Abstract
Bacteria serve as the central arena for understanding how gene networks and proteins process information and control cellular behaviors. Recently, much effort has been devoted to the investigation of specific bacteria gene circuits as functioning modules. The next challenge is the integrative modeling of complex cellular networks composed of many such modules. A tractable integrative model of the sophisticated decision-making signal transduction system that determines the fate between sporulation and competence is presented. This model provides an understanding of how information is sensed and processed to reach an “informative” decision in the context of cell state and signals from other cells. The competence module (ComK dynamics) is modeled as a stochastic switch whose transition rate is controlled by a quorum-sensing unit. The sporulation module (Spo0A dynamics) is modeled as a timer whose clock rate is adjusted by a stress-sensing unit. The interplay between these modules is mediated via the Rap assessment system, which gates the sensing units, and the AbrB–Rok decision module, which creates an opportunity for competence within a specific window of the sporulation timer. The timer is regulated via a special repressilator-like inhibition of Spo0A* by Spo0E, which is itself inhibited by AbrB. For some stress and input signals, this repressilator can generate a frustration state with large variations (fluctuations or oscillations) in Spo0A* and AbrB concentrations, which might serve an important role in generating cell variability. This integrative framework is a starting point that can be extended to include transition into cannibalism and the role of colony organization.
Keywords: Bacillus subtilis, competence, gene networks, sporulation, stochasticity
Even for bacteria, life can be both complex and stressful. How do they cope with adversity? Can these coping strategies be described at the molecular level? Many bacterial strains respond to stress, such as starvation or DNA damage, by forming endospores, which is a cellular response involving many genes. In Bacillus subtilis, sporulation entails the activity of >500 genes over the course of ≈10 h. The process ends in death by lysis of the “mother cell” and the formation of a dormant daughter cell (the spore) that can resist hazards like heat, radiation, and toxic chemicals, but also cannot take advantage immediately of favorable circumstances to reproduce. Sporulation is not initiated automatically upon nutrient limitation, but instead it is the end result of a series of steps that might be described as cellular decisions regarding how to best cope with the stress (1, 2). Initially, it appears a variety of other tactics to survive are used, including the activation of flagellar motility to seek new food sources by chemotaxis, the secretion of antibiotics and other chemical weapons to destroy microbes competing for the same ecological niche, and the secretion of hydrolytic enzymes to scavenge extracellular proteins and polysaccharides. The cells also assess various internal conditions before beginning to sporulate. The assessments include checking chromosome integrity and the state of chromosomal replication, presumably to ensure that once committed spore formation can actually be completed.
After alternative responses prove inadequate to relieve the stress, sporulation is the fate chosen by a majority of the cells. For example, in the case of the domesticated B. subtilis 168 strain studied here, 50–70% of the cells make the commitment to sporulation. The genetic material released to the media by lysis of the sporulationg cells is not wasted by the colony. On their path toward sporulation, the individual cells can opt for the differentiated state of competence, triggered by ComK (the competence master regulator) exceeding a certain threshold level (3, 4). In this state the cell can take up exogenous DNA from lysed cells. This material can then be used for DNA repair and occasionally even as new genetic information to enable resisting the encountered stress. Competence is not a permanent genetic state, and after several hours the cell switches back to vegetative growth on its path toward sporulation (5). In the case of B. subtilis 168, under stressful conditions ≈10% of the cells are in the competence state and a cell spends ≈20 h in this state before switching back into the vegetative state (5, 6). Variability in the duration of competence has been suggested to be of phisiological importance in a recent study by Süel and collaborators (7).
Considerable research effort has been devoted to untangling the components of the signal transduction decision-making system that allows the individual cell to decide whether to wait, take a chance and escape into competence, or commit to sporulation. It is now understood that the cell decision follows careful sensing, advanced cell–cell communication using a variety of peptide pheromones (autocrine agents), and elaborate information processing to assess information about the colony density, the collective progression toward sporulation, and the inclination to escape into competence (8). The cell assesses these signals in the context of its own present and past stress and its progression toward sporulation.
An advanced sensing system integrates and converts the cell stresses into a phosphate flow through a cascade of kinases, termed the phosphorelay, which ends with the accumulation of the sporulation master regulator protein Spo0A* (phosphrylated Spo0A) (9). The level of Spo0A* defines three stages in the decision-making process (10). When the levels of Spo0A* are low, transitions into competence are blocked by the AbrB–Rok circuit, a repressor of ComK. The “early assessment stage” is performed by the Rap system together with two two-component sensing systems (11, 12). The assessment involves regulation of the probability to escape into competence, after ComK inhibition is lifted, and regulation of the progression toward sporulation. After Spo0A* reaches a threshold level S1, the cell enters into the “decision stage” (13), during which the AbrB–Rok circuit opens a time window of opportunity (termed the “competence window”) to escape into competence or make a final commitment to sporulation. The transitions into competence are stochastic, and because the transition probability depends on the individual cell-specific physiological parameters of stress, external information, and noise level, it can be termed a “molecular decision.” The third and final “commitment stage,” is reached when Spo0A* is accumulated above an even higher threshold level S2 (14). The SinI–SinR circuit (also acts by a cascade of inhibitions) is turned on, leading to inhibition of ComK and terminating any attempts to competence transition. We see that the bacteria's decision relies on a combination of deterministic and stochastic events processed by many modules that sense information, process information, and regulate cell responses.
Years of intensive experimental studies have identified tens of key regulatory genes and quantified the associated physiological parameters that are involved in the sporulation-competence decision process of the domesticated B. subtilis 168. More recently, these findings have led to the development of tractable quantitative models of some of the elements (or module circuits) of this highly interconnected genetic network. Considerable new attention in a series of articles by Elowitz and collaborators (5, 6) and others (15) has been given to modeling the role of the Comk–ComS master switch in the transition into and return from competence. The generic dynamics of typical two-component systems like those that participate in the quorum sensing and stress detection mechanism has been modeled and studied by Alon and collaborators (16). Other modeling studies of the phosphorelay cascades of kinases by Huang and Ferrell (17) show that a cooperative-like behavior can be obtained without the hallmarks of molecular cooperativity under equilibrium conditions. Modeling of protein-antagonist motifs such as the Sin or the Rap modules by Arkin and collaborators (18, 19) has demonstrated how the dynamical modes of these circuits depend on the physiological parameters.
These theoretical studies have paved the way to meet the next challenges posed by the sporulation-competence decision system: the competence model needs to be extended to include the crucial regulating effect of the input signals from the early assessment system. In addition, it is now relevant to develop a tractable quantitative model of the sporulation path that includes the phosphorelay cascade and the dynamics of the sporulation master protein Spo0A*. Once the modeling of the competence and the sporulation genetic circuits (modules) is completed, the next and greater challenge is to analyze the interaction between these modules: to model and investigate the dynamics of the Rap and the AbrB–Rok systems.
Such integrative model reveals the interplay between the decision-making system as a general task-performing system and the local functions executed by its component module circuits, as the signals propagate throughout the network to reach the decision about the cell's fate (20–22). The integrative modeling also allow us to understand how the external information about the cell stress, the status and inclinations of other cells, and the colony density is processed to reach an “informed” decision. Finally, such an analysis provides a sound basis for studying how cell–cell communication can couple the intracellular decision-making genetic circuits of the individual cells. The essential aspect of how individual decision circuits of cooperating cells and the interplay between individual stresses and the state of the colony as a whole has not been studied from this perspective before.
As we point out in Discussion, the integrative framework presented here can be extended to include the return from competence and cannibalism after additional experimental information about these interesting phenomena will be obtained. Regarding the “return problem,” there are new results that indicate the putative role of RapH in regulating the transition back from competence into the vegetative state (23). Although we focus here on the sporulation/competence decision, it is important to bring up a third option: cannibalism. Recently, it has been shown that cells further along on the path toward sporulation can produce and secrete antibacterial factors that block sibling cells from sporulating and cause them to lyse, thus killing them (24, 25). Such cannibalistic cells coming to this fateful decision to kill their brothers then feed on the nutrients released by the lysed cells while impeding progression toward sporulation. Current evidence indicates that cannibalism is initiated during the decision stage, after Spo0A* starts to repress AbrB.
Sporulation/Competence Road Map: The Decision-Making Network
The decision-making network presented in Fig. 1 is an elaborate signal transduction system that determines the cell fate by sensing and processing information about the cell stress, the colony density (quorum-sensing peptides), and the stress status and inclinations of neighboring cells (peptide pheromones). The input signals are processed by the combined action of a highly complex regulatory network of interaction between genes and proteins, including activation and inhibition of genes and phosphorylation and dephosphorylation of proteins.
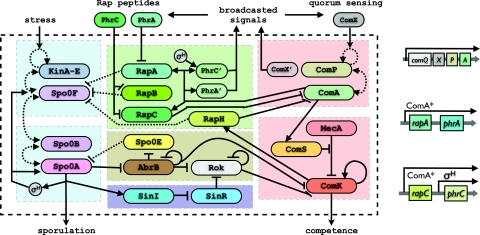
Fig. 1.
The sporulation-competence signal transduction network. The network modules are described in detail in the sections on The Competence Module, The Sporulation Module, The Rap Module and Early Assessment, and The Decision Stage and Final Commitment. The sporulation module is shown on the left (blue), and the competence module is shown on the right (red). Each module is composed of a sensing unit and a regulatory unit. The KinA-E stress-sensing unit and the Spo0A timer comprise the sporulation module, and the Spo0P–ComA quorum-sensing unit and the ComK switch comprise the competence module. The two main modules interact via the Rap communication and information processing module (green), the AbrB–Rok decision module (brown), and the SinR-SinI commitment unit (purple). The input and output signals are represented by the wide solid lines that cross the cross the outer black envelope. The two sensing units of the sporulation and competence modules together with the Rap module perform early assessment of internal and external signals and send out the pheromone communication signals. There are internal input signals to the Rap module from the sporulation timer (σH) and the competence switch (ComK). Solid lines indicate positive (with arrow head) and negative (with perpendicular bar) transcription regulation or (in)activation upon binding, and the dashed line represent phosphorylation (with arrow head) or dephosphorylation (with perpendicular bar). On the right, the promoters regulating the production of pheromones used to broadcast the signals to the environment are represented. ComS and Rok repression under overexpression of ComK are not depicted, because they are not important in the decision process.
To simplify the analysis of this complex system, we first model and analyze the operation of the two key modules: sporulation and competence. To facilitate understanding we note that the sporulation module acts as a timer that measures the progression toward sporulation with an adjustable clock rate. The competence module acts as a stochastic switch, with an adjustable escape probability. The sporulation progression is measured by the accumulated concentration of Spo0A*. Next, we identify, model, and analyze the operation of the Rap communication and information processing system and the AbrB–Rok circuit.
Spo0A* accumulation is determined by a cascade of kinases transferring phosphate to the sporulation master regulator Spo0A (9, 26–29). Phosphate is transferred down the relay, leading to an accumulation of Spo0A*. The outcome is that the clock rate of the sporulation timer is adjusted by the cell stress. Spo0A* acts as a transcriptional activator of both Spo0A and Spo0F via the sigma factor σH. Spo0B rapidly transfers phosphate between these two response regulators, allowing any regulation of Spo0F to directly affect the levels of Spo0A* (30).
The competence switch consists of a self-activator master regulator ComK and a degradation complex MecA/ClpP/ClpC that continuously acts to keep ComK at low levels (3, 31). This degradation is regulated by competitive binding of peptide ComS. It has been proposed that the ComK–ComS–MecA circuit can act as an excitable system, a bistable system, or both, depending on parameters (4–6, 15, 32). Either excitable or bistable, the module acts as a stochastic switch. Entrance into competence can be described as activation over an effective free-energy barrier whose height depends on the concentrations of ComK and ComS (15). In addition to the previously widely studied ComK–ComS–MecA core circuit, the competence module consists of the ComP–ComA quorum-sensing two-component system that activates the production of ComS in response to the level of the quorum-sensing pheromone ComX (33).
The sporulation clock rate and the competence escape waiting time are further regulated by the Rap module (23, 34, 35), composed of many (11 sometimes redundant) motifs. Generally speaking, the Rap decreases the clock rate of the sporulation timer and increases the waiting time of the competence switch by dephosphorylation of Spo0F and inactivation of ComA, respectively. The Rap is also regulated by Spo0A*, which enhances the production of some of the pheromones (e.g., PhrC) via σH (36). More recently, it has been discovered that the Rap system is regulated by ComK (enhancer of RapH that dephosphorylate Spo0F), presumably to prevent sporulation during competence.
The AbrB–Rok decision circuit (a ComK repressor), blocks competence for levels of Spo0A* outside of a certain window. When the Spo0A* concentration accumulates above the threshold level S1, the competence repressor AbrB is rapidly inhibited by Spo0A*, and the cell is allowed to enter into competence (13). The competence transition probability is determined by the level of ComS defined in the early assessment. In parallel, the inhibition of AbrB also leads to the derepression of the dephosphatase Spo0E (37, 38). Consequently, because Spo0E dephosphorylates Spo0A*, the inhibition of AbrB leads to a decrease in the clock rate of the sporulation timer.
However, even if the rate is slowed down, the concentration of Spo0A* can continue to increase. When it reaches a higher threshold level S2 the cell enters the final sporulation commitment. Above this threshold, Spo0A* suppresses the inhibition of Rok via the SinI–SinR module (14, 39). Consequently, the level of Rok, an inhibitor of ComK, increases to a level that blocks transitions into competence.
Put together, the combined effect of the AbrB and Rok circuits is to allow cells on the road toward sporulation an opportunity to escape into competence during a time window S1 < Spo0A* < S2 (40). During this time window, the clock rate of the timer slows down while the escape waiting time is reduced, giving the cell a fair chance to escape into competence before the final commitment to sporulation.
The Competence Module
The master regulator ComK activates its own transcription, in a positive feedback loop, once its concentration crosses a certain threshold (32). Active degradation by a special degradation complex MecA/ClpP/ClpC keeps the ComK concentration low and thus prevents its overexpression, which would lead to competence. By interfering with ComK degradation, ComS can allow the ComK concentration to cross the threshold for self-activation, leading to overexpression and consequently transition into competence (3). Upon entering into the competent state the concentration of ComK reaches high levels. Because overexpression of ComK has a negative effect on ComS, it will ultimately determine the exit from competence. The operation of this ComK–MecA–ComS circuit has been studied in detail by modeling it as a dynamical system with two variables, the concentrations of ComK and ComS (4–6, 15). The circuit can act either as an excitable system or a stochastic switch. Here, we focus on the decision to enter competence, that is, we focus on the regulation of the transition probability (41). We treat the concentration of ComS as a control parameter and describe the switch operation by the following dynamical equation of ComK:
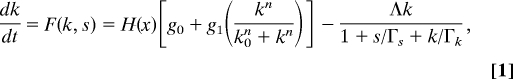 |
where k and s are the ComK and ComS concentrations, g0 and g1 are the basal and activated synthesis rates, Λ is the maximum degradation rate of ComK, k0 is the ComK concentration for half-activation of the feedback loop, and Γs and Γk are concentrations of ComS and ComK for half-maximal degradation. H(x) = x0m/(x0m + xm) is a Hill function performing the external regulation of the transcription of ComK, where x, x0, and m are the signal repressing ComK expression (AbrB + Rok, as explained in the section on The Decision Stage and Final Commitment), its concentration for half-maximal repression and the Hill coefficient of this repression. In Fig. 2 B and C we show the nullclines for Eq. 1, where ComS is taken as a varying control parameter. As we see, for a range of values of s/Γs such that, ComS1 < s/Γs < ComS2, the cell can make transitions from the vegetative state into competence. Below this range the vegetative state is the only available option, and above this range the cell necessarily goes into competence. We note that when the system is treated as a two-variable dynamical system the competent state can be an unstable fixed point (system is excitable), allowing the system to show well-defined competence cycles around the fixed point. Here, we have a 1D system, with ComS taken as a control parameter, so the competent state does not show cycles, but instead makes transitions into competence, which is the focus here.
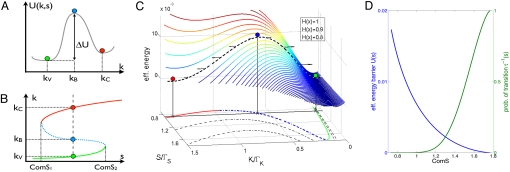
Fig. 2.
The function of the competence switch described by Eq. 1 when ComS acts as a control parameter. (A) We illustrate the effective potential U(k,s), the integral of F(k,s) along the gray dashed line in B or the perpendicular black line in C, indicating the considered value of ComS. (B) We show the schematic shape of the nulclline (equilibrium solutions) k*(s), such that F(k*(s),s) = 0 as function of the control parameter s. The green segment below ComS2 corresponds to the vegetative state, and the red segment above ComS1 corresponds to the competent state. The latter can be either metastable (the bistable case presented here) or unstable (excitable case). The blue dashed segment corresponds to unstable (or saddle for the excitable case) point, the barrier between the two solutions. (C) We show the effective potential barrier ΔU(s). Note that for a given value of s, ΔU(s) is the difference in the value of U(k,s) between the barrier point kB and the vegetative state kV. (D) We show the effective potential U(s) and the probability of competence transition τ−1 (s). Because the energy barrier decreases with an increase in s, the probability of transitions into competence becomes higher. The parameters used are typical of excitable systems: g0/ΛΓk = 0.03, g1/ΛΓk = 0.5, and k0/Γk = 0.7.
The transitions into competence can be formulated as an activation problem; a threshold concentration has to be crossed by fluctuations in the ComK level for the transition into competence to happen. The situation can be mapped to the escape problem of a particle over a potential barrier under the effect of noise. In this picture Eq. 1 can be viewed as the motion of an overdamped (high friction) mechanical particle moving in one dimension, the concentration k being the analogue of the axis in which the particle moves and the rate F(k,s) = dk/dt the equivalent of a force acting on the particle. Within this analogy, for a given value of s, the particle is moving in a potential U(k) defined by U(k,s) = −∫ F(k,s)dk.
The activation barrier is ΔU(s) = U(kB) − U(kV), where kV and kB correspond to the values of k for vegetation and barrier, respectively, as shown in Fig. 2 A and C. Using Kramers activation theory, the probability per unit time of escape into competence, τ−1 is proportional to exp[−ΔU(s)/ε] where ε is the effective noise and the prefactor is determined by the rate constants at the vicinity of the vegetative and barrier states. Because the concentration of ComS is increased in response to the quorum-sensing signal, the activation barrier ΔU(s) goes down, and consequently the escape time τ is reduced (with stronger nonlinear dependence).
The external quorum sensing signal ComX leads to enhancement in the ComS production through the ComA–ComP quorum-sensing module shown in Fig. 1. This two-component system is a typical sensing motif where a signal is translated into the activation of a response regulator. Such a two-component system is widely used by bacteria for sensing external input signals. The system consists of an intramembrane histidine kinase ComP, which autophosphorylates upon binding to an external input signal ComX. The phosphate is then rapidly transferred to a response regulator ComA. Detailed studies of the operation of a generic two-component system and the ComA–ComP system with additional relevant references are included in SI Appendix.
ComA* acts as a transcription factor that activates, among many targets, the competence inducer ComS. ComP can also dephosphorylate ComA* (phosphorylated ComA) in the absence of ComX (42). Therefore, the ComA–ComP module operates as a quorum-sensing gate that activates the production of ComS, inducing competence, only above a threshold concentration of ComX (Fig. 3B). We also note that the production of ComS must not exceed levels that would eliminate the vegetative state (kept below ComS2, as defined earlier). Experiments show, however, that levels of ComS in the cell would have to increase dramatically (6-fold) to notice this effect (6). The regulator ComA interacts with the Rap module in the assessment stage. The gate characteristics of the ComA—ComP-sensing module are regulated by the Rap system (43) as is shown in The Rap Module and Early Assessment section.
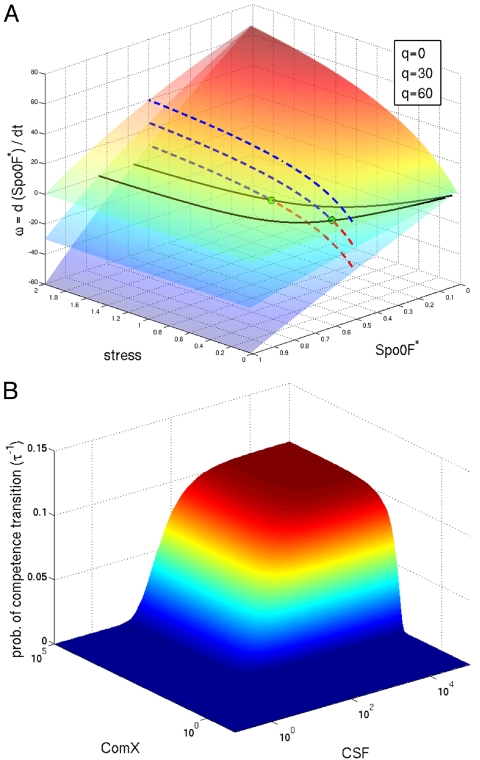
Fig. 3.
Progression toward sporulation ω and probability of competence transition τ−1 as a function of the sensed signals. (A) Rate of Spo0F phosphorylation as a function of the stress signal and the SpooF* levels for different levels of Spo0F dephosphorylation q (by Spo0B and RapAB). Black solid lines represent ω = 0 and dashed lines represent ω(stress) for Spo0F* = 0.5. Green dots indicate the value of stress necessary for Spo0F* to continue growing. If the level of stress falls below this value, there will be a reduction on the levels of Spo0F*. (B) Probability of competence transition τ−1 as a function of quorum-sensing pheromone ComX and Rap pheromone C (CSF). Competence needs the presence of both of these inducers, and the probability increases with higher levels of ComX and CSF. If the pheromones are present in excess, the probability of competence transition reaches a limit defined by the maximum production of ComS and the noise in the system. Parameters are F = N = 1, K1 = 1, K'1 = 0.1 for the phosphorelay, A = 2, Kd = 1, Kp = 0.1, Kb = 5 for the quorum-sensing mechanism, maximal ComS production is S = 1.8, and the parameters for the Hill function regulating RapC and ComS production are a0 = 1 and m = 2. Parameters are explained in SI Appendix.
The Sporulation Module
The sporulation module, which acts as a timer, controls the cell progression toward sporulation (9). The master regulator of this module, Spo0A, upon phosphorylation activates the transcription of itself and the other response regulator Spo0F via σH, in a positive feedback loop activated when Spo0A* > SAct (29). The module is build of two submodules, the Kin–Spo0F and the Spo0B–Spo0A two-component systems, which are coupled in series by Spo0B quickly transferring phosphate between Spo0F and Spo0A, as is analyzed in detail in SI Appendix.
Kin–Spo0F is a stress-sensing system, the structure of which is similar to that of the ComA–ComP two-component system. The schematic diagram shown in Fig. 1 represents the operation of at least five different histidine kinases (KinA–KinE), each autophosphorylating in response to a different stress signal. In the absence of or low stress levels, the histidine kinases KinA–E, can also dephosphorylate Spo0F*. Therefore, the rate of production of Spo0F* ω = d(Spo0F*)/dt has a sigmoid dependence on the stress level. This result, together with the dephosphorylation of Spo0F* by Rap, implies that the Kin–Spo0F sensing system acts as a gate that turns on the sporulation timer only above some minimum stress level. The clock rate ω is regulated by a competition between the positive effect of stress level and the negative effect of the Rap input [dephosphorylation of Spo0F* (34)], as detailed in the next section.
When a sufficiently high stress level is encountered, phosphate is transferred to the Spo0B histidine kinase, which rapidly transfers the phosphate to the response regulator Spo0A, beginning the accumulation of Spo0A*. The accumulation rate Ω = d(Spo0A*)/dt of Spo0A* (the clock rate of the sporulation timer) changes during the progression of sporulation. When Spo0A* accumulates above the threshold concentration SAct, Ω rapidly increases from a base level Ω0 to a higher-level Ωup.
The Rap Module and Early Assessment
The cell uses a sophisticated integrated system composed of the ComA–ComP- and Kin–Spo0F-sensing modules the Rap system to make early assessments (23, 35), as shown in Fig. 1. The Rap communication and information processing module, the core component of this integrated system, consists of many Rap proteins (11 have been identified so far in B. subtilis) that dephosphorylate Spo0F, inactivate ComA, or both (34, 44). Rap proteins usually share a promoter and are cotranscribed with their own associated Rap pheromone, located downstream. A typical example is the RapA–PhrA pair that is cotranscribed when activated by ComA*. As illustrated in Fig. 1, pheromone PhrA is produced first as a precursor PhrA′, which is later modified into its active form PhrA upon being exported out of the cell. The external active signal PhrA is imported into the cell and acts by binding to its own associated protein RapA and inhibiting its activity. The concentration of active signal PhrA in the vicinity of the cell is composed of pheromone produced by the cell itself and other cells around it. Therefore, the RapA–PhrA circuit acts as a comparator element and provides information about the individual concentration of ComA* in the cell vs. the group level of ComA* in the vicinity (19). RapA then adjusts the clock rate of the sporulation timer accordingly, by dephosphorylation of Spo0F*. For sufficiently high colony density, i.e., there is sufficient concentration of ComX in the vicinity to produce enough ComA*, RapA adjusts the clock rate to be slower when there are many cells around inclined to escape into competence.
The model and analysis of the operation characteristics of the RapA–PhrA element are detailed in SI Appendix. Schematically, the steady-state concentration of RapA a, is given by
where pA is the concentration of the active form of the pheromone PhrA, g is the total concentration of RapA, proportional to the synthesis rate of RapA and PhrA that is activated by ComA, and K is a constant embodying the rates of production, export, and import of the pheromone, its binding constant to RapA and, most important, the gain in the signal coming from the pheromone secreted by the neighboring cells.
This is a typical example in which a Rap protein is transcribed only by ComA*. Other Rap proteins, like RapC, also shown in Fig. 1, have more complex computational elements. In addition to the cotranscription of the Rap protein and its associated pheromone by ComA*, the transcription of the pheromone can be further enhanced by Spo0A*. The pheromones can have an additional promoter activated by the sigma factor σH, induced by Spo0A* (36). Hence the RapC–PhrC circuit adds two internal input signals, the levels of ComA* and Spo0A*, to set the rate of pheromone secretion. The rate of secretion of PhrC carries information about the cell's likelihood to escape into competence. Therefore, the RapC–PhrC element also provides the cell with an assessment of the intentions of its neighbors to escape into competence vs. its own. The model and analysis of the operation characteristics of the RapC–PhrC element are detailed in SI Appendix.
Whereas RapA–PhrA regulates the progression toward sporulation by dephosphorylation of Spo0F*, RapC–PhrC regulates both the progression toward spoulation (via the inhibition of RapB by PhrC) and the probability of competence transitions by inactivation of ComA. PhrC is therefore especially important in sporulation and competence control. This pentapeptide, widely termed competence and sporulation factor (CSF), promotes at high concentrations both competence and sporulation by protecting ComA* from the inhibiting effect of RapC and protecting Spo0F* from the inhibiting effect of RapB.
From the colony's perspective, CSF has a balancing and synchronizing effect, accelerating the production of ComA* and Spo0F* in cells that are behind schedule. CSF thus makes the probability to enter competence more uniform throughout the colony and synchronizes the sporulation progression (clock rates). For example, in cells exposed to weaker stress RapB inhibits the progression toward sporulation. By inhibiting the effect of the RapB, CSF received from the neighbors provides the cell with more equal opportunity to progress toward sporulation. We note that CSF has similar effect to provide latecomer cells also with better opportunity to enter into competence, thus executing an “affirmative action.”
In Fig. 3 we show the results of model simulations of the combined functioning of the integrated phosphorelay–Rap quorum-sensing system in executing the early assessment process. We show the level of the output of this circuit, the sporulation progression ω and the probability of competence transition τ−1, as function of the input signals stress, ComX and CSF. The level of stress necessary for progression to sporulation (larger ω) depends on the level of dephosphorylation of Spo0F* by RapA and RapB (Fig. 3A). The probability of a competence transition is increased with higher levels of pheromones ComX and CSF, but reaches a maximum level ultimately defined by the maximum activation of ComS and the level of noise in the system (Fig. 3B). Experiments indeed show that even under optimal conditions only ≈15% of the cells in a colony enter competence.
The Decision Stage and Final Commitment
The AbrB–Rok decision module, shown in Fig. 1, has unique operational principles, because its functions are executed by a cascade of transcription inhibitions. Activation of this module means that the cascade of inhibitions is turned on. The module is composed of three genes, two of which, AbrB and Rok, are self-inhibitory and both inhibit the competence master regulator ComK. AbrB also inhibits Rok. The third protein, Spo0E, starts to dephosphorylate Spo0A* when its inhibition by AbrB is removed (37, 38). AbrB itself is inhibited when the concentration of Spo0A* crosses the threshold S1. The decision regulator AbrB is an unstable protein involved in the repression of a wide variety of genes, in addition to those belonging to the decision-making system. Because of this instability, the concentration of AbrB decreases fast when the gene transcription is inhibited.
Because AbrB is self-inhibitory and unstable, upon repression by Spo0A* its concentration decreases relatively fast and the inhibition of ComK is lifted, allowing the cell to escape into competence (13). However, lower levels of AbrB also reduce the inhibition of Rok. At sufficiently low levels of AbrB, the concentration of Rok increases to a level that once again inhibits of ComK, preventing competence transitions. In Fig. 4A we show the dependence of the concentrations of AbrB and Rok on the level of Spo0A* (see SI Appendix for detailed description of the modeling and the analysis of the delicate interplay between the effects of AbrB and Rok in regulating ComK). There is a computational advantage of combining self-inhibition with high unrepressed transcription levels that is shared by the AbrB and Rok motifs: it establishes an upper bound in the steady-state level of the protein. In the case of AbrB there is a transition to a lower level when inhibited by Spo0A*, whereas in the case of Rok there is a transition to a higher level when the inhibition by AbrB is removed.
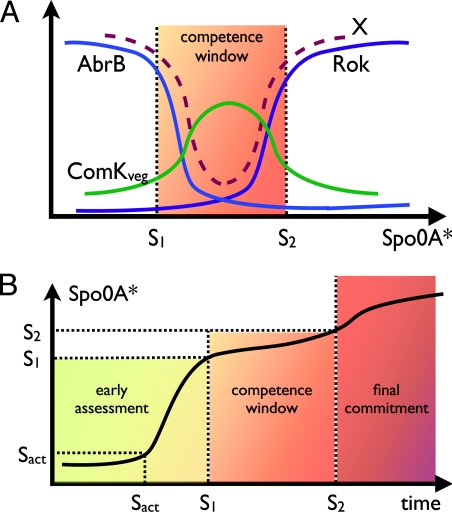
Fig. 4.
The effects of the AbrB–Rok module on the competence switch and the sporulation timer. (A) We show the level of AbrB and RoK as function of Spo0A* that is an inhibitor of AbrB. Spo0A* concentration exceeds the threshold level S1 and lead to a decrease in the AbrB concentration and consequently to an increase in the concentration of Rok that is inhibited by AbrB. Both AbrB and Rok acts as transcription inhibitors of the competence regulator ComK. Hence the competence window is determined by x, the combined concentration of AbrB and Rok, which has low values between S1 and S2. The effect of the reduction of x is an increase in the level of ComKveg (the vegetative ComK concentration) and consequently increase in the switching rate τ−1. (B) We show the dynamical behaviors of Spo0F, Spo0A, and Spo0A* as function of time for constant stress level. We note that the increase in the growth rate of Spo0F is the outcome of the increased level of Spo0A*, which acts as an up-regulator of Spo0F (via σH).
Both AbrB and Rok have binding sites in the ComK promoter. Because there is evidence that the binding sites of AbrB and Rok in the comK promoter overlap (45), only one molecule of those can bind at a time. If we normalize the AbrB and Rok concentrations by their binding constant, we can add them in a single ComK repression signal x, which is used in Eq. 1 in the inhibition of ComK. The decrease in x during the competence window lifts the inhibition of ComK, leading to an increase in the vegetative value of ComK (ComKveg), and consequently to a substantial increase in the probability per unit time of escape into competence τ−1, as shown in Fig. 4A.
When the cell enters the competence window (Spo0A* > S1), the Spo0A* inhibition of AbrB leads to an increase in the level of Spo0E, because its inhibition by AbrB is lifted (37, 38). This increase leads to relatively rapid dephosphorylation of Spo0A* by Spo0E and a decrease in the clock rate Ω to a lower value Ωwindow. The latter is determined by the difference between the phosphate relay rate ωup and the rate of Spo0A* dephosphorylation by Spo0E.
In Fig. 4B we show the time dependence of Spo0A*, which measures the sporulation progression. For low stress level and/or fast dephosphorylation of Spo0F by the Rap system, ωup is relatively low, so the cell can spend a long time in the competence window. Here, we assume that S1 is sufficiently larger than SAct, so that the cell spends a significant time in the second period of the early assessment stage, during which it can secrete higher levels of CSF. However, we note that currently there is no concrete information about the values of these two threshold levels. An interesting situation can be observed if S1 is close to SAct. In such cells (either wild-type or engineered mutants), dephosphorylation of Spo0A* should start before ω can reach its maximal value ωup.
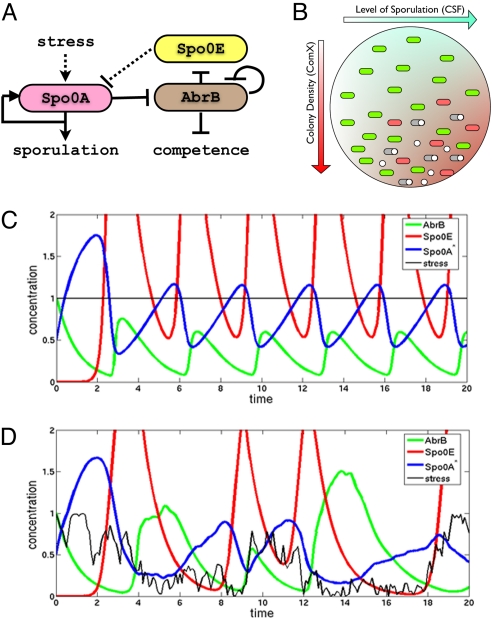
If the stress level and the inputs from the Rap system are such that ωup is low, the level of Spo0A* could decrease after it crosses S1. The reason is that the triplet Spo0A–AbrB–Spo0E could form a unique repressilator-type motif (as is detailed and modeled in SI Appendix). The outcome can be a “frustration state” during which the levels of these three proteins would vary greatly, according to fluctuations in ω. The response to a constant ω could be oscillatory, and this system could then be used to amplify noise signals coming from all of the sensors, generating a great variability of Spo0A* throughout the colony and resulting in different decisions by individuals and a variety of phenotypes throughout the colony (Fig. 5).
Fig. 5.
The repressilator-like Spo0A-AbrB-Spo0E circuit can generate phenotypical variability. (A) Repressilator-like circuit regulating both sporulation and competence. (B) Illustration of the effects of the effects of colony density (ComX) and average level of sporulation in the colony (CSF). Higher levels of such pheromones cause phenotypical variability driven by noise. (C) Response of the circuit for an input stress signal with no noise can show oscillations. Parameters: N = 6, gb = 100,000, b0[infi]b = 0.5, b0[infi]e = 0.075, ge = 50, a0 = 0.075. (D) Response of the system for a noisy input signal can show great alternation of the species involved, being responsible for the phenotypical variation observed. Parameters: N = 6, gb = 100,000, b0[infi]b = 0.75, b0[infi]e = 0.1, ge = 5, a0 = 0.05. Parameters are explained in SI Appendix.
During the decision stage, the concentration of Spo0A* continues to increase at a rate (Ωwindow). When it reaches a second threshold S2 the cell enters the final sporulation commitment (Fig. 4B). When increasing above S2, Spo0A* turns on the SinI–SinR module that will result in blocking competence completely. More specifically, Spo0A* represses ComK, inducing a shift in a series of inhibitions. ComK inhibitor Rok is initially kept at lower to intermediate levels by a repressor SinR. High levels of Spo0A* induce the production of SinI, which binds to SinR to form an inactive complex (14, 39). Inactivation of SinR then causes the derepression of Rok, whose high levels lead to complete inactivation of ComK and the blocking of competence.
Rok is also inhibited by overexpression of ComK. The functional role of this inhibition is to allow a cell that escaped into the competent state before the final commitment to decouple from the inhibitory effect of Rok. Because at this stage the concentration of AbrB is also low, the transition back from competence is not regulated by the sporulation module. It is observed that the exit from competence is always to the vegetative state, meaning that competent cells do not sporulate. Recently, a missing regulatory mechanism that prevents sporulation in competent cells was proposed. The involved factor is RapH, which dephosphorylates Spo0F* (23). Unlike the other Rap proteins described earlier, which are activated by ComA, the transcription of RapH is activated by high concentrations of ComK. During competence the activation of RapH is high, and its dephosphorylation of Spo0F is rapid, leading to a flow in the reverse direction of the phosphate relay and a decrease in the level of Spo0A*. RapH also decreases the level of ComA, and in turn the level of the competence inducer ComS, thus participating in regulating the transition back from competence. The emerging picture is that the process of transition back from competence, which is not studied here, also involves the Rap system. In other words, it could also involve assessment of signals from other cells.
Discussion
The cellular sporulation-competence decision process is an elaborate signal transduction system with two main functions: (i) to determine the cell fate based on sensing and processing external information according to the cell stress and stored information and (ii) to send messages to other cells about progression toward sporulation and inclinations into switching into competence. A tractable quantitative model of the sporulation-competence system is introduced as a general task-performing network composed of functional modules, which enables us to understand how the external information is sensed and processed to reach informative decisions.
To simplify the system complexity, we first modeled, analyzed the operation, and characterized the functions of the two key modules: the sporulation timer and the competence stochastic switch. Each module is composed of a sensing unit and a regulating submodule that includes a self-activated master regulator (Spo0A* for sporulation and ComK for competence) whose operation is controlled by input from the sensing system. The sensing units of both modules are gated by the Rap system. Special effort was devoted to untangle the complexity of this sophisticated communication and information processing system.
Next, the inhibition of inhibition operation principles of the AbrB–Rok decision module were unraveled by modeling its dynamical activity. This module performs two tasks. One task is gating the competence transitions to be allowed only within an specific competence window between two values of Spo0A*, the measure of the sporulation timer. The AbrB–Spo0A part of the decision module regulates the clock rate of the sporulation timer. Because Spo0A is inhibited by Spo0E that is inhibited by AbrB that is inhibited by Spo0A* these three genes form a special repressilator circuit (46) with one gene being controlled by external input and self-activated (Spo0A) and another being self-inhibited (AbrB). Ordinarily, this circuit simply leads to a decrease in the rate of accumulation of Spo0A* when AbrB is sufficiently inhibited. We also found that this repressilator circuit can lead to an “undecided” frustrated state in which the values of AbrB exhibit large dynamical oscillations and fluctuations. Because the AbrB dynamics in the frustrated state is sensitive to the cell conditions, on the colony level the AbrB-Spo0A circuit may play an important role in generating variability among the colony.
Preliminary colony-level results regarding the early assessments were presented. In general, the colony-level problem is to understand the collective sporulation-competence processes, given the repertoire of individual cells, based on their complex and intricate cellular signal transduction systems and genetic circuits. Each cell has its own microenvironment and hence behaves differently than other cells in the colony. Also, it is clear that cells often behave stochastically as it has been carefully established in our previous study of the competence process, where genetic noise is necessary to drive some cells (at least temporarily) into the competent state (4–6, 15, 32). It may very well be the case that stochastic behavior at the single-cell level and cell variability have an important functional role as they are necessary to drive optimum behavior of the colony as a whole. In the context of game theory, this variability is referred to as a mixed strategy.
It is important to emphasize that the theoretical research efforts toward understanding sporulation and competence have been carried out on the domesticated strain B. subtilis 168. Over years of growth under benign laboratory conditions it has lost many essential genes associated with cooperative behaviors, and therefore the sporulating and competent cells are distributed nearly uniformly in the colony (5). In contrast, as was shown by Dubnau and Losick (47), colonies of the wild-type B. subtilis 3610 strain exhibit intricate colony organization when driven toward sporulation. This colony organization involves the formation of specially structured groups of spores at specific locations, presumably to increase the chance of survival after germination, posing a higher challenge for theoretical investigations.
Finally, we note that although we do not study here the return form competence or the transition into cannibalism, this framework can be extended to include these phenomena once sufficient experimental information is obtained. Recently, it has been shown that cells ahead on the path toward sporulation, when Spo0A* exceeds a threshold level, become cannibalistic and feed on the nutrients released by neighboring cells they lyse, impeding their progression toward sporulation. It is likely that cells become cannibalistic after the commitment to sporulation. If cells, however, become cannibalistic for lower levels of Spo0A* (that corresponds to the competence window), they can decide between competence and cannibalism. From game theory perspective, it would be reasonable that competent cells were immune to cannibalism, because otherwise the transition to competence would be too risky, but it is not known whether this is indeed the case.
Supplementary Material
Acknowledgments.
We thank Dr. Gürol Süel and Dr. Colin Ingham for illuminating conversations about the experimental aspects of this system. This work was supported by National Science Foundation Grant PHY-0822283 (to the Center for Theoretical Biological Physics), the Tauber Family Foundation, and the Maguy-Glass chair in physics of complex systems.
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2006.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912185106/DCSupplemental.
References
- 1.Veening J, Hamoen LW, Kuipers OP. Phosphatases modulate the bistable sporulation gene expression pattern in Bacillus subtilis. Mol Microbiol. 2005;56:1481–1494. doi: 10.1111/j.1365-2958.2005.04659.x. [DOI] [PubMed] [Google Scholar]
- 2.Stephens C. Bacterial sporulation: A question of commitment? Curr Biol. 1998;8:R45–R48. doi: 10.1016/s0960-9822(98)70031-4. [DOI] [PubMed] [Google Scholar]
- 3.Hamoen LW, Venema G, Kuipers OP. Controlling competence in Bacillus subtilis: Shared use of regulators. Microbiology. 2003;149:9–17. doi: 10.1099/mic.0.26003-0. [DOI] [PubMed] [Google Scholar]
- 4.Leisner M, Stingl K, Frey E, Maier B. Stochastic switching to competence. Curr Opin Microbiol. 2008;11:553–559. doi: 10.1016/j.mib.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Süel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 6.Süel GM, Kulkarni RP, Dworkin J, Garcia-Ojalvo J, Elowitz MB. Tunability and noise dependence in differentiation dynamics. Science. 2007;315:1716–1719. doi: 10.1126/science.1137455. [DOI] [PubMed] [Google Scholar]
- 7.Cagatay T, Turcotte M, Elowitz MB, Garcia-Ojalvo J, Süel GM. Architecture-dependent noise discriminates functionally analogous differentiation circuits. Cell. 2009;139:512–522. doi: 10.1016/j.cell.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Grossman AD. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Microbiol. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 9.Burbulys D, Trach KA, Hoch JA. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 10.Fujita M, González-Pastor JE, Losick R. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henke JM, Bassler BL. Bacterial social engagements. Trends Cell Biol. 2004;14:648–656. doi: 10.1016/j.tcb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Dwyer DJ, Kohanski MA, Collins JJ. Networking opportunities for bacteria. Cell. 2008;135:1153–1156. doi: 10.1016/j.cell.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn J, Roggiani M, Dubnau D. The major role of Spo0A in genetic competence is to down-regulate abrB, an essential competence gene. J Bacteriol. 1995;177:3601–3605. doi: 10.1128/jb.177.12.3601-3605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai U, Mandic-Mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein–protein interaction. Genes Dev. 1993;7:139–148. doi: 10.1101/gad.7.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Schultz D, Ben Jacob E, Onuchic JN, Wolynes PG. Molecular-level stochastic model for competence cycles in Bacillus subtilis. Proc Natl Acad Sci USA. 2007;104:17582–17587. doi: 10.1073/pnas.0707965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinar G, Milo R, Martínez MR, Alon U. Input output robustness in simple bacterial signaling systems. Proc Natl Acad Sci USA. 2007;104:19931–19935. doi: 10.1073/pnas.0706792104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CY, Ferrell JE., Jr Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voigt CA, Wolf DM, Arkin AP. The Bacillus subtilis sin operon: An evolvable network motif. Genetics. 2005;169:1187–1202. doi: 10.1534/genetics.104.031955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischofs IB, Hug JA, Liu AW, Wolf DM, Arkin AP. Complexity in bacterial cell–cell communication: Quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay. Proc Natl Acad Sci USA. 2009;106:6459–6464. doi: 10.1073/pnas.0810878106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf DM, Arkin AP. Motifs, modules, and games in bacteria. Curr Opin Microbiol. 2003;6:125–134. doi: 10.1016/s1369-5274(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 21.Barabási A, Oltvai ZN. Network biology: Understanding the cell's functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 22.Kashtan N, Alon U. Spontaneous evolution of modularity and network motifs. Proc Natl Acad Sci USA. 2005;102:13773–13778. doi: 10.1073/pnas.0503610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits WK, et al. Temporal separation of distinct differentiation pathways by a dual specificity Rap-Phr system in Bacillus subtilis. Mol Microbiol. 2007;65:103–120. doi: 10.1111/j.1365-2958.2007.05776.x. [DOI] [PubMed] [Google Scholar]
- 24.Claverys J, Håvarstein LS. Cannibalism and fratricide: Mechanisms and raisons d'être. Nat Rev Microbiol. 2007;5:219–229. doi: 10.1038/nrmicro1613. [DOI] [PubMed] [Google Scholar]
- 25.Be'er A, et al. Deadly competition between sibling bacterial colonies. Proc Natl Acad Sci USA. 2009;106:428–433. doi: 10.1073/pnas.0811816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bijlsma JJE, Groisman EA. Making informed decisions: Regulatory interactions between two-component systems. Trends Microbiol. 2003;11:359–366. doi: 10.1016/s0966-842x(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 27.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 28.Fabret C, Feher VA, Hoch JA. Two-component signal transduction in Bacillus subtilis: How one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siranosian KJ, Grossman AD. Activation of spo0A transcription by sigma H is necessary for sporulation but not for competence in Bacillus subtilis. J Bacteriol. 1994;176:3812–3815. doi: 10.1128/jb.176.12.3812-3815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzeng Y, Zhou XZ, Hoch JA. Phosphorylation of the Spo0B response regulator phosphotransferase of the phosphorelay initiating development in Bacillus subtilis. J Biol Chem. 1998;273:23849–23855. doi: 10.1074/jbc.273.37.23849. [DOI] [PubMed] [Google Scholar]
- 31.Fujita M, Losick R. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 2005;19:2236–2244. doi: 10.1101/gad.1335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maamar H, Dubnau D. Bistability in the Bacillus subtilis K-state (competence) system requires a positive feedback loop. Mol Microbiol. 2005;56:615–624. doi: 10.1111/j.1365-2958.2005.04592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comella N, Grossman AD. Conservation of genes and processes controlled by the quorum response in bacteria: Characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis. Mol Microbiol. 2005;57:1159–1174. doi: 10.1111/j.1365-2958.2005.04749.x. [DOI] [PubMed] [Google Scholar]
- 34.Perego M. A peptide export–import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Core L, Perego M. TPR-mediated interaction of RapC with ComA inhibits response regulator-DNA binding for competence development in Bacillus subtilis. Mol Microbiol. 2003;49:1509–1522. doi: 10.1046/j.1365-2958.2003.03659.x. [DOI] [PubMed] [Google Scholar]
- 36.McQuade RS, Comella N, Grossman AD. Control of a family of phosphatase regulatory genes (phr) by the alternate sigma factor sigma-H of Bacillus subtilis. J Bacteriol. 2001;183:4905–4909. doi: 10.1128/JB.183.16.4905-4909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafikhani SH, Leighton T. AbrB and Spo0E control the proper timing of sporulation in Bacillus subtilis. Curr Microbiol. 2004;48:262–269. doi: 10.1007/s00284-003-4186-2. [DOI] [PubMed] [Google Scholar]
- 38.Ohlsen KL, Grimsley JK, Hoch JA. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci USA. 1994;91:1756–1760. doi: 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafikhani SH, Mandic-Mulec I, Strauch MA, Smith I, Leighton T. Postexponential regulation of sin operon expression in Bacillus subtilis. J Bacteriol. 2002;184:564–571. doi: 10.1128/JB.184.2.564-571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leisner M, Stingl K, Rädler JO, Maier B. Basal expression rate of comK sets a “switching window” into the K-state of Bacillus subtilis. Mol Microbiol. 2007;63:1806–1816. doi: 10.1111/j.1365-2958.2007.05628.x. [DOI] [PubMed] [Google Scholar]
- 41.Leisner M, Kuhr J, Rädler JO, Frey E, Maier B. Kinetics of genetic switching into the state of bacterial competence. Biophys J. 2009;96:1178–1188. doi: 10.1016/j.bpj.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tortosa P, et al. Specificity and genetic polymorphism of the Bacillus competence quorum-sensing system. J Bacteriol. 2001;183:451–460. doi: 10.1128/JB.183.2.451-460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auchtung JM, Lee CA, Grossman AD. Modulation of the ComA-dependent quorum response in Bacillus subtilis by multiple Rap proteins and Phr peptides. J Bacteriol. 2006;188:5273–5285. doi: 10.1128/JB.00300-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bongiorni C, Ishikawa S, Stephenson S, Ogasawara N, Perego M. Synergistic regulation of competence development in Bacillus subtilis by two Rap-Phr systems. J Bacteriol. 2005;187:4353–4361. doi: 10.1128/JB.187.13.4353-4361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albano M, et al. The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions. J Bacteriol. 2005;187:2010–2019. doi: 10.1128/JB.187.6.2010-2019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 47.Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.