Abstract
Plants under herbivore attack are able to initiate indirect defense by synthesizing and releasing complex blends of volatiles that attract natural enemies of the herbivore. However, little is known about how plants respond to infestation by multiple herbivores, particularly if these belong to different feeding guilds. Here, we report the interference by a phloem-feeding insect, the whitefly Bemisia tabaci, with indirect plant defenses induced by spider mites (Tetranychus urticae) in Lima bean (Phaseolus lunatus) plants. Additional whitefly infestation of spider-mite infested plants resulted in a reduced attraction of predatory mites (Phytoseiulus persimilis) compared to attraction to plants infested by spider mites only. This interference is shown to result from the reduction in (E)-β-ocimene emission from plants infested by both spider mites and whiteflies. When using exogenous salicylic acid (SA) application to mimic B. tabaci infestation, we observed similar results in behavioral and chemical analyses. Phytohormone and gene-expression analyses revealed that B. tabaci infestation, as well as SA application, inhibited spider mite-induced jasmonic acid (JA) production and reduced the expression of two JA-regulated genes, one of which encodes for the P. lunatus enzyme β-ocimene synthase that catalyzes the synthesis of (E)-β-ocimene. Remarkably, B. tabaci infestation concurrently inhibited SA production induced by spider mites. We therefore conclude that in dual-infested Lima bean plants the suppression of the JA signaling pathway by whitefly feeding is not due to enhanced SA levels.
Keywords: herbivore-induced plant volatiles, induced plant defense, insect-plant interactions, phytohormones, terpene synthase
An important indirect defense of plants against herbivores is the emission of plant volatiles that provide important foraging cues for natural enemies of the herbivore (1, 2). Attraction of parasitoids or predators by herbivore-induced plant volatiles (HIPVs) has been well-demonstrated in many plant species both in the laboratory (2) and in the field (3). With regard to the underlying mechanisms, it has been demonstrated that the octadecanoid pathway, with the plant hormone jasmonic acid (JA) as central component, plays an important role in regulating HIPV emission (4, 5), although the shikimic acid and ethylene pathways can play roles as well (6–10). For example, in Lima bean plants, a transient increase of endogenous JA in leaves is involved in the induced synthesis of HIPVs (11), and application of exogenous JA to leaves leads to the induction of a volatile blend similar to the HIPV blend induced by spider mites (12). Conversely, blocking JA synthesis or its action results in the reduction of volatile emission (7), and consequently interferes with the attraction of predators to herbivore-damaged plants (5). However, only few studies on indirect plant defense have considered plants attacked by multiple herbivore species (13–15), whereas this is a widespread phenomenon in nature (16, 17). This is especially interesting when it concerns herbivore species that belong to different feeding guilds, such as parenchymal cell content feeders and phloem feeders (18).
Plants respond to herbivores belonging to different feeding guilds by activating distinct signal-transduction pathways. For instance, chewing herbivores predominantly activate the jasmonic acid (JA) signaling pathway, whereas phloem-feeding insects, such as whiteflies and aphids, frequently activate the salicylic acid (SA) signaling pathway (19–21). Moreover, evidence is accumulating that the SA and JA signaling pathways can mutually affect each other. For example, SA suppresses JA-dependent defense gene expression (22–24), possibly through inhibiting JA synthesis or its action (25). Similarly, JA has been shown to negatively affect SA-dependent gene expression (22). In some cases, synergistic effects between the two signaling pathways have been described (26). Cross-talk between different defense signaling pathways has important consequences for the evolution of plant defense, as it can influence the amount of damage suffered by plants and subsequently influence selection pressure on defense response (27).
Based on the observations that infestation by phloem-feeding insects may induce SA-dependent genes while suppressing JA-dependent genes (19–21), and possible cross-talk between JA and SA signaling pathways (28), we hypothesized that infestations by phloem-feeding insects would affect the emission of HIPVs induced by herbivores whose feeding behavior triggered JA-mediated responses, and will consequently interfere with the induction of HIPV-mediated indirect plant defense. Here, we demonstrate that infestation by the generalist whitefly, Bemisia tabaci, interferes with the indirect defense of Lima bean plants in response to generalist spider mites (Tetranychus urticae) through inhibition of the JA signaling pathway induced by the latter.
Results
Predatory Mite Olfactory Preference Behavior.
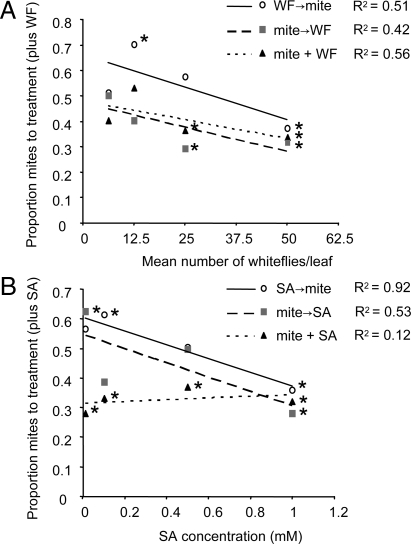
We first investigated the choice of predatory mites between plants infested with spider mites only and plants infested with spider mites plus whiteflies, to establish the effects of whitefly density and the time sequence of feeding by spider mites and whiteflies. Increasing the whitefly density negatively correlated with the attraction of predatory mites to plants infested with whiteflies and spider mites. This occurred for all three sequential combinations of spider mites and whiteflies; that is, (i) first spider mite infestation followed by whitefly infestation, (ii) first whitefly infestation followed by spider mite infestation and (iii) simultaneous infestation with spider mites and whiteflies (Fig. 1A). At densities <13 adults per leaf, B. tabaci did not negatively interfere with the attraction of predatory mites to T. urticae-infested plant volatiles in the Y-tube two-choice olfactory bioassays (Fig. 1A). However, at higher densities, B. tabaci feeding resulted in reduced attraction and at 50 adults per leaf, B. tabaci interfered with predatory mite attraction, regardless of feeding sequence (Fig. 1A).
Fig. 1.
Behavior of predatory mites in Y-tube olfactometer. Attraction of the predatory mite P. persimilis to the volatiles emitted from plants treated with T. urticae and B. tabaci (A) or T. urticae and SA (B), when volatiles from T. urticae-infested plants were offered as an alternative. (A) Effect of whitefly density on predatory mite behavior for three different infestation sequences of T. urticae and B. tabaci (B) Effect of SA dose on predatory mite behavior for three different sequences for T. urticae infestation and SA treatment. P. persimilis responses are presented as numbers of P. persimilis that chose for the treated plants (plus whitefly or SA) divided by the total number of responding P. persimilis (n = 80). Data points marked with an asterisk indicate significant differences from a 50:50 distribution (binomial test; *, P < 0.05). WF, whitefly B. tabaci; mite, T. urticae.
In addition, we used exogenous SA application to mimic the whitefly infestation. When plants were simultaneously exposed to T. urticae infestation and SA application, SA had a significantly negative effect on the attraction of predatory mites at all four SA doses studied (Fig. 1B). In plants that had either first been infested with T. urticae and subsequently been sprayed with SA or those that had been treated in the reverse order, the lowest dose of SA (0.01 mM) enhanced the attraction of predatory mites; at higher SA dosages, the positive effect of SA on predator attraction disappeared, and at a dose of 1.0 mM SA a significant, negative effect on the attraction of predatory mites was recorded (Fig. 1B).
Taken together, B. tabaci at a density of 50 adults per leaf or SA at a dose of 1.0 mM significantly reduced the attraction of predatory mites to spider-mite-induced Lima bean volatiles, irrespective of the treatment sequence. We, therefore, used the latter B. tabaci density and SA dose for subsequent bioassays in which plants were simultaneously exposed to T. urticae and B. tabaci or SA.
Volatile Blends from Dual-Damaged Lima Bean Plants.
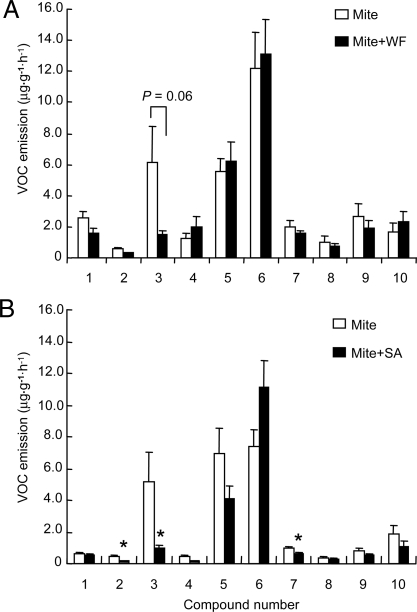
Gas chromatography-mass spectrometry (GC-MS) analysis revealed that 10 major compounds were consistently released from plants simultaneously treated with T. urticae and B. tabaci or SA, and those infested by T. urticae only (Fig. 2). Quantitative analysis showed that the amount of the monoterpene (E)-β-ocimene from plants infested with both T. urticae and B. tabaci was lower as compared to the amount emitted from T. urticae-infested plants (ANOVA, F1, 16 = 4.0, P = 0.06; Fig. 2A). The amounts of (E)-β-ocimene, as well as (Z)-β-ocimene and an unidentified compound were significantly lower in plants treated with T. urticae plus SA compared to plants infested with T. urticae only (Fig. 2B).
Fig. 2.
Compounds identified in the headspace of Lima bean plants. (A) Comparison of mean (± SE) (n = 9) emission rate of volatiles from plants infested with T. urticae only and plants simultaneously infested with T. urticae and B. tabaci. (B) Comparison of mean (± SE) emission rate of volatiles from plants infested with T. urticae only and plants simultaneously treated with T. urticae and SA. Peak numbers represent: 1, (Z)-3-hexenyl acetate; 2, (Z)-β-ocimene; 3, (E)-β-ocimene; 4, linalool; 5, (E)-4,8-dimethyl-1,3,7-nonatriene; 6, methyl salicylate; 7, C10H16O (unknown); 8, indole; 9, β-caryophyllene; 10, (E, E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene. Asterisks represent significant differences (*, P < 0.05) from T. urticae-infested plants as determined by Fisher protected least significant difference (PLSD) test of ANOVA. SA, salicylic acid; WF, whitefly B. tabaci; mite, spider mite T. urticae.
Effects of (E)-β-Ocimene on Predator Behavior.
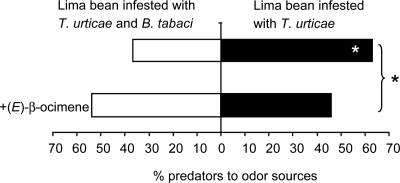
In a two-choice olfactory bioassay, predatory mites preferred the odor blend from T. urticae-infested plants over the blend from plants infested with both T. urticae and B. tabaci (Binomial test, P = 0.02); however, predatory mites did not discriminate between them when the odor blend from dual-damaged plants was supplemented with additional (E)-β-ocimene (Fig. 3). The preference distribution of predatory mites for these two-choice situations (with or without (E)-β-ocimene supplementation) was significantly different (GLM, F1, 7 = 12.47, P = 0.013; Fig. 3). These data demonstrate that the reduced emission of (E)-β-ocimene by dual-damaged plants caused the reduced attraction of predatory mites.
Fig. 3.
Effect of (E)-β-ocimene on the choices of P. persimilis between volatiles emitted from plants infested with T. urticae and plants simultaneously infested with T. urticae and B. tabaci, in a Y-tube olfactometer. Choices between odor sources were statistically analyzed with a two-sided binomial test, and choices before and after adding (E)-β-ocimene (indicated by the accolade) was analyzed with GLM, the significance of which is indicated behind the bracket (*, P < 0.05).
Quantification of Endogenous JA and SA.
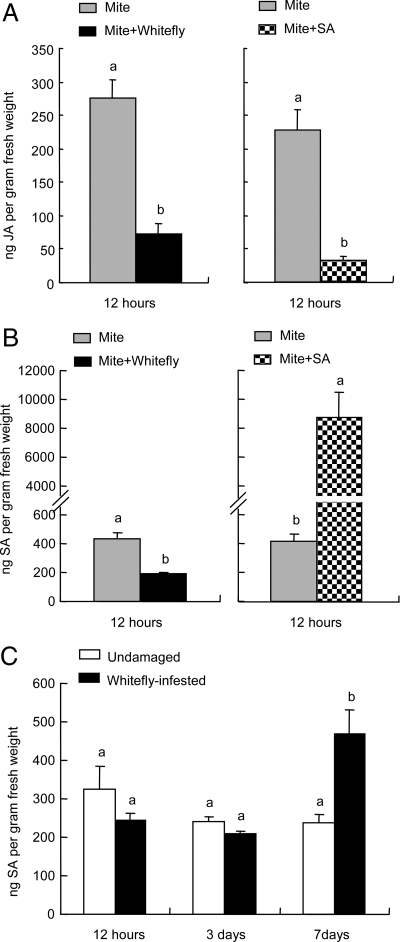
Subsequently, we quantified the amounts of endogenous JA and SA in plants infested with T. urticae only and plants simultaneously treated with T. urticae and B. tabaci or SA. We sampled the leaves 12 h after the onset of T. urticae infestation in each treatment because endogenous JA concentration exhibits a transient burst at this time point when Lima bean plants are wounded or damaged by spider mites. The amount of JA was significantly reduced in leaves infested with T. urticae and B. tabaci compared to leaves infested with T. urticae only (ANOVA, F1, 7 = 35.2, P < 0.001; Fig. 4A). Similarly, the amount of JA in leaves treated with T. urticae and SA was significantly decreased as compared to leaves infested with T. urticae only (F1, 6 = 40.5, P < 0.001; Fig. 4A). In undamaged plants the level of JA was below the detection limit.
Fig. 4.
Phytohormone analysis of plants of different treatments. Quantification of endogenous JA (A) and SA (B and C) levels in Lima bean leaves after different treatments. (A) JA levels in leaves infested with T. urticae and those simultaneously infested with T. urticae and B. tabaci for 12 h. (B) SA levels in leaves infested with T. urticae and those simultaneously treated with T. urticae infestation and SA application for 12 h. (C) The amounts of SA in undamaged and B. tabaci-infested leaves at different time points. Values are the mean (± SE) of 3–5 biological replicates. Different letters above bars indicate significant differences in the quantities between control and treatment (Fisher's PLSD test of ANOVA, P < 0.05).
The amount of SA was significantly reduced in leaves infested with T. urticae and B. tabaci compared to leaves infested with T. urticae only (ANOVA, F1, 8 = 34.4, P < 0.001; Fig. 4B). In contrast, the SA titer was significantly higher in leaves treated with T. urticae and SA compared to leaves infested with T. urticae only (ANOVA, F1, 6 = 27.3, P = 0.002; Fig. 4B).
To determine whether or not B. tabaci infestation induces SA in Lima bean plants, we assessed the kinetics of the SA titer in B. tabaci-infested or undamaged Lima bean leaves. The SA titer in leaves infested with B. tabaci for 12 h or 3 days did not differ from the titer in undamaged control leaves. In contrast, after 7 days of B. tabaci infestation the SA titer in infested leaves was 2.0 times larger than in undamaged controls (ANOVA, P = 0.03; Fig. 4C).
Gene-Expression Changes in Response to B. tabaci Infestation or SA Treatment.
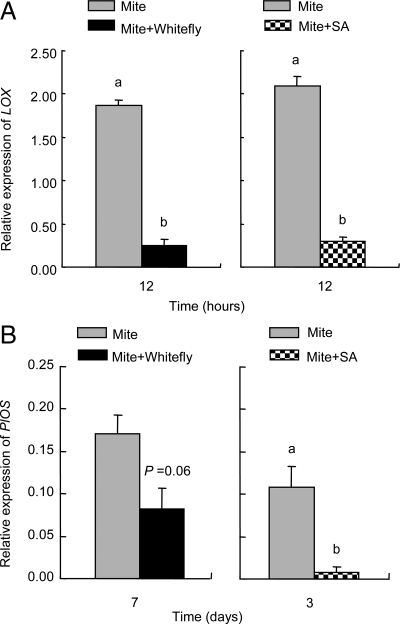
By using quantitative RT-PCR, we quantified the transcript levels of two genes in leaves treated with T. urticae and B. tabaci or SA, compared to leaves infested with T. urticae only. Lipoxygenase (LOX) is a key enzyme in the biosynthesis of JA along the octadecanoid pathway (29). In Lima beans, JA regulates the expression of PlOS that codes for the enzyme β-ocimene synthase (30). Both the infestation by B. tabaci and the exogenous application of SA significantly reduced LOX transcript levels induced by T. urticae (ANOVA, Whitefly: F1, 4 = 71.3, P = 0.001; SA: F1, 4 = 47.2, P = 0.002; Fig. 5A). B. tabaci caused a marginally significant reduction, while SA led to a significant reduction in T. urticae-induced PlOS transcript levels (ANOVA, Whitefly: F1, 4 = 6.5, P = 0.06; SA: F1, 4 = 12.4, P = 0.02; Fig. 5B).
Fig. 5.
Gene transcription quantification. qRT-PCR data for LOX (A) and PlOS (B) gene expression. (A) Transcript levels of LOX in leaves infested with T. urticae only and leaves simultaneously treated with T. urticae and B. tabaci or SA application. (B) Transcript levels of PlOS in leaves infested with T. urticae only and leaves simultaneously treated with T. urticae and B. tabaci or SA application. LOX and PlOS transcript levels have been normalized to the amount of PlACT1 transcripts in each sample. Values are the mean (± SE) of three biological replicates. Different letters above bars indicate significant differences in the transcript levels between treatments (Fisher's PLSD test of ANOVA, P < 0.05).
Discussion
Our results demonstrate that whitefly infestation of spider mite-infested Lima bean plants interferes with the attraction of predatory mites, irrespective of the infestation sequence (Fig. 1A). Moreover, our data show that interference with predator attraction was due to a reduction in (E)-β-ocimene emission from dual-infested plants (Figs. 2A and 3). This terpenoid is known to attract the predatory mite P. persimilis (6, 31). Similarly, the application of SA also interfered with spider-mite induced predator attraction, irrespective of treatment sequence, and also resulted in a reduced (E)-β-ocimene emission (Figs. 1B and 2B). These data suggest that the interference with indirect plant defense caused by B. tabaci infestation may be mediated by the SA signaling pathway.
A few studies documented effects of infestation by phloem-feeding insects on plant responses induced by other herbivore species (14, 32, 33). For instance, B. tabaci suppressed the emission of three terpenoids that were induced by simultaneously feeding caterpillars in cotton plants (33). In contrast, the aphid Myzus persicae caused an increased emission of volatiles triggered by spider mites in pepper plants, and consequently increased the attraction of predators to plants infested with aphids and spider mites (14). However, the signal-transduction mechanisms underlying these effects of phloem-feeding insects remain unknown, although interactions between different defense signal-transduction pathways have been suggested to play a role (33).
It is important to note that additional B. tabaci infestation might influence the performance of spider mites, as competition between the two herbivores might alter nutritional or morphological plant traits. Such an influence could have resulted in a reduction in the damage inflicted by spider mites, leading to a reduction in plant volatile emission (34, 35). However, we have observed that spider mites laid more eggs on B. tabaci-infested or SA-treated plants as compared to uninfested plants (see SI Text and Fig. S1), which shows that B. tabaci infestation or SA application did not negatively interfere with the feeding behavior of spider mites. Therefore, the reduction in (E)-β-ocimene emission was not caused by a reduction in spider mite feeding intensity on dual-infested plants. Thus, Lima bean plants that are infested by B. tabaci provide the spider mites with an increased reproduction rate and a reduced attraction of predators. This means that the infestation with B. tabaci, as well as the application of SA, reduces both the direct and the indirect defense and consequently, spider mites are expected to prefer B. tabaci-infested or SA-treated plants over uninfested leaves. This indeed is the case: in a two-choice assay, spider mites preferred to settle on leaves from a plant that had been infested by B. tabaci or treated with SA over uninfested leaves (see SI Text and Fig. S2).
Herbivore-induced plant volatile emission is known to be mainly regulated by the octadecanoid or JA signal-transduction pathway (4, 5). For instance, in Lima bean plants, spider mites induced the accumulation of JA and SA levels, of which JA played a key role in regulating volatile emission (11, 12), although SA also played a role (8). However, herbivores belonging to different feeding guilds have been shown to elicit distinct defense pathways in plants. Chewing insects induce JA-dependent defense responses, whereas piercing-sucking insects frequently induce SA-dependent defense responses (19–21). Considering the well-known cross-talk between JA and SA signaling pathways (28), it might be expected that B. tabaci-induced responses had an impact on the JA-regulated volatile emission. Indeed, we demonstrate that B. tabaci infestation inhibited JA synthesis induced by spider mites and JA-regulated (E)-β-ocimene emission. Bemisia tabaci infestation not only suppressed the expression of a key gene involved in the JA signaling pathway (LOX, 36), but also suppressed the JA production triggered by spider mites (Figs. 3A and 4A). Furthermore, the transcript level of the JA-regulated PlOS gene coding for β-ocimene synthase was also suppressed by B. tabaci infestation in dual-damaged plants (Fig. 4B), which correlates well with the reduction in (E)-β-ocimene emission (Fig. 2A). Unexpectedly, B. tabaci infestation at the same time interfered with the SA induction by spider mites (Fig. 4B). Indeed, we observed that even if Lima bean plants were only infested by B. tabaci this did not induce SA production 12 h after infestation (Fig. 4C). Also in plants infested with spider mites plus whiteflies, but not in plants only infested with spider mites, the SA level increases with longer infestation time ranging from 12 h until 7 days (see SI Text and Fig. S3). After 3 days of infestation, the PlOS transcript level is not yet reduced relative to plants infested with spider mites only, while at 7 days the PlOS transcript level in plants infested with both herbivores is half of that recorded for plants only infested with spider mites (see SI Text and Fig. S4). From these data, we conclude that the interference by B. tabaci with the plant's indirect defense is due to the suppression of the JA signaling pathway. Considering that external SA application highly mimicked the effects of B. tabaci infestation in behavioral, volatile, and phytohormone assays, this suggests that SA-related signaling is involved in the interference caused by B. tabaci. Further work is needed to identify the mechanisms along which whitefly feeding reduces the biosynthesis of both JA and SA and the possible involvement of other phytohormones.
Considering the polyphagous nature and wide distribution of B. tabaci, it is reasonable to assume that interactions with other herbivores are common. As B. tabaci densities in the field have been reported to be much higher than those used in the present study (37), it is likely that under natural conditions the interference by B. tabaci in indirect plant defense against other herbivores can be even stronger than observed in our experiments.
It is well-established that HIPVs function as important cues in interactions between plants and the natural enemies of herbivores. The discovery that combined infestations by phloem-feeding insects and cell-content feeding herbivores that commonly occur under natural circumstances affect plant-carnivore interactions, demonstrates that chemically mediated interactions in nature are more intricate than previously thought (38). To understand the selective pressures operating in the evolution of indirect plant defense, plant responses to multiple herbivores should be addressed. The fitness advantages to JA-inducing herbivores are obvious because the interference by phloem-feeding insects reduces the predation risk imposed by predators, and moreover, the associated suppression of JA-mediated direct defense may benefit herbivore growth rate (Figs. S1 and S2) (39). However, from the plant's perspective, defenses against multiple herbivores belonging to different feeding guilds might result in higher costs that exert selection on the evolution of indirect plant defenses.
Materials and Methods
Plants.
Lima bean plants (Phaseolus lunatus L., cv Sieva) were grown in a greenhouse compartment at 25 ± 5 °C, 50–70% R.H., and a photoperiod of 16L:8D. Plants were used in experiments when the two primary leaves were fully unfolded, which occurred between 10–15 days after sowing.
Insects and Mites.
Two-spotted spider mites, Tetranychus urticae (Acari: Tetranychidae), were reared on Lima bean plants in a greenhouse compartment (25 ± 5 °C, R.H.50–70%, 16L:8D). Whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), were maintained on Poinsettia (Euphorbia pulcherrima) plants in a separate greenhouse compartment (25 ± 5 °C, R.H.50–70%, 16L:8D). Predatory mites, Phytoseiulus persimilis (Acari: Phytoseiidae), were reared on detached Lima bean leaves infested with spider mites in Petri dishes in a climate cabinet (23 ± 2 °C, R.H.50–70%, 16L:8D). Female predatory mites were used 1–3 days after the final molt for experiments. Before experiments, predatory mites were starved for 2–3 h by individually confining them in Eppendorf tubes.
Olfactometer Experiments.
Responses of predatory mites to plant volatiles were tested in a Y-tube olfactometer that has been extensively described elsewhere (31, 40). In short, two odor sources are used to generate two laminar airflows in a Y-shaped glass tube. Individual adult female predatory mites are released at the downwind side of the tube and their choice for either odor source is recorded. Each odor comparison was repeated on 3–4 days with 20 predatory mites per day.
Spider Mite and Whitefly Infestation.
In these experiments, the density of T. urticae was fixed at 10 adults per leaf, which is sufficient to induce the attraction of predatory mites (35), while different B. tabaci densities were used. In total, four densities of B. tabaci were used, being 13, 25, 50, and 100 adults per two-leaf-plant. During the treatment period, plants were placed in a cage (30 × 40 × 60 cm); and whiteflies were released into the cage and allowed to feed and oviposit on the plant. For each density of B. tabaci, 24 potted Lima bean plants were infested with T. urticae and B. tabaci in three different sequences. (i) First mites then whiteflies: plants were initially infested with T. urticae mites for 2 days. Subsequently, T. urticae remained on the leaves while the plants were infested with adult B. tabaci whiteflies (treatment 1) or kept free of B. tabaci infestation (control 1) for 7 days. (ii) First whiteflies then mites: plants were initially infested with adult B. tabaci (treatment 2) or left uninfested (control 2) for 7 days. After that, B. tabaci remained on leaves while both treated and control plants were infested with T. urticae mites for 2 days. (iii) Mites and whiteflies simultaneously: plants were infested simultaneously with T. urticae and B. tabaci (treatment 3), or infested with T. urticae only (control 3) for 7 days. Before bioassays, B. tabaci adults were removed from the plants by aspiration, while T. urticae remained.
Mite and SA Treatment.
In these experiments, the density of T. urticae was fixed at 10 adults per leaf, while the SA concentration was varied. Four SA (Sigma–Aldrich) concentrations were assayed, that is, 0.01, 0.1, 0.5, and 1.0 mM. For each SA dose, 24 potted Lima bean plants were inoculated with T. urticae and exogenous SA in three different sequences. (i) First mites then SA: plants were first infested with T. urticae mites for 2 days. Subsequently, these plants were sprayed with 1.25 mL/leaf of SA solution (containing 0.1% Tween 20 as surfactant; treatment 4) or water (containing 0.1% Tween 20; control 4). Three days later, the plants were used for bioassays. (ii) First SA then mites: plants were first sprayed with 1.25 mL/leaf of SA solution (treatment 5) or water (control 5). Three days later, both treated and control plants were infested with T. urticae mites for 2 days and subsequently the plants were used for bioassays. (iii) Mites and SA simultaneously: plants were simultaneously inoculated with T. urticae and treated with 1.25 mL/leaf of SA solution (treatment 6) or water (control 6). Three days later, the plants were used for bioassays. Four detached leaves of each treatment were used as an odor source.
Collection of Headspace Volatiles.
Volatiles emitted by Lima bean plants were collected using a dynamic headspace collection system similar as described by Bruinsma et al. (41). One plant was transferred to a 5-L glass vessel (Duran). Purified air was split into two air streams with a constant flow of 200 mL/min. The system was purged for 1 h with purified air before attaching a tube filled with 120 mg Tenax TA (Grace-Alltech) to the air outlet in the lid to trap the headspace volatiles. Headspace collections were carried out in a climate chamber at 23 ± 1 °C, 60 ± 5% R.H. Volatile collection lasted for 2 h.
Chemical Analysis of Volatiles.
Headspace samples were analyzed as described in detail by Bruinsma et al. (41). First, traps were flushed with helium (30 mL/min) for 15 min to remove moisture and oxygen. Samples were thermally desorbed at 220 °C for 5 min and refocused on a sorbent trap at 0 °C. Volatiles were injected on the analytical column (Rtx-5ms, 30 m × 0.25 mm ID, 1.0-μm film thickness, Restek) with a split flow of 20 mL/min by heating the cold trap to 250 °C for 3 min. The temperature programs of the GC were as follows: 40 °C (3-min hold), 10 °C min−1 to 280 °C (3-min hold). The column effluent was ionized by electron impact ionization (70 eV). Mass scanning was done from 33 to 250 m/z. Compounds were identified by comparing the mass spectra with those of authentic standards or with NIST 05 and Wiley library spectra. Quantification of identified compounds was based on comparison with a set of authentic compounds injected in different concentrations ranging from 100 ng to 10 μg/μL methanol. Response factors were linear for all reference compounds within this concentration range.
Effects of (E)-β-Ocimene on Predatory Mite Behavior.
In the Y-tube olfactometer, we investigated the behavioral preference of P. persimilis females when offered a choice between the volatiles from plants infested with T. urticae and volatiles from plants simultaneously infested with T. urticae and B. tabaci. After individually testing 20 predators, synthetic (E)-β-ocimene (Sigma–Aldrich) emitted from a 5-μL glass microcapillary (release rate = 125 μg/h) was added downwind of the plants infested with T. urticae and B. tabaci. Another set of 20 P. persimilis were subsequently tested on an individual basis. The experiment was repeated on 4 different days with independent sets of odor sources and predators.
Quantification of Endogenous JA and SA.
The quantification of endogenous JA and SA followed a protocol from Schulze et al. (42). Samples were analyzed on a Finnigan GCQ Instrument (Thermo Electron) running in a CI-negative ion mode, as described by Schulze et al. (42).
Quantitative Real-Time PCR.
Total RNA extraction, purification, and cDNA synthesis were done as described by Zheng et al. (43). To quantify lipoxgenase (LOX) and P. lunatus ocimene synthase (PlOS) transcript levels, real-time quantitative RT-PCR was performed in a Rotor-Gene 6000 machine (Corbett Research) with a 72-well rotor. The amplification reactions were performed in 25 μL final volume containing 12.5 μL ABsolute TM QPCR SYBR Green Mix (ABgene), 1 μL forward primer (2 μM) and reverse primer (2 μM) pairs (final primers concentration: 80 nM), and 1 μL cDNA (10 ng/μL) first strand template. The PCR program was the same as described by Zheng et al. (43). The gene-specific primers of LOX (GenBank accession X63521), PlOS (GenBank accession EU194553) and PlACT1 (GenBank accession DQ159907) as housekeeping gene were designed with the Beacon Designer software (Premier Biosoft International) set to an annealing temperature of 56 °C. LOX primers were F-LOX (5′-GGAATGGGACAGGGTTTATG-3′) and R-LOX (5′- CAAAGTCACTGGGCTTCTCA-3′). Its predicted length was 176 bp. PlOS primers were F-PlOS (5′-TGCATGGGTCTCAGTCTCTG-3′) and R- PlOS (5′- TGCTGCTTCCCCTCTCTCTA-3′). Its predicted length was 189 bp. PlACT1 primers were F-PlACT1 (5′-CCAAGGCTAACCGTGAAAAG-3′) and R-PlACT1 (5′-AGCCAGATCAAGACGAAGGA-3′). Its predicted length was 208-bp. These primer sequences were blasted against the NCBI nucleotide and EST database to ascertain that they are not homologous to other genes. The LOX and PlOS expression relative to PlACT1 expression were quantified by comparing the threshold cycle for each PCR to their respective dilution series and dividing the resulting quantities.
Statistical Analysis.
Binomial tests were performed to analyze the Y-tube olfactometer experiments. When analyzing the differences in the choice distributions of predators across treatments, a Generalized Linear Model with a logit-link function and binomial distribution of error variance was used. Predators that did not make a choice were excluded from the analysis. Fisher protected least significant difference (PLSD) tests of ANOVA was used to analyze volatile and phytohormone data. The data of gene expression were log-transformed and statistically analyzed by a one-way ANOVA.
Supplementary Material
Acknowledgments.
We thank Leo Koopman, Rieta Gols and Leon Westerd for culturing the insects and mites. This work was supported by the Earth and Life Sciences Foundation VICI Grant no. 865.03.002 (to M.D.), which is subsidized by the Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907890106/DCSupplemental.
References
- 1.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 2.Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- 3.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 4.Boland W, Hopke J, Donath J, Nuske J, Bublitz F. Jasmonic acid and coronatin induce odor production in plants. Angew Chem Int Ed Engl. 1995;34:1600–1602. [Google Scholar]
- 5.Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dicke M, et al. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions - involvement of host plant in its production. J Chem Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- 7.Engelberth J, et al. Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 2001;125:369–377. doi: 10.1104/pp.125.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 2000;41:391–398. doi: 10.1093/pcp/41.4.391. [DOI] [PubMed] [Google Scholar]
- 9.van Poecke RMP, Dicke M. Induced parasitoid attraction by Arabidopsis thaliana: Involvement of the octadecanoid and the salicylic acid pathway. J Exp Bot. 2002;53:1793–1799. doi: 10.1093/jxb/erf022. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi JAG, Ozawa R, Shimoda T, Takabayashi J, Nishioka T. Exogenous ACC enhances volatiles production mediated by jasmonic acid in lima bean leaves. FEBS Lett. 2001;509:332–336. doi: 10.1016/s0014-5793(01)03194-5. [DOI] [PubMed] [Google Scholar]
- 11.Koch T, Krumm T, Jung V, Engelberth J, Boland W. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dicke M, Gols R, Ludeking D, Posthumus MA. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol. 1999;25:1907–1922. [Google Scholar]
- 13.Shiojiri K, Takabayashi J, Yano S, Takafuji A. Infochemically mediated tritrophic interaction webs on cabbage plants. Popul Ecol. 2001;43:23–29. [Google Scholar]
- 14.Moayeri HRS, Ashouri A, Poll L, Enkegaard A. Olfactory response of a predatory mirid to herbivore induced plant volatiles: Multiple herbivory vs. single herbivory. J Appl Entomol. 2007;131:326–332. [Google Scholar]
- 15.De Boer JG, Hordijk CA, Posthumus MA, Dicke M. Prey and non-prey arthropods sharing a host plant: Effects on induced volatile emission and predator attraction. J Chem Ecol. 2008;34:281–290. doi: 10.1007/s10886-007-9405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poelman EH, Van Loon JJA, Dicke M. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plants Sci. 2008;13:534–541. doi: 10.1016/j.tplants.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Soler R, et al. Influence of presence and spatial arrangement of belowground insects on host-plant selection of aboveground insects: A field study. Ecol Entomol. 2009;34:339–345. [Google Scholar]
- 18.Dicke M, Van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol. 2009;5:317–324. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- 19.Moran PJ, Thompson GA. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001;125:1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007;143:866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempema LA, Cui XP, Holzer FM, Walling LL. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007;143:849–865. doi: 10.1104/pp.106.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol. 1998;39:500–507. [Google Scholar]
- 23.Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT. Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta. 1999;209:87–95. doi: 10.1007/s004250050609. [DOI] [PubMed] [Google Scholar]
- 24.Koornneef A, et al. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 2008;147:1358–1368. doi: 10.1104/pp.108.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA. Salicylic-acid inhibits synthesis of proteinase-inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Wees SCM, De Swart EAM, Van Pelt JA, Van Loon LC, Pieterse CMJ. Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2000;97:8711–8716. doi: 10.1073/pnas.130425197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stinchcombe JR, Rausher MD. Diffuse selection on resistance to deer herbivory in the ivyleaf morning glory, Ipomoea hederacea. Am Nat. 2001;158:376–388. doi: 10.1086/321990. [DOI] [PubMed] [Google Scholar]
- 28.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 29.Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arimura GI, et al. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 2008;146:965–973. doi: 10.1104/pp.107.111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mumm R, Posthumus MA, Dicke M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008;31:575–585. doi: 10.1111/j.1365-3040.2008.01783.x. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Saona C, Chalmers JA, Raj S, Thaler JS. Induced plant responses to multiple damagers: Differential effects on an herbivore and its parasitoid. Oecologia. 2005;143:566–577. doi: 10.1007/s00442-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Saona C, Crafts-Brandner SJ, Canas LA. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J Chem Ecol. 2003;29:2539–2550. doi: 10.1023/a:1026314102866. [DOI] [PubMed] [Google Scholar]
- 34.Gouinguené S, Alborn H, Turlings TCJ. Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J Chem Ecol. 2003;29:145–162. doi: 10.1023/a:1021984715420. [DOI] [PubMed] [Google Scholar]
- 35.Gols R, Roosjen M, Dijkman H, Dicke M. Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J Chem Ecol. 2003;29:2651–2666. doi: 10.1023/b:joec.0000008010.40606.b0. [DOI] [PubMed] [Google Scholar]
- 36.Arimura GI, et al. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 37.Liu T-X. Population dynamics of Bemisia argentifolii (Homoptera: Aleyrodidae) on spring collard and relationship to yield in the lower Rio Grande valley of Texas. J Econ Entomol. 2000;93:750–756. doi: 10.1603/0022-0493-93.3.750. [DOI] [PubMed] [Google Scholar]
- 38.De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 39.Li CY, Williams MM, Loh YT, Lee GI, Howe GA. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 2002;130:494–503. doi: 10.1104/pp.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takabayashi J, Dicke M. Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol Exp Appl. 1992;64:187–193. [Google Scholar]
- 41.Bruinsma M, Pang BP, Mumm R, Van Loon JJA, Dicke M. Comparing induction at an early and late step in signal transduction mediating indirect defence in Brassica oleracea. J Exp Bot. 2009;60:2589–2599. doi: 10.1093/jxb/erp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulze B, Lauchli R, Sonwa MM, Schmidt A, Boland W. Profiling of structurally labile oxylipins in plants by in situ derivatization with pentafluorobenzyl hydroxylamine. Anal Biochem. 2006;348:269–283. doi: 10.1016/j.ab.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Zheng SJ, Van Dijk JP, Bruinsma M, Dicke M. Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): Dynamics of BoLOX expression patterns during insect and pathogen attack. Mol Plant-Microbe Interact. 2007;20:1332–1345. doi: 10.1094/MPMI-20-11-1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.