Abstract
Astrocytes and one of their products, IL-6, not only support neurons but also mediate inflammation in the brain. Retinoid-related orphan receptor-α (RORα) transcription factor has related roles, being neuro-protective and, in peripheral tissues, anti-inflammatory. We examined the relation of RORα to astrocytes and IL-6 using normal and RORα loss-of-function mutant mice. We have shown RORα expression in astrocytes and its up-regulation by pro-inflammatory cytokines. We have also demonstrated that RORα directly trans-activates the Il-6 gene. We suggest that this direct control is necessary to maintain IL-6 basal level in the brain and may be a link between the neuro-supportive roles of RORα, IL-6, and astrocytes. Furthermore, after inflammatory stimulation, the absence of RORα results in excessive IL-6 up-regulation, indicating that RORα exerts an indirect repression probably via the inhibition of the NF-κB signaling. Thus, our findings indicate that RORα is a pluripotent molecular player in constitutive and adaptive astrocyte physiology.
Keywords: inflammation, staggerer, microglia
Astrocytes are highly polyvalent cells that play pivotal roles in physiological and pathological processes in the CNS. In addition to critical functions in brain homeostasis, development, and neuronal activity, astrocytes have a role in brain inflammatory processes that is becoming increasingly understood (1–3). Astrocytes are among the effector cells of innate immunity in the CNS and acquire the capacity to produce high levels of inflammatory mediators, including IL-6. This cytokine can exert completely opposing effects, either promoting neuronal survival or triggering neurodegeneration and cell death (4). Expression of IL-6, like most inflammatory mediators, is principally driven by the NF-κB signaling pathway. NF-κB activity is reduced by several regulators, including the transcription factor retinoid acid-related orphan receptor-α (RORα) (5).
RORα is a nuclear receptor thought to act as a constitutive activator of transcription (6). Although it is widely expressed throughout the body, in the brain it appears to be restricted to some neuron populations of the cerebellum, inferior olive, hippocampus, thalamus, cortex, hypothalamus, and olfactory bulb, and also in retinal ganglion cells (7). RORα is particularly highly expressed in Purkinje cells, in which it plays a crucial role in differentiation and survival processes (8, 9). The staggerer (Rorasg/sg) mutant mouse carries a deletion in the Rora gene that leads to extensive cerebellar neurodegeneration associated with an inflammatory reaction as well as effects in other body systems (10–14). An important aspect of the staggerer phenotype is abnormal innate immunity characterized by an increased susceptibility to systemic LPS treatment (15, 16). This abnormal inflammatory reaction is not surprising given the anti-inflammatory action of RORα mediated through inhibiting NF-κB (5).
In light of the role of RORα in the regulation of the inflammatory phenomenon in the periphery, we asked whether it could have a similar role in the CNS and looked for RORα expression in glial cells involved in the brain inflammatory reaction, i.e., astrocytes and microglia.
Here, we show RORα expression and modulation in astrocytes but not in microglia. We further demonstrate that RORα plays a dual role in the control of Il-6 gene expression in astrocytes. We found that RORα directly trans-activates Il-6 gene expression in non-reactive astrocytes and indirectly inhibits Il-6 expression through the NF-κB pathway in activated astrocytes.
Results
Expression and Regulation of RORα in Astrocytes.
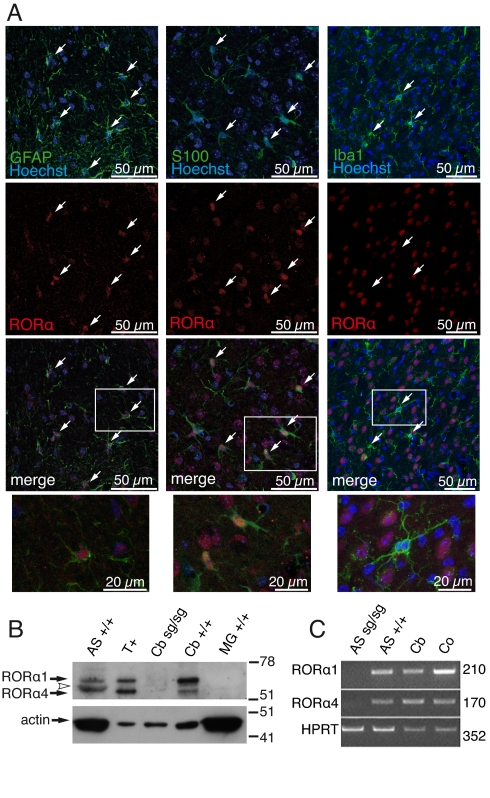
To determine in which glial cell type RORα is expressed, astrocytes and/or microglia, we looked for RORα in brain slices. We found RORα localized in the nucleus of astrocytes throughout the brain near RORα-expressing or non-expressing neurons (Fig. 1A and Fig. S1). No RORα expression was evidenced in microglia (Fig. 1A Right). To further characterize the expression of RORα in glial cells, we analyzed the presence of the 2 mouse-specific isoforms, RORα1 and RORα4, in nuclear extracts by Western blot and in total RNAs using RT-PCR from highly purified cultured cells. In cerebellar astrocyte and tissue extracts, we detected both isoforms with a strong expression for RORα1 in contrast to RORα 4, which was barely detectable in astrocytes. RORα4 migrated near a slightly smaller unidentified protein, probably as a result of a non-specific antibody cross-reaction because of its presence in the Rorasg/sg cerebellum extracts (Fig. 1B). Here again, no RORα was detected in microglia extract, confirming the absence of RORα in these cells. RT-PCR experiments confirmed the expression of the isoform transcripts in astrocytes (Fig. 1C).
Fig. 1.
Expression of RORα in astrocytes. (A) Localization of RORα in brain. Sagittal sections of cerebellum (Left) and cortex (Middle and Right) from 21-d-old Rora+/+ mice. Astrocytes were labeled using anti-GFAP (green) or anti-S100 (green) antibodies, microglia using anti-Iba-1 (green), and nuclei using Hoechst 33258 (blue). Expression of RORα was revealed with anti-RORα (red). RORα and nucleus co-localize in astrocytes but not in microglia (arrows). Higher-magnification images of framed areas are in the merged images (Bottom). (B) Western blot analysis of RORα in enriched nuclear extracts from WT (+/+) and staggerer (sg/sg) cerebellum (Cb) and from astrocyte (AS) and microglia (MG) cultures. Twelve micrograms of tissue and 25 μg of cell extracts were electrophoresed. T+ are in vitro-synthesized RORα1 and RORα4 isoforms. The arrowhead indicates protein cross-reacting with anti-RORα antibody. (C) Amplification of RORα1 and RORα4 transcripts in cultivated astrocytes. Total RNA from highly purified staggerer (sg/sg) and WT (+/+) astrocyte cultures (AS) and from WT cortex (Co) and WT cerebellum (Cb) as positive controls were used. Predicted sizes for the amplified fragment are indicated (Right).
Taken together, these results indicate that in vivo RORα is expressed in astrocytes but not in microglia, in addition to its previously documented neuronal expression (7).
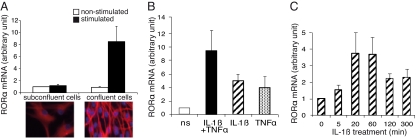
Transcriptional up-regulation of RORα after LPS treatment or hypoxia has been demonstrated in various cells that express RORα (17, 18). We investigated the effects of IL-1β and TNF-α, 2 proinflammatory cytokines, on Rora transcription in astrocytes. Primary cultures of astrocytes undergo an autonomous slow maturation process involving changes to their morphology and biochemical phenotype from dividing to confluent cells (19). This process is particularly remarkable in cultures of cerebellar astrocytes. Initially taking on a broad flat form corresponding to immature cells, astrocytes compact into a bipolar form as their expression of GFAP increases (Fig. 2A). At this stage, the cultures have reached confluence and the cells are non-proliferative and mature. In cultures of mature (i.e., confluent) astrocytes, cytokine treatment consistently up-regulated RORα expression compared with untreated cells. Induction of RORα mRNA expression by IL-1β and TNF-α was 9 times higher in mature cells compared with immature cells (Fig. 2A). At the concentrations used, IL-1β and TNF-α induced similar RORα mRNA increases, 5 and 4 fold respectively, compared with non-stimulated cultures. Effects were additive when the 2 cytokines were associated (Fig. 2B). RORα mRNA increased as early as 5 min after IL-1β treatment, peaking between 20 and 60 min after induction, and then declined (Fig. 2C). Thus, confluent cell cultures were used in further studies.
Fig. 2.
Modulation of RORα mRNA by pro-inflammatory cytokines in astrocyte cultures. Highly purified C57BL/6 astrocyte cultures were used. They were treated with IL-1β (20 ng/mL) and TNF-α (50 ng/mL) alone or in combination for 1 h and assayed for RORα mRNA using the real-time RT-PCR technique. RORα mRNAs are expressed in an arbitrary unit defined in SI Methods. (A) Effect of cell confluence on the induction of RORα mRNA by IL-1β combined to TNF-α. (Bottom) Photomicrographs of cerebellar astrocytes labeled by GFAP in sub-confluent (Left) and confluent (Right) cultures and the corresponding levels of RORα mRNA in non-stimulated and stimulated cultures (Top) expressed as the ratio of mRNAs to non-stimulated sub-confluent cultures. The mean of RORα mRNA basal levels of un-stimulated sub-confluent cultures was arbitrarily set to 1. Confluent astrocytes express a high level of GFAP, stained in red, and are highly responsive to cytokine stimulation compared with sub-confluent cells. (B) Effect of cytokine on RORα mRNA expression in confluent cultures. IL-1β and TNF-α were used alone and combined. ns: non-stimulated. Pro-inflammatory cytokines modulate RORα mRNA expression with an additive effect when combined. (C) Time course of RORα mRNA expression induced by IL-1β. RORα mRNA levels were expressed as the ratio to cultures stimulated at point 0 of the kinetic, arbitrarily set to 1. Values are the mean ± SEM of 3 to 5 independent cultures.
Bi-Directional Regulation of IL-6 Expression in Staggerer Astrocytes.
Several reports have demonstrated the role of RORα in regulation of the inflammatory response in the periphery (5, 16, 20), and the contribution of the NF-κB signaling pathway to the regulation of CNS inflammation in astrocytes is now well documented (3). Astrocytes are considered to be the major source of IL-6, whose expression is partly driven by the transcription factor NF-κB (4). Taken together, these observations led us to look for a link between RORα activity and the expression of the Il-6 gene in cultured astrocytes.
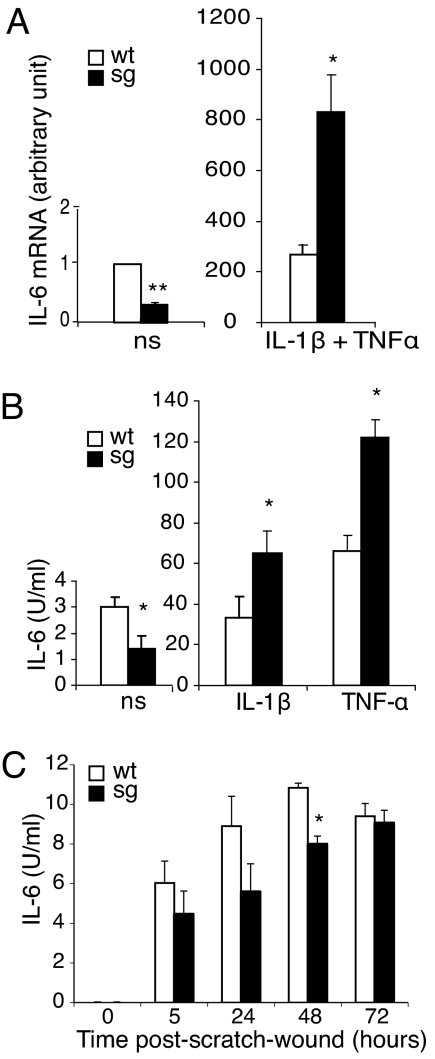
We thus compared IL-6 expression triggered by IL-1β plus TNF-α treatment in astrocyte cultures with (Rora+/+) and without (Rorasg/sg) endogenous RORα (Fig. 3). Cytokine treatment strongly increased levels of IL-6 mRNAs in both Rorasg/sg and WT astrocytes (Fig. 3A). In the absence of RORα that normally inhibits the NF-κB signaling pathway, stimulation was more efficient in Rorasg/sg astrocytes: Rorasg/sg IL-6 mRNAs were 3.1 fold higher compared with Rora+/+ (Fig. 3A Right). Similarly, IL-6 secreted in Rorasg/sg astrocyte supernatants after IL-1β or TNF-α treatment were 2-fold greater compared with Rora+/+ (Fig. 3B Right).
Fig. 3.
Expression and secretion of IL-6 before and after pro-inflammatory stimulation in WT and staggerer (sg) astrocyte cultures. Confluent astrocyte cultures were used. (A) IL-6 mRNA in non-stimulated (ns) and IL-1β plus TNF-α (20 ng/mL and 50 ng/mL, respectively)-stimulated cultures. Levels of mRNA, expressed as the ratio to non-stimulated cultures, arbitrarily set to 1, were determined using the real-time RT-PCR after 1 h of incubation with the stimulating cytokines. Values are the means ± SEM of 4 to 6 independent cultures, with 2 replicates. (B) IL-6 secreted in non-stimulated (ns), IL-1β (20 ng/mL), or TNF-α (50 ng/mL)-stimulated cultures. Levels of IL-6 were determined in supernatants after 18 h of incubation by using a biological assay. Values are the means of 3 independent cultures with 2 replicates. Staggerer and WT resting and activated astrocytes express and secrete differential IL-6 levels. (C) IL-6 secreted after layer wounding. WT and staggerer astrocyte monolayers were scratched and assayed for IL-6 in supernatants 5 h, 24 h, 48 h, and 72 h later. Wounded as well as resting staggerer astrocytes produce lower IL-6 levels compared with WT. Values are the means ± SEM of 3 independent cultures. Statistical analysis of staggerer versus WT was performed by Mann-Whitney U test: *, P < 0.05 and **, P < 0.01.
Strikingly, in the absence of stimulation, basal expression of both IL-6 mRNAs and protein was consistently lower in Rorasg/sg compared with Rora+/+: 3 and 2.2 fold, respectively (Fig. 3A Left and Fig. 3B Left). Thus, without any exogenous inflammatory stimulus, the loss of RORα function parallels a loss of IL-6 expression. This suggested that basal IL-6 production by resting cultured astrocytes is driven by a pathway other than NF-κB, possibly involving RORα. To confirm this hypothesis, we tested the “scratch-wound” model in which the stimulus consists of lesioning the astrocyte layer (21). We showed that the wound triggered IL-6 production without activation of the NF-κB pathway (Fig. S2) and induced less secretion of IL-6 in Rorasg/sg astrocytes compared with Rora+/+ (Fig. 3C). This down-regulation of IL-6 in the absence of NF-κB activation suggested that RORα could directly control Il-6 expression.
Il-6 Gene Trans-Activation by RORα.
To further investigate the role of RORα on IL-6 regulation and assess the hypothesis of RORα trans-activation of Il-6, we sought a ROR response element (RORE) half-site motif, PuGGTCA flanked by an AT-rich sequence, on the human Il-6 promoter (DNA Strider software). Sequence analysis revealed the presence of a putative RORE located between nucleotides -1,148 bp and -1,154 bp from the transcription start site. Two and 3 putative ROREs were also found in the mouse and in the rat Il-6 promoter sequence, respectively (Table 1).
Table 1.
Sequences of putative ROREs in Il-6 promoter
| Species | Sequence | Localization (bp) | Accession no. |
|---|---|---|---|
| Human | CTTATTGGGTCA | 54 | M22111 |
| Mouse | ATTTCCAGGTCA | 391 | M20572 |
| AAACTCAGGTCA | 773 | ||
| Rat | AATACTAGGTCA | 841 | M26745 |
| TTAGAAGGGTCA | 1,899 | ||
| AAACTCAGGTCA | 2,454 |
The localization numbers indicate RORE positions in the GenBank/EMBL sequences.
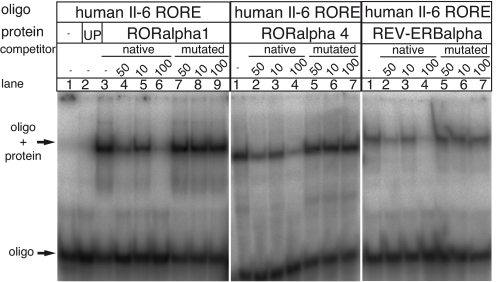
We tested the ability of RORα1 and RORα4 to bind to the putative RORE by using electrophoretic mobility-shift assays (EMSAs). We introduced REV-ERBα, a closely related orphan nuclear receptor that binds to the same motif as RORα but functions in an opposing manner, i.e., as a repressor (22). As shown in Fig. 4, specific DNA-protein complexes with a retarded migration were formed with RORα1, RORα4, and REV-ERBα binding to the synthetic 32P-labeled oligonucleotides representing the human Il-6 RORE. The specificity of the binding was assessed by competition assays using native or mutated unlabeled human Il-6 RORE. Increasing excess (10-, 50-, or 100-fold) of unlabeled Il-6 RORE increasingly inhibited the complex formation in contrast to the mutated form that did not compete.
Fig. 4.
RORα1, RORα4, and REV-ERBα specifically bind to a putative RORE of the Il-6 promoter. EMSA was performed with radiolabeled double-stranded oligonucleotides that contained the putative RORE of the human Il-6 (hIL-6) promoter. Radiolabeled hIl-6 RORE oligonucleotides were incubated with RORα1 (Left, lanes 3–9), RORα4 (Center), REV-ERBα (Right), no proteins (Left, lane 1) or with un-programmed reticulocyte (UP) lysate as control (Left, lane 2). Competition assays were carried out by incubating radiolabeled hIl-6 RORE oligonucleotides with RORα1 (Left, lanes 4–9), RORα4 (Center, lanes 2–7), or REV-ERBα (Right, lanes 2–7) in the presence of unlabeled native or mutated hIl-6 RORE oligonucleotides at 10-, 50-, or 100-fold molar excess. Arrows indicate migration of double-stranded oligonucleotides (Lowerr) and DNA-protein complexes (Upper).
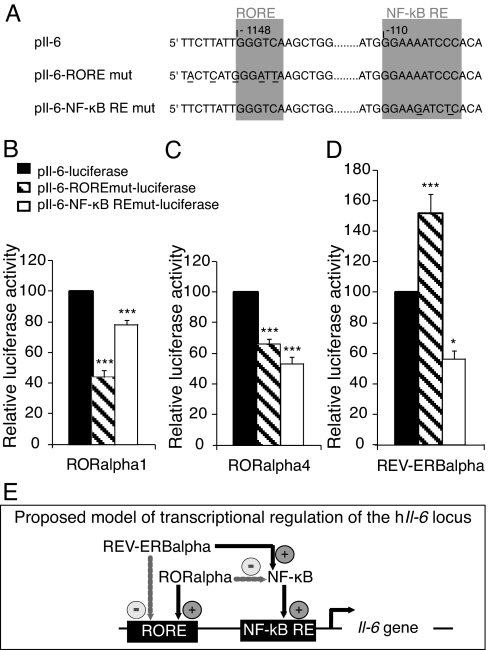
Modulation of transcriptional activity at the hIl-6 promoter by RORα1, RORα4, and REV-ERBα as effectors was measured using a hIl-6 promoter coupled to the luciferase reporter gene transfected into Cos-7 cells. In addition, we used point-mutated variants in the response elements of NF-κB and RORα to dissect each protein effect on transcription of the reporter (Fig. 5A). As shown in Figs. 5 B and C, point mutation within the RORE significantly diminished the luciferase expression by 66% and 44% in the presence of RORα1 or RORα4, respectively, compared with the activity of the native hIl-6 promoter. In contrast to RORα, REV-ERBα increased by 1.5-fold the expression of the luciferase when RORE was mutated (Fig. 5D), suggesting that the transgenic REV-ERBα removed the endogenous RORα blockade effect on the NF-κB pathway. These results strongly suggest that RORα and REV-ERBα could competitively bind to the Il-6 promoter and act respectively as a transactivator and a repressor of Il-6 expression. Point mutation within the NF-κB response element attenuated the luciferase activity by 23%, 47%, and 44% when co-transfected with RORα1, RORα4, or REV-ERBα, respectively (Figs. 5 B–D). These decreases indicate that NF-κB activates the Il-6 promoter activity whatever the effector.
Fig. 5.
Trans-activation of Il-6 by RORα and REV-ERBα. (A) Sequences of native response elements (pIl-6) and response elements point mutated in the RORα (pIl-6-RORE mut) and in the NF-κB (pIl-6- NF-κB RE mut). (B-D) Differential modulation of IL-6 promoter by RORα1, RORα4, and REV-ERBα. Cos-7 cells were transiently co-transfected with reporter plasmids carrying one copy of the native (pIl-6-luciferase) or point-mutated human Il-6 promoters (pIl-6-RORE mut-luciferase or pIl-6-NF-κB RE mut-luciferase) and the expression plasmid vector of the 2 RORα isoforms and of REV-ERBα for 48 h. Transfected cells were then assayed for luciferase activity. Values expressed as percentage of the native reporter pIl-6-Luc are the mean ± SEM of 3 independent experiments with 6 replicates each. Statistical analysis was performed by 1-way ANOVA followed by Scheffé multiple comparison test: *, P < 0.05, **, P < 0.01, ***, P < 0.001, mutated versus native promoter. (E) Proposed model of transcriptional regulation of the human Il-6 promoter. RORα may negatively regulate Il-6 expression through the NF-κB pathway. Alternatively, RORα may trans-activate Il-6 expression by interacting with a RORE in the promoter. For these 2 pathways, RORα competes with REV-ERBα that binds the same response elements with a repressor activity.
Taken together, our results suggest a dual direct and indirect transcriptional regulation of the Il-6 locus by RORα (Fig. 5E).
Discussion
Although both astrocytes and RORα play key roles in neuronal development, survival, and function (2, 8), it has been assumed that these roles were distinct as RORα has previously been identified and studied only in neurons (7). In this study, we use a loss-of-function mutation of RORα (i.e., staggerer) to investigate the role of RORα in astrocytes and in the regulation of IL-6, which has important neurodevelopmental and neuroprotective properties, as well as mediating neuro-inflammation (23). We found that RORα is expressed in astrocytes and exerts dual control on the Il-6 gene: RORα directly up-regulates IL-6 expression yet indirectly suppresses cytokine-induced IL-6 up-regulation by inhibiting the NF-κB pathway.
In this study we demonstrate that RORα is specifically expressed, among glial cells, in the nuclei of astrocytes throughout the CNS and particularly in the hippocampus, cortex, and cerebellum. In addition, we also identified the expression of both RORα1 and RORα4 isoforms in cultures of highly purified astrocytes from the cerebellum and cortex. However, whether the dual expression we observed is co-localized in single astrocytes remains unknown. These data expand previous studies in which high expression of these RORα isoforms was obtained from the cerebellum and was assumed to be exclusively of neuronal origin (24). In addition, in agreement with our results, a recent study of the astrocyte transcriptome included RORα transcripts (25).
Our data also reveal RORα function in astrocytes. The rapid induction and temporal modulation of RORα transcripts following pro-inflammatory stimulation fit well with the properties expected of transcriptional activity. In addition, cytokine induction of RORα was not only additive in combined cytokine treatment (IL-1β and TNF-α), but also correlated with astrocyte maturity. This indicates that both cytokines activate the same transduction pathway and that the signal transduction response to cytokine stimulation is more effective in mature astrocytes. We also demonstrate that loss of RORα function (i.e., Rorasg/sg) is associated with increased astrocytic pro-inflammatory-induced IL-6 expression. This is consistent with previous studies in which RORα over-expression decreased the expression of NF-κB-related genes, including Il-6, and adds that RORα also negatively regulates the NF-κB pathway in astrocytes, as well as in the peripheral tissues (5, 16, 20).
In summary, our demonstration of RORα expression in astrocytes together with the up-regulation of RORα and IL-6 following an inflammatory stimulus suggests that, in addition to its role in neuronal maturation and survival (8, 9), RORα has an anti-inflammatory role in the nervous system, as it does in peripheral tissues, through the regulating astrocyte function.
A surprising finding in our study was that, in comparison to control (i.e., Rora+/+), loss of RORα function (i.e., Rorasg/sg) resulted in less IL-6 expression in un-stimulated astrocytes (i.e., basal level) and in cultured astrocytes stimulated in a manner (i.e., scratch-wound) that did not induce NF-κB signaling. This suggests that RORα promotes IL-6 synthesis. In silico search through the proximal part of the mouse Il-6 promoter revealed 2 consensus DNA binding sites for RORα, with one of them perfectly conserved between rat and mouse. We demonstrated that RORα trans-activates the Il-6 gene, with RORα1 and RORα4 isoforms differentially increasing transcriptional activity. The weaker effect of RORα4 is consistent with the findings of previous studies that showed differential trans-activation activity for the 2 RORα isoforms as a result of distinct DNA-binding properties dictated by their different amino-terminal domains (6, 20). The direct control of Il-6 gene expression by RORα is further argued by 2 results obtained with REV-ERB, another transcription factor, which represses trans-activation mediated by RORα by blocking RORα DNA binding sites (22, 26). First, in our studies, REV-ERBα bound to the same site as RORα in the Il-6 promoter. Second, in the study of Ramakrishnan et al., ectopic expression of a dominant negative version of REV-ERBβ in skeletal muscle culture not only decreased the expression of all studied NF-κB target genes except for Il-6, but also up-regulated mRNAs for both RORα and IL-6 (27). This induction of Il-6 mRNA in a context in which the NF-κB signaling pathway is blocked and RORα up-regulated is consistent with a direct effect of RORα on Il-6.
These findings raise questions about the biological significance of the bi-directional control of astrocytic Il-6 gene expression by RORα.
In a non-inflammatory context, astrocytes produce low levels of IL-6. IL-6 displays hallmarks of neurotrophic factor that have been demonstrated by numerous animal models and in vitro studies (23, 28–31). In the unique mouse model in which IL-6 appears to be detrimental, astrocyte IL-6 over-expressing transgenic mice, neuroprotective mechanisms come into place after injury (32, 33). In normal physiological conditions, IL-6 levels in the brain remain low (4). Although various central nervous cell types can produce IL-6, astrocytes are increasingly recognized for their impact on neuronal function and viability and are probably the main source of basal level of IL-6 in the normal brain. Based on the RORα direct control of IL-6 and the IL-6 down-regulation in resting RORα deficient astrocytes, we envision a link between the neuroprotective functions of IL-6 and RORα with the neuron supportive function of astrocytes.
In the acute phase of the inflammatory response, astrocytes are also considered as the main source of brain IL-6 and are now viewed as effector cells of the brain inflammatory reaction. As such, they express the NF-κB signaling pathway, which plays a key role in brain inflammation (3). Our data add that, upon pro-inflammatory cytokine trigger, astrocyte expression of the Rora is rapidly increased, which in turn would not only enhance IL-6 up-regulation in the early phase of the inflammatory reaction, but also inhibit NF-κB signaling to limit the ongoing inflammatory reaction. Thus our data are supported by, and provide a mechanism to explain, the effects of selectively inactivating astroglial NF-κB after spinal cord injury in vivo: greater up-regulation of IL-6 expression during early astrocyte activation compared with WT mice (34).
In the staggerer, the relevance of IL-6 on cerebellar neurodegeneration can be just speculative in the absence of a reference model, i.e., a double-mutant bearing the staggerer mutation and invalidated for the Il-6 gene (11, 35). The death of Purkinje cells is triggered by an intrinsic death mechanism resulting from the loss-of-function mutation in the Rora, although the secondary massive degeneration of granular neurons is a result of the loss of their target neurons. The absence of astrocytic RORα function probably exacerbates the associated inflammatory reaction because of the abnormally high levels of IL-6 that have been detected in the degenerative cerebellum of these mice (36). Studies of new mouse models constructed by transgenesis will enable a better understanding of the true contribution of IL-6 to the pathophysiology of cerebellar neurons in the staggerer mice.
In conclusion, our study provides evidence that RORα is expressed in astrocytes and that astrocytic RORα exerts bi-directional control on production of a key mediator of brain inflammation and of the neuron-glia interaction, i.e., IL-6. The use of common players, RORα and IL-6, in astrocyte-neuron or astrocyte-immune cell interactions in resting and reactive astrocytes illustrates how a single cell type (i.e., the astrocyte) can readily change its function by activating an autoregulatory loop. In this loop, RORα plays a bi-directional role by positively regulating Il-6 promoter transcription, plus acting as a negative regulator of NF-κB signaling. Taken together, our findings indicate that RORα is a multipotent molecular player of constitutive and adaptive mechanisms of the astrocyte physiology.
Methods
Detailed methods for all approaches used are given in the SI Methods.
Cell Cultures, Wounding, and IL-6 Assay.
Highly purified cerebellar astrocytes were prepared from 5-d-old pups of staggerer litters as described previously (37). Cells were treated with murine IL-1β (20 ng/mL) and/or TNF-α (50 ng/mL; R&D Systems). Cells were wounded by scraping a 2-μL pipette tip on the confluent cell layer in one straight 0.5-cm line. Microglia were derived from postnatal day-1 mouse cortices as previously described (38). IL-6 secreted in supernatants was determined in a biological assay using the IL-6-dependent B-cell hybridoma B9.
Immunohistochemistry.
Mice were trans-cardially perfused with 4% paraformaldehyde. Twenty-five-micrometer brain sections were incubated with primary antibodies to GFAP (Sigma), S-100 (Sigma), NeuN (Chemicon), or Iba-1 (Wako) and goat anti-RORα (Santa Cruz Biotechnology). Sections were analyzed using a Leica SP5 confocal microscope.
Oligonucleotides.
See SI Methods for detailed description of oligonucleotides.
Non-Quantitative PCR and RT-PCR.
RNAs (0.1–0.5 μg/sample) were reverse-transcribed into cDNA using the Reverse Transcription System (Promega). For non-quantitative PCR, cDNAs were amplified on a Perkin–Elmer DNA Thermal Cycler. Real-time PCR was performed on duplicate samples of cDNA using SybrGreen (Abgene) and an ICycler IQ thermal cycler (Bio-Rad). 18S-rRNA levels were used to normalize amounts of cDNA. Quantification was carried out using the Δ-ΔCt method (39).
Plasmid Construction and Site-Directed Mutagenesis.
The hRORα1, hRORα4, and hREV-ERBα cDNA fragments were inserted into the pSG5 vector (Stratagene). The full-size Il-6 promoter reporter gene p1168h.IL6P-luc+ containing the 1,168-bp human Il-6 promoter and the recombinant plasmid p1168h.IL6Pm NF-κB-luc+ has been previously described (40). P1168h.IL6P-luc+ was mutated by QuikChange PCR site-directed mutagenesis (Stratagene).
EMSA.
EMSAs were performed as described previously (41). Briefly, radiolabeled double-stranded oligonucleotides containing the putative RORE of the human Il-6 promoter were incubated with RORα1, RORα4, and REV-ERBα proteins. For competition experiments, native or mutated unlabeled double-stranded oligonucleotides were added simultaneously. The gels were analyzed with a PhosphoImager apparatus and ImageQuant software.
Transient Transfection and Luciferase Assay.
Cos-7 cells were transfected with reporter DNA (luciferase under control of native or mutated Il-6 promoter), effector DNA (pSG5-RORα1, pSG5-RORα4, or pSG5-REV-ERBα), and pSVBGal using FuGENE 6 transfection reagent (Roche Diagnostics). The luciferase assay was carried out according to the manufacturer's instruction (Promega Biotec). Activation is expressed relative to luciferase activity after co-transfection of the reporter and the empty vector alone. This activity was normalized to the co-expressed β-galactosidase levels.
Western Blot Analysis.
Twelve or 25 μg of enriched fractions of nuclear proteins from astrocytes, microglia, and brain tissues were analyzed by Western blot. Membranes were incubated with the primary antibodies goat polyclonal anti-RORα (Santa Cruz Biotechnology) or mouse monoclonal anti-ß-actin (Sigma), followed by incubation with anti-goat (Thermo Fisher Scientific) or anti-mouse (Jackson ImmunoResearch) horseradish peroxidase-conjugated secondary antibodies.
Statistical Analysis.
Statistical analyses were carried out using StatView software (Abacus Concepts).
Supplementary Material
Acknowledgments.
We thank Rachel Sherrard and Ann Lohof for their helpful discussion and critical reading of the manuscript. This work was supported by funds from the Centre National de la Recherche Scientifique (CNRS) and Université Pierre et Marie Curie, and by a grant from the Fédération de la Recherche sur le Cerveau. N.J. was supported by fellowships from the association France-Alzheimer, the Association pour la Recherche sur le Cancer, the Neuropôle de Recherche Francilien, and the région Ile de France. S.J. was supported by the Délégation Générale pour l'Armement and the CNRS.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911782106/DCSupplemental.
References
- 1.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 2.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 3.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- 5.Delerive P, et al. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giguere V, et al. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- 7.Ino H. Immunohistochemical characterization of the orphan nuclear receptor ROR alpha in the mouse nervous system. J Histochem Cytochem. 2004;52:311–323. doi: 10.1177/002215540405200302. [DOI] [PubMed] [Google Scholar]
- 8.Gold DA, Gent PM, Hamilton BA. ROR alpha in genetic control of cerebellum development: 50 staggering years. Brain Res. 2007;1140:19–25. doi: 10.1016/j.brainres.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 9.Boukhtouche F, et al. Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation. J Neurosci. 2006;26:1531–1538. doi: 10.1523/JNEUROSCI.4636-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidman RL, Lane PW, Dickie MM. Staggerer, a new mutation in the mouse affecting the cerebellum. Science. 1962;137:610–612. doi: 10.1126/science.137.3530.610. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton BA, et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature. 1996;379:736–739. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- 12.Lemaigre-Dubreuil Y, Brugg B, Chianale C, Delhaye-Bouchaud N, Mariani J. Over-expression of interleukin-1 beta-converting enzyme mRNA in staggerer cerebellum. Neuroreport. 1996;7:1777–1780. doi: 10.1097/00001756-199607290-00017. [DOI] [PubMed] [Google Scholar]
- 13.Vernet-der Garabedian B, Lemaigre-Dubreuil Y, Delhaye-Bouchaud N, Mariani J. Abnormal IL-1beta cytokine expression in the cerebellum of the ataxic mutant mice staggerer and lurcher. Brain Res Mol Brain Res. 1998;62:224–227. doi: 10.1016/s0169-328x(98)00268-x. [DOI] [PubMed] [Google Scholar]
- 14.Journiac N, Doulazmi M, Pajak F, Mariani J, Vernet-der Garabedian B. Quantitative analysis of microglial cells in the degenerating cerebellum of the staggerer (RORA(sg/sg)) mutant mouse. J Neurogenet. 2005;19:143–154. doi: 10.1080/01677060600569762. [DOI] [PubMed] [Google Scholar]
- 15.Kopmels B, et al. Evidence for a hyperexcitability state of staggerer mutant mice macrophages. J Neurochem. 1992;58:192–199. doi: 10.1111/j.1471-4159.1992.tb09295.x. [DOI] [PubMed] [Google Scholar]
- 16.Stapleton CM, et al. Enhanced susceptibility of staggerer (RORalphasg/sg) mice to lipopolysaccharide-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L144–L152. doi: 10.1152/ajplung.00348.2004. [DOI] [PubMed] [Google Scholar]
- 17.Besnard S, et al. Expression and regulation of the nuclear receptor RORalpha in human vascular cells. FEBS Lett. 2002;511:36–40. doi: 10.1016/s0014-5793(01)03275-6. [DOI] [PubMed] [Google Scholar]
- 18.Chauvet C, Bois-Joyeux B, Danan JL. Retinoic acid receptor-related orphan receptor (ROR) alpha4 is the predominant isoform of the nuclear receptor RORalpha in the liver and is up-regulated by hypoxia in HepG2 human hepatoma cells. Biochem J. 2002;364:449–456. doi: 10.1042/BJ20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu VW, Schwartz JP. Cell culture models for reactive gliosis: New perspectives. J Neurosci Res. 1998;51:675–681. doi: 10.1002/(SICI)1097-4547(19980315)51:6<675::AID-JNR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Migita H, Satozawa N, Lin JH, Morser J, Kawai K. RORalpha1 and RORalpha4 suppress TNF-alpha-induced VCAM-1 and ICAM-1 expression in human endothelial cells. FEBS Lett. 2004;557:269–274. doi: 10.1016/s0014-5793(03)01502-3. [DOI] [PubMed] [Google Scholar]
- 21.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 22.Retnakaran R, Flock G, Giguere V. Identification of RVR, a novel orphan nuclear receptor that acts as a negative transcriptional regulator. Mol Endocrinol. 1994;8:1234–1244. doi: 10.1210/mend.8.9.7838156. [DOI] [PubMed] [Google Scholar]
- 23.Gadient RA, Otten UH. Interleukin-6 (IL-6)–a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 24.Matysiak-Scholze U, Nehls M. The structural integrity of ROR alpha isoforms is mutated in staggerer mice: Cerebellar coexpression of ROR alpha1 and ROR alpha4. Genomics. 1997;43:78–84. doi: 10.1006/geno.1997.4757. [DOI] [PubMed] [Google Scholar]
- 25.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bois-Joyeux B, et al. Modulation of the far-upstream enhancer of the rat alpha-fetoprotein gene by members of the ROR alpha, Rev-erb alpha, and Rev-erb beta groups of monomeric orphan nuclear receptors. DNA Cell Biol. 2000;19:589–599. doi: 10.1089/104454900750019344. [DOI] [PubMed] [Google Scholar]
- 27.Ramakrishnan SN, Lau P, Burke LJ, Muscat GE. Rev-erbbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: Evidence for cross-talk between orphan nuclear receptors and myokines. J Biol Chem. 2005;280:8651–8659. doi: 10.1074/jbc.M413949200. [DOI] [PubMed] [Google Scholar]
- 28.Zhong J, Dietzel ID, Wahle P, Kopf M, Heumann R. Sensory impairments and delayed regeneration of sensory axons in interleukin-6-deficient mice. J Neurosci. 1999;19:4305–4313. doi: 10.1523/JNEUROSCI.19-11-04305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penkowa M, et al. Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia. 1999;25:343–357. [PubMed] [Google Scholar]
- 30.Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- 32.Campbell IL, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penkowa M, et al. Astrocyte-targeted expression of interleukin-6 protects the central nervous system during neuroglial degeneration induced by 6-aminonicotinamide. J Neurosci Res. 2003;73:481–496. doi: 10.1002/jnr.10681. [DOI] [PubMed] [Google Scholar]
- 34.Brambilla R, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrup K, Mullen RJ. Staggerer chimeras: Intrinsic nature of Purkinje cell defects and implications for normal cerebellar development. Brain Res. 1979;178:443–457. doi: 10.1016/0006-8993(79)90705-4. [DOI] [PubMed] [Google Scholar]
- 36.Brugg B, et al. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci USA. 1995;92:3032–3035. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etienne-Manneville S, Chaverot N, Strosberg AD, Couraud PO. ICAM-1-coupled signaling pathways in astrocytes converge to cyclic AMP response element-binding protein phosphorylation and TNF-alpha secretion. J Immunol. 1999;163:668–674. [PubMed] [Google Scholar]
- 38.Flode AM, Combs CK. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. J Neurosci Methods. 2007;164:218–224. doi: 10.1016/j.jneumeth.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanden Berghe W, Francesconi E, De Bosscher K, Resche-Rigon M, Haegeman G. Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-kappaB-dependent mechanism. Mol Pharmacol. 1999;56:797–806. [PubMed] [Google Scholar]
- 41.Chauvet C, et al. The gene encoding fibrinogen-beta is a target for retinoic acid receptor-related orphan receptor alpha. Mol Endocrinol. 2005;19:2517–2526. doi: 10.1210/me.2005-0153. [DOI] [PubMed] [Google Scholar]
- 42.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.