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, et al. Prevalence and phenotpye consequence of FRAXA and FRAXE alleles in a large, ethnically diverse, special education-needs population. American Journal of Medical Genetics. 1999;64:495–507. doi: 10.1086/302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Petersen MR. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proceedings of the National Academy of sciences. 1992;89:9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, Ojarikre O, Biggin C, Skuse D, Burgoyne P, Wilkinson L. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects of cognitive function in mice. Nature Genetics. 2005;37(6):625–629. doi: 10.1038/ng1577. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of fragile X permutation. Nature Genetics. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Driscoll DA, Salvin J, Sellinger B, Budarf ML, McDonald-McGinn DM, Zackai EH, Emanuel BS. Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: implications for genetic counseling and prenatal diagnosis. Journal of Medical Genetics. 1993;30:813–817. doi: 10.1136/jmg.30.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham I, Hunt AR, Collins JE, Bruskiewich R, Beare DM, et al. The DNA sequence of human chromosome 22. Nature. 1999;402:489–495. doi: 10.1038/990031. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Hu D, Eliez S, Bellugi U, Galaburda A, Korenberg J, Mills D, Reiss AL. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. doi: 10.1212/01.WNL.0000148598.63153.8A. [DOI] [PubMed] [Google Scholar]
- Einfeld SL, Tonge BJ, Florio T. Behavioral and emotional disturbance in individuals with Williams syndrome. American Journal of Mental Retardation. 1997;102:45–53. doi: 10.1352/0895-8017(1997)102<0045:BAEDII>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Menon V, White CD, Schmitt JE, Reiss AL. Functional brain imaging study of mathematical reasoning abilities in velocardiofacial syndrome (del22q11.2) Genetics in Medicine. 2001;3(1):49–55. doi: 10.1097/00125817-200101000-00011. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: A volumetric MRI study. American Journal of Psychiatry. 2000;157:409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Ensing GJ, Loker J, Moore C, Leppert M, Keating M. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proceedings of the National Academy of Sciences. 1993;90(8):3226–3230. doi: 10.1073/pnas.90.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences. 2003;100(12):7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangiskakis JM, Ewart AK, Morris CA, Mervis CB, Bertrand J, Robinson BF, Klein BP, Ensing GJ, Evertt LA, Green ED, Pröschel C, Gutowski NJ, Noble M, Atkinson DL, Odelberg SJ, Keating MT. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 1996;86(1):59–69. doi: 10.1016/s0092-8674(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL. Cognitive profiles associated with the fra(X) syndrome in males and females. American Journal of Medical Genetics. 1991;38(4):542–547. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. Psychiatric disorders associated with fragile X in the young female. Pediatrics. 1993;91:321–329. [PubMed] [Google Scholar]
- Gabel LA, Won S, Kawai H, McKinney M, Tartakoff AM, Fallon JR. Visual experience regulates transient expression and dendritic localization of Fragile X Mental Retardation Protein. Journal of Neuroscience. 2004;24(47):10579–10583. doi: 10.1523/JNEUROSCI.2185-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi C, Figerio E, Burt DM, Cazzaniga I, Perrett DI, Borgatti R. Facial expression recognition in Williams syndrome. Neuropsychologia. 2003;41:733–738. doi: 10.1016/s0028-3932(02)00178-1. [DOI] [PubMed] [Google Scholar]
- Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Current Opinion in Genetics and Development. 2006;16:270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here’s looking at you kid: Neural systems underlying face and gaze processing in Fragile X syndrome. Archives of General Psychiatry. 2004;61:281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- Good C, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RSJ, Oreland L, Skuse DH. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126(11):2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, Morris MA, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neuroscience. 2005;8(11):1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, Erba HW, Ringel J, Hayashi KM, Patnaik S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Annals of Neurology. 2008;63(1):40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray V, Karmiloff-Smith A, Funnell E, Tassabehji M. In-depth analysis of spatial cognition in Williams syndrome: A critical assessment of the role of the LIMK1 gene. Neuropsychologia. 2006;44:679–685. doi: 10.1016/j.neuropsychologia.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Boyett-Anderson JM, Menon V, Reiss AL. Reduced basal forebrain and hippocampal activation during memory encoding in girls with fragile X syndrome. NeuroReport. 2004;15(10):1579–1583. doi: 10.1097/01.wnr.0000134472.44362.be. [DOI] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, Piven J, Reiss AL. Early white-matter abnormalitites of the ventral frontostriatal pathway in fragile X syndrome. Developmental medicine and child neurology. 2009 doi: 10.1111/j.1469–8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Mills D, Yam A, Hoeft F, Bellugi U, Reiss AL. Genetic influences on sociability: Heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. Journal of Neuroscience. 2009;29(4):1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberecht MF, Menon V, Warsofsky IS, White CD, Dyer-Friedman J, Glover GH, Neely EK, Reiss AL. Functional neuroanatomy of visuo-spatial working memory in Turner syndrome. Human Brain Mapping. 2001;14:96–107. doi: 10.1002/hbm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment and research. Baltimore, MD: The Johns Hopkins University Press; 2002. [Google Scholar]
- Hart SJ, Davenport MJ, Hopper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006;129:1125–1136. doi: 10.1093/brain/awl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Nakajima T, Takagi M, Fukuhara N, Abe H. Human cerebellar activation in relation to saccadic eye movements: A functional magnetic resonance imaging study. Ophthalmologica. 2002;216:399–405. doi: 10.1159/000067551. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hirota H, Matsuoka R, Chen XN, Salandanan LS, Lincoln A, Rose FE, Sunahara M, Osawa M, Bellugi U, Korenberg JR. Williams syndrome deficits in visual spatial processing linked to GTF2IRD1 and GTF2I on Chromosome 7q11.23. Genetics in Medicine. 2003;5(4):311–321. doi: 10.1097/01.GIM.0000076975.10224.67. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golari G, Ng D, Mills D, Korenberg J, Bellugi U, Galaburda A, Reiss AL. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. Journal of Neuroscience. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Human Brain Mapping. 2007;28:543–554. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in Turner syndrome. Journal of Neuroscience. 2006;26(26):7007–7013. doi: 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A, Gaijart N, De Zeeuw CI. LIMK1 and CLIP-115: Linking cytoskeletal defects to Williams syndrome. BioEssays. 2004;26(2):141–150. doi: 10.1002/bies.10402. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Koekkoek B, Akhmanova A, Krugers H, Dortland B, Miedema M, van Alphen A, Kistler WM, Jaegle M, Koutsourakis M, Van Camp N, Verhoye M, van der Linden A, Kaverina I, Grosveld F, De Zeeuw CI, Galjart N. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP-115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nature Genetics. 2002;32:116–127. doi: 10.1038/ng954. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural abnormalities in Fragile-X mental retardation syndrome. Cerebral Cortex. 2000;10(10):1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Jacobs PA. The chromosome complement of human gametes. In: Milligan SR, editor. Oxford Reviews of Reproductive Biology. New York: Oxford University Press; 1992. pp. 4–72. [PubMed] [Google Scholar]
- Jernigan TL, Bellugi U. Anomalous brain morphology on magnetic resonance images in Williams syndrome and Down syndrome. Archives of Neurology. 1990;47:529–533. doi: 10.1001/archneur.1990.00530050049011. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR. Cerebral morphometric distinctions between Williams and Down syndromes. Archives of Neurology. 1993;50(2):186–191. doi: 10.1001/archneur.1993.00540020062019. [DOI] [PubMed] [Google Scholar]
- Jin P, Alisch RS, Warren ST. RNA and microRNAs in fragile X mental retardation. Nature Cell Biology. 2004;6(11):1048–1053. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nature Neuroscience. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Jones KL. Smith’s Recognizable Patterns of Human Malformations. 5. New York: W.B. Saunders; 1997. [Google Scholar]
- Kapp ME, Von Noorden GK, Jenkins R. Strabismus in Williams syndrome. American Journal of Opthalmology. 1995;119(3):355–360. doi: 10.1016/s0002-9394(14)71180-8. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, Geraghty M, Kaufmann WE, Pearlson GD. Regional cortical white matter reductions in velocardiofacial syndrome: A volumetric MRI analysis. Biological Psychiatry. 2001;49:677–684. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kates W, Miller A, Abdulsabur N, Antshel K, Conchelos J, Fremont W, Roizen N. Temporal lobe anatomy and psychiatric symptoms in Velocardiofacial Syndrome (22q11.2 Deletion Syndrome) J Am Acad Child Adolesc Psychiatry. 2006;45(5):587–595. doi: 10.1097/01.chi.0000205704.33077.4a. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: Correlations in fragile X syndrome. American Journal of Medical Genetics. 1999;83:286–295. [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cerebral Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biological Psychiatry. 2003;54(6):636–646. doi: 10.1016/s0006-3223(03)00289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 2004;42:1971–1978. doi: 10.1016/j.neuropsychologia.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Kirk-Neely E, Reiss AL. Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cerebral Cortex. 2004;14:174–180. doi: 10.1093/cercor/bhg116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Lightbody AA, Reiss AL. Cholinergic dysfunction in fragile X syndrome and potential intervention: A preliminary 1H MRS study. American Journal of Medical Genetics Part A. 2009;149A:403–407. doi: 10.1002/ajmg.a.32697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian EW, Corbin F, Woerly S, Rousseau F. The fragile X mental retardation protein is associated with ribosomes. Nature Genetics. 1996;12:91–93. doi: 10.1038/ng0196-91. [DOI] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, Berman KF. Genetic contributions to human gyrification: Sulcal morphometry in Williams syndrome. Journal of Neuroscience. 2005;25(34):7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Anderman E, Faubert J, Chaudhuri A. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004a;63:1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Zangenehpour S, Mullen K, Holden JJA, Der Kaloustian VM, Andermann E, Chaudhuri A. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004;127:591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Research Reviews. 2007;53:27–38. doi: 10.1016/j.brainresrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, Talor AK, Glover GH, Reiss AL. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: Relation to behavioral and molecular measures. American Journal of Psychiatry. 2001;158:1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Mazzocco MMM, Zee DS. Ocular motor indicators of executive dysfunction in fragile X and Turner syndromes. Brain and Cognition. 2007;63:203–220. doi: 10.1016/j.bandc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Salek-Haddadi A, Lund TE, Laufs H, Carmichael D. Modeling large motion events in fMRI studies of patients with epilepsy. Magnetic Resonance Imaging. 2007;25:894–901. doi: 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Bellugi U. Musical abilities in individuals with William syndrome. Music Perception. 1998;15:357–389. [Google Scholar]
- Marenco S, Siuta MA, Kippenhan JS, Grodofsky S, Chang W, Kohn P, Mervis CB, Morris CA, Weinberger DR, Meyer-Lindenberg A, Pierpaoli C, Berman KF. Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proceedings of the National Academy of Sciences. 2007;104(38):15117–15122. doi: 10.1073/pnas.0704311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Whitfield-Gabrieli S, Reiss AL. Artifact repair of fMRI data from high motion clinical subjects (abstract) NeuroImage. 2007;36(Supp 1):S142. [Google Scholar]
- Mazzocco MMM. Math learning disability and MD subtypes: Evidence from studies of Turner syndrome, fragile X syndrome and neurofibromatosis type 1. Journal of Learning Disabilities. 2001;34(6):520–533. doi: 10.1177/002221940103400605. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu W, MacDonald J, Wang J, Falls D. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35(1):121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in fragile X syndrome: Relation to FMR1 gene expression. Proceedings of the National Academy of Sciences. 2004;101(10):3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu M, Xavier R, Demay M, Russell R, Factor S. TBX1 is for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Becerra AM. Language and Communicative Development in Williams Syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:3–15. doi: 10.1002/mrdd.20140. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nature Neuroscience. 2005;8(8):991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behavior. Nature Reviews Neuroscience. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Michaelovsky E, Gothelf D, Korostishevsky M, Frisch A, Burg M, Carmel M, Steinberg T, Inbar D, Apter A, Weizman A. Association between a common haplotype in the COMT gene region and psychiatric disorders in individuals with 22q11.2DS. International Journal of Neuropsychopharmacology. 2007;22:1–13. doi: 10.1017/S1461145707008085. [DOI] [PubMed] [Google Scholar]
- Mitnick RJ, Bello JA, Shprintzen RJ. Brain anomalies in velocardio-facial syndrome. American Journal of Medical Genetics (Neuropsychiatry Genetics) 1994;54:100–106. doi: 10.1002/ajmg.1320540204. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, Galaburda AM, Reiss AL. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biological Psychiatry. 2006;62:256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;1:2070–2076. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, LeBihan D, Cohen L, Dehaene S. Brain anatomy in Turner syndrome: Evidence for impaired social and spatial-numerical networks. Cerebral Cortex. 2004;14(8):840–850. doi: 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
- Moretti R, Bava A, Torre P, Antonello RM, Cazzato G. Reading errors in patients with cerebellar vermis lesions. Journal of Neurology. 2002;249:461–468. doi: 10.1007/s004150200040. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: Correlation with neurocognitive performance. Neurology. 1998;50:121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Daly E, Haxby JV, Allen G, White BJ, McIntosh AR, Powell CM, Horwitz B, Rapoport SI, et al. X-chromosome effects on female brain: A magnetic resonance imaging study of Turner’s syndrome. Lancet. 1993;342:1197–1200. doi: 10.1016/0140-6736(93)92184-u. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Archives of General Psychiatry. 1999;56(10):940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252(5009):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Óskarsdóttir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Archives of Disease in Childhood. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger 5-HTTLPR polymorphism impact human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Proschel C, Blouin MJ, Gutowski NJ, Ludwig R, Noble M. LIMK1 is predominantly expressed in neural tissues and phosphorylates serine, threonine and tyrosine residues in vitro. Oncogene. 1995;11(7):1271–1281. [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nature Medicine. 1995;1(2):159–167. doi: 10.1038/nm0295-159. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Aylward E, Freund LS, Joshi PK, Bryan RN. Neuroanatomy of fragile X syndrome: The Posterior Fossa. Annals of Neurology. 1991;29:26–32. doi: 10.1002/ana.410290107. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: Analyzing gene-brain-behavior relationships in child developmental psychopathologies. Development and Psychopathology. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: Brain anatomy parallels cognition and behavior in Williams syndrome. The Journal of Neuroscience. 2004;24(21):5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Patwardhan A, Haberecht M. Brain imaging in neurogenetic conditions: Realizing the potential of behavioral neurogenetics research. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:186–197. doi: 10.1002/1098-2779(2000)6:3<186::AID-MRDD6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, Bellugi U. Neuroanatomy of Williams syndrome: A high-resolution MRI study. Journal of Cognitive Neuroscience, Supplement. 2000;12:65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Freund L, Tseng JE, Joshi PK. Neuroanatomy in fragile X females: The posterior fossa. American Journal of Human Genetics. 1991;49(2):279–288. [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Hall SS. Fragile X syndrome: Assessment and treatment implications. Child and Adolescent Psychiatric Clinics of North America. 2007;16(3):663–675. doi: 10.1016/j.chc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Lee J, Freund L. Neuroanatomy of fragile X syndrome: The temporal lobe. Neurology. 1994;44:1317. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL. Neurodevelopmental effects of X monosomy: A volumetric imaging study. Annals of Neurology. 1995;38(5):731–738. doi: 10.1002/ana.410380507. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Human Brain Mapping. 2002;16(4):206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans SM, Roeltgen DP, Kushner H, Ross JL. Executive function in females with Turner syndrome. Developmental Neuropsychology. 1997;13:23–40. [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. Transition to young adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. American Journal of Medical Genetics. 1998;79:140–147. [PubMed] [Google Scholar]
- Rosenthal G, Gilman S, Koeppe RA, Kluin KJ, Markel DS, Junck L, Gebarski SS. Motor dysfunction in olivopontocerebellar atrophy is related to cerebral metabolic rate studies with positron emission tomography. Annals of Neurology. 1988;24(3):414–419. doi: 10.1002/ana.410240310. [DOI] [PubMed] [Google Scholar]
- Ross J, Zinn A, McCauley E. Neurodevelopmental and psychosocial aspects of Turner syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:135–141. doi: 10.1002/1098-2779(2000)6:2<135::AID-MRDD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Phillip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, Aurias A, Raymond FL, Clayton-Smith J, Hatchwell E, McKeown C, Beemer FA, Dallapiccola B, Novelli G, Hurst JA, Ignatius J, Green AJ, Winter RM, Brueton L, Brondum-Nielsen K, Scambler PJ. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. Journal of Medical Genetics. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional funtions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiology of Learning and Memory. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Scambler PJ, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R, Wilson DI, Goodship JA, Cross IE, Burn J. Velocardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eliez S, Bellugi U, Reiss AL. Analysis of cerebral shape in Williams syndrome. Archives of Neurology. 2001;58:283–287. doi: 10.1001/archneur.58.2.283. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eliez S, Warsofsky IS, Bellugi U, Reiss AL. Enlarged cerebellar vermis in Williams syndrome. Journal of Psychiatric Research. 2001;35(4):225–229. doi: 10.1016/s0022-3956(01)00024-3. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Watts K, Eliez S, Bellugi U, Galaburda AM, Reiss AL. Increased gyrification in Williams syndrome: evidence using 3D MRI methods. Developmental Medicine and Child Neurology. 2002;44:292–295. doi: 10.1017/s0012162201002109. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Skuse DH, James RS, Bishop DVM, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs RA. Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nature Genetics. 2008;40(6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. Journal of Child Neurology. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. MicroRNA: MicroRNAs reach out into dendrites. Current Biology. 2006;16(4):R121–R123. doi: 10.1016/j.cub.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Abnormal prefrontal cortex function during response inhibitin in Turner syndrome: Functional magnetic resonance imaging evidence. Biological Psychiatry. 2002;53:107–111. doi: 10.1016/s0006-3223(02)01488-9. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. American Journal of Medical Genetics. 2000;91:144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Teisl JT, Reiss AL, Mazzocco MM. Maximizing the sensitivity of a screening questionnaire for determining fragile X at-risk status. American Journal of Medical Genetics. 1999;83:281–285. [PubMed] [Google Scholar]
- Tezenas Du Montcel S, Mendizabai H, Ayme S, et al. Prevalence of 22q11 microdeletion. Journal of Medical Genetics. 1996;33:719. doi: 10.1136/jmg.33.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lönnqvist J, Standerskjöld-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nature. 2001;4(12):1–6. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64(1):196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Engle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, Critchley H, Owen MJ, Henry J, Murphy KC, Murphy DGM. Structural brain anomalies associated with deletion at chromosome 22q11. British Journal of Psychiatry. 2001;178:412–419. doi: 10.1192/bjp.178.5.412. [DOI] [PubMed] [Google Scholar]
- Van Amelsvoort T, Schmitz N, Daly E, Deeley Q, Critchley H, Henry J, Robertson D, Owen M, Murphy KC, Murphy DG. Processing facial emotion in adults with velo-cardio-facial syndrome: functional magnetic resonance imaging. British Journal of Psychiatry. 2006;189:560–561. doi: 10.1192/bjp.bp.105.019876. [DOI] [PubMed] [Google Scholar]
- Van der Geest JN, Lagers-van Haselen GC, vanHagen JM, Brenner E, Govaerts LCP, de Coo IFM, Frens MA. Visual depth processing in Williams-Beuren syndrome. Experimental Brain Research. 2005;166(2):200–209. doi: 10.1007/s00221-005-2355-1. [DOI] [PubMed] [Google Scholar]
- Van der Geest JN, Lagers-van Haselen GC, vanHagen JM, Govaerts LCP, de Coo IFM, de Zeeuw CI, Frens MA. Saccade dysmetria in Williams-Beuren syndrome. Neuropsychologia. 2004;42:569–576. doi: 10.1016/j.neuropsychologia.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Van Dyke DL, Wiktor A, Roberson JR, Weiss L. Mental retardation in Turner syndrome. Journal of Pediatrics. 1991;118(3):415–417. doi: 10.1016/s0022-3476(05)82159-6. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Xhang FP. Identification of a gene (FMR-1) containing a CGG repeat coincident wit ha breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vicari S, Bellucci S, Carlesimo GA. Visual and spatial long-term memory: Differential pattern of impairments in Williams and Down syndromes. Developmental Medicine and Child Neurology. 2005;47:305–311. doi: 10.1017/s0012162205000599. [DOI] [PubMed] [Google Scholar]
- Wang PP, Bellugi U. Evidence from two genetic syndromes for a dissociation between verbal and visual-spatial short-term memory. Journal of Clinical and Experimental Neuropsychology. 1994;16:317–322. doi: 10.1080/01688639408402641. [DOI] [PubMed] [Google Scholar]
- Wang PP, Doherty S, Hesselink JR, Bellugi U. Callosal morphology concurs with neurobehavioral and neuropathological findings in two neurodevelopmental disorders. Archives of Neurology. 1992;49:407–411. doi: 10.1001/archneur.1992.00530280101029. [DOI] [PubMed] [Google Scholar]
- Wang PP, Woodin MF, Kreps-Falk R, Moss EM. Research on behavioral phenotypes: velocardiofacial syndrome (deletion 22q11.2) Developemental Medicine and Child Neurology. 2000;42:422–427. doi: 10.1017/s0012162200000785. [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Archives of General Psychiatry. 2008;65(11):1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M, Pankau R, Amm M, Gosch A, Wessel A. The spectrum of ocular features in the Williams-Beuren syndrome. Clinical Genetics. 1996;49(1):28–31. doi: 10.1111/j.1399-0004.1996.tb04320.x. [DOI] [PubMed] [Google Scholar]
- Worley P. Making proteins at the synapse: Activity-regulated translation and CPEB. Neuron. 1998;21:936–938. doi: 10.1016/s0896-6273(00)80609-1. [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Garg V, Matsuoka R, Thomas T, Srivastava D. A molecular pathway revealing a genetic bass for uman cardiac and craniofacial defects. Science. 1999;283:1158–1161. doi: 10.1126/science.283.5405.1158. [DOI] [PubMed] [Google Scholar]