Abstract
The immune receptor signaling pathway is used by nonimmune cells, but the molecular adaptations that underlie its functional diversification are not known. Circulating platelets use the immune receptor homologue glycoprotein VI (GPVI) to respond to collagen exposed at sites of vessel injury. In contrast to immune cell responses, platelet activation must take place within seconds to successfully form thrombi in flowing blood. Here, we show that the GPVI receptor utilizes a unique intracellular proline-rich domain (PRD) to accelerate platelet activation, a requirement for efficient platelet adhesion to collagen under flow. The GPVI PRD specifically binds the Src-family kinase Lyn and directly activates it, presumably through SH3 displacement. In resting platelets, Lyn is constitutively bound to GPVI in an activated state and platelets lacking Lyn exhibit defective collagen adhesion like that of platelets with GPVI receptors lacking the PRD. These findings define a molecular priming mechanism that enables an immune-type receptor to adopt a hemostatic function. These studies also demonstrate that active kinases can constitutively associate with immune-type receptors without initiating signal transduction before receptor ligation, consistent with a recent molecular model of immune receptor signaling in which receptor ligation is required to bring active kinases to their receptor substrates.
The response of platelets to vessel injury is essential to prevent posttraumatic blood loss but also underlies the pathophysiology of cardiovascular diseases, such as heart attack and stroke (1). Platelet activation requires the generation of rapid and coordinated intracellular signals that culminate in cell-matrix and cell-cell adhesion and thrombus formation within seconds (2). Collagen, the most abundant and thrombogenic subendothelial matrix protein exposed by vessel injury, provides both a primary activating stimulus and an adhesive surface for the initiation of platelet thrombi in the arterial system. Understanding these events has therapeutic potential, as blocking this early step may prevent the formation of pathologic thrombi associated with the coronary and cerebral arteries (3).
Molecular and genetic studies have established that collagen-induced platelet activation is triggered by the platelet-specific surface receptor glycoprotein VI (GPVI). GPVI is an Ig (Ig)-like domain-containing receptor that is structurally and functionally homologous to immune receptors but is expressed exclusively on platelets and megakaryocytes (4). Extensive pharmacologic and genetic studies have demonstrated that GPVI signaling in response to collagen is highly analogous to that of the related multisubunit T-cell receptor and the IgE and IgA receptors (5). GPVI ligands, such as collagen or the snake-venom toxin convulxin, induce receptor clustering that facilitates the phosphorylation of the tandem tyrosines found in the immunotyrosine activating motif (ITAM) of the noncovalently associated FcRγ chain adaptor by Src-family tyrosine kinases (SFKs), such as Lyn and Fyn. ITAM phosphorylation stimulates the recruitment and activation of the intracellular tyrosine kinase Syk and the assembly of a signaling complex that includes the adaptors linker for activated T-cells (LAT) and Src homology (SH)2 domain-containing leukocyte phosphoprotein of 76 kDa (Slp-76), culminating in the activation of phospholipase C (PLC)γ2 (5, 6). This pathway, like the established G protein-coupled signaling pathways that mediate platelet activation by thrombin and ADP, results in the elevation of intracellular calcium levels and platelet activation responses, including granule release and integrin conformational changes. In contrast to immune receptors that form stable immune synapses and transmit signals, which generate cellular responses over several hours, platelet activation by GPVI takes place within seconds, allowing circulating platelets to firmly adhere to exposed collagen (7, 8). Whether and how platelets have adapted the immune receptor homologue GPVI to meet the accelerated temporal requirements inherent in hemostasis is unknown.
GPVI, like homologous immune receptors, contains conserved transmembrane residues that are required for its association with the FcRγ chain, the essential signal transducing component of the receptor complex (9). However, a feature of GPVI that is unique among FcRγ-coupled receptors is a conserved intracellular class I (R/K)xxPxxP proline-rich domain (PRD) of the amino acid sequence K/RPLPPLP that constitutes a core binding motif for SH3 domains, such as those found on SFKs (10) [supporting information (SI) Fig. S1A]. Biochemical studies in cell lines have demonstrated that the GPVI PRD mediates binding to the SFKs Lyn and Fyn (11, 12). These studies have demonstrated variable degrees of reduced signaling by GPVI receptors lacking this motif (11, 13). However, the role of the GPVI PRD in platelets and hemostasis has not been defined.
To study the function of the GPVI PRD, we generated mice with platelets that expressed wild-type GPVI or GPVI lacking the PRD. Our studies reveal that the PRD does not alter the strength of sustained GPVI signaling but instead accelerates platelet activation kinetics, a feature required for the efficient adhesion of platelets to collagen under flow conditions. Molecular studies to explain the basis for signal acceleration by the GPVI PRD reveal that this domain specifically binds the SH3 domain of Lyn. We find that GPVI PRD binding directly activates Lyn and that GPVI-bound Lyn in resting platelets is in an active state before receptor ligation. These studies provide insight into the molecular mechanism by which platelets have adapted the immune-receptor signaling pathway to function under the temporal constraints of the hemostatic system.
Results
Loss of the GPVI PRD Does Not Alter Sustained GPVI Signaling in Platelets.
To examine the role of the GPVI PRD in platelet signaling, we used retroviral vectors to express WT GPVI and a mutant lacking the PRD (PD GPVI) (Fig. S1B) in GPVI-deficient hematopoietic progenitors that were subsequently transferred to irradiated host mice, as previously described (14). In chimeric animals reconstituted with vectors that encoded WT or PD GPVI, GFP+ platelets coexpressed surface GPVI (Fig. S1C). The levels of GPVI expression were similar for WT GPVI and PD GPVI, and these levels were comparable to those found on human platelets (Fig. S1 C and D).
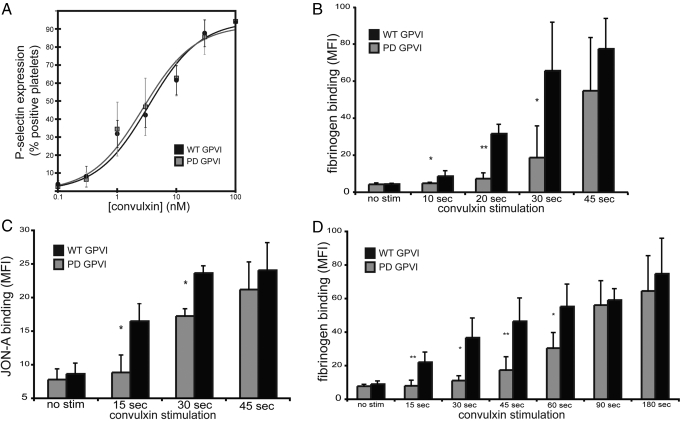
To test the role of the GPVI PRD in platelets, we exposed platelets from reconstituted animals to varying concentrations of the GPVI agonist convulxin for 10 min and measured platelet activation by the surface expression of P-selectin that occurs following intracellular granule release. No significant difference in platelet activation by convulxin was detected between platelets expressing WT GPVI and PD GPVI receptors under these conditions (Fig. 1A and Fig. S2A). The ability of PD GPVI platelets to spread and form lallelipodia on type-I collagen was not altered compared to that of WT GPVI platelets, whereas in vector control, GPVI-deficient platelets did not adhere well to collagen and did not spread on collagen (Fig. S2 B and C). Together, these results indicate that the GPVI PRD is not required for sustained signaling in response to convulxin or collagen.
Fig. 1.
The intracellular PRD is required for rapid platelet activation by GPVI receptors but not sustained GPVI signaling. (A) Dose-response curve of platelet activation mediated by WT and PD GPVI signaling. Platelets from GPVI-deficient mice expressing WT GPVI or PD GPVI receptors were stimulated with the indicated concentrations of convulxin for 10 min, and activation was measured using flow cytometry to detect the expression of surface P-selectin. (B) Time-course of platelet fibrinogen binding by WT and PD GPVI-expressing platelets following convulxin stimulation. Platelets were stimulated with 10-nM convulxin in the presence of Alexa-Fluor 647-conjugated fibrinogen for times indicated, fixed, and analyzed by flow cytometry. (C) Time-course of JON-A binding following 10-nM convulxin stimulation. (D) Time-course of fibrinogen binding following stimulation with 10 μg/ml CRP. In all experiments, only GFP+, GPVI-expressing platelets were analyzed. *, P < 0.05; **, P < 0.01; error bars indicate mean ± SD; n = 3 to 6 experiments for each time-point indicated, using four animals for WT or PD GPVI.
GPVI PRD Is Required for Rapid Platelet Activation by GPVI.
The GPVI PRD has been shown to bind the SFKs Lyn and Fyn, a finding which suggested that this domain might accelerate the initiation of GPVI signaling by facilitating the recruitment of Lyn and Fyn to the FcRγ chain (11, 12). To test the importance of the PRD in accelerating GPVI-mediated platelet activation, platelets were stimulated with convulxin, and the degree of platelet activation at varying time-points was analyzed. Platelet activation was determined by the binding of the integrin αIIbβ3 ligand fibrinogen, or by the activation state-specific anti-αIIb antibody JON-A. Consistent with the findings illustrated in Fig. 1A, at 45 s the degree of activation was identical for WT GPVI and PD GPVI platelets (see Fig. 1 B and C). At time-points of up to 30 s following convulxin stimulation, however, platelet activation was significantly impaired in platelets expressing PD GPVI receptors compared with those expressing WT GPVI receptors (see Fig. 1 B and C). A delay in activation of PD GPVI platelets was also observed in response to stimulation with collagen-related peptide (CRP), a synthetic collagen peptide specific for GPVI (15), whereas sustained signaling at time-points of 90 and 180 sec was identical (see Fig. 1D).
Loss of the PRD Delays Proximal GPVI Signal Transduction in RBL-2H3 Cells.
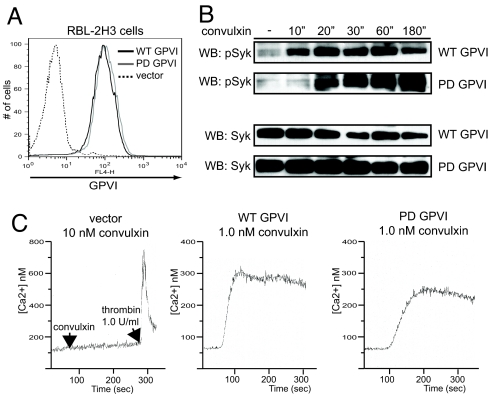
Retroviral complementation of GPVI-deficient bone marrow yields a limited number of animals that are chimeric, and these reconstituted mice often have a low percentage of platelets that express GPVI receptors. Therefore, we could not biochemically test the mechanism of delayed PD GPVI signaling in platelets. To biochemically characterize PD GPVI signaling kinetics, we used lentiviral vectors to stably express equivalent levels of WT GPVI and PD GPVI receptors in RBL-2H3 cells (Fig. 2A). The RBL-2H3 mast cell line expresses endogenous FcRγ chain and has been used by our laboratory and others to study GPVI signaling (9, 13). Following stimulation with convulxin, RBL-2H3 cells expressing PD GPVI exhibited delayed phosphorylation of Syk, the kinase activated following FcRγ ITAM phosphorylation, relative to that of WT GPVI expressing cells (Fig. 2B). However, total Syk phosphorylation at later time-points was unchanged (see Fig. 2B). Additionally, elevation of intracellular Ca2+ was slower following GPVI stimulation in PD GPVI expressing cells compared to WT GPVI-expressing cells (Fig. 2C).
Fig. 2.
PD GPVI receptors exhibit delayed Syk phosphorylation and calcium signaling. (A) Lentiviral vectors were used to express WT GPVI or PD GPVI or vector alone (vector) in RBL-2H3 cells and surface expression of GPVI was detected by flow cytometry. (B) Time-course of GPVI-mediated activation of Syk following convulxin stimulation in WT GPVI and PD GPVI-expressing RBL-2H3 cells. GPVI-expressing RBL-2H3 cells were stimulated with convulxin for the time-points indicated, and whole-cell lysate was analyzed by immunoblotting (WB) with antibodies against activated Syk (pSyk) and total Syk. (C) Ca2+ mobilization in RBL-2H3 cells expressing WT and PD GPVI and vector control. Cells were stimulated with convulxin and intracellular Ca2+ mobilization was measured by the ratio of Fura-2 emissions in a fluorimeter. Curves from representative experiments are shown; n = 3 measurements.
GPVI PRD Is Required for Efficient Platelet Adhesion to Collagen Under Flow.
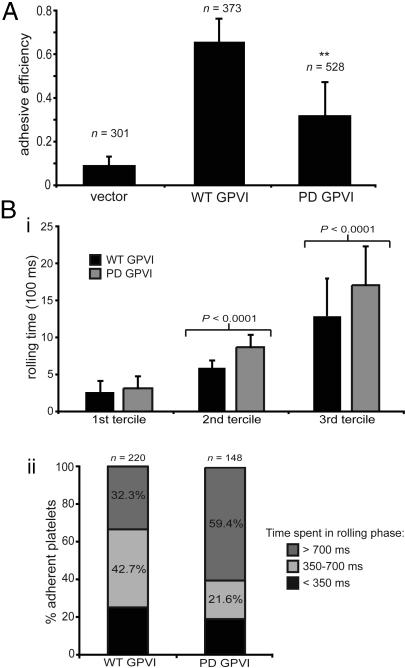
Under experimental conditions of blood flow over a collagen-coated surface, GPVI signaling is essential for the transition from platelet rolling, mediated by the GPIb-von Willebrand Factor interaction, to firm adhesion, mediated by activated α2β1 integrin-collagen interaction (2, 7, 16). To determine the role of the GPVI PRD during GPVI-collagen signaling under flow conditions, whole blood from reconstituted animals was flowed over a type-I collagen patch in a microfluidic chamber, and platelet rolling and adhesion were analyzed in real time. To analyze platelet adhesion on an individual cell basis, each flow experiment was analyzed frame-by-frame, and all rolling platelets were tracked to determine if and when they firmly adhered to the collagen surface (Fig. S3). An “adhesive efficiency” for each experiment was determined by a/(a + r), where a equals the number of platelets that permanently adhered and r equals the number of platelets that rolled along collagen but did not adhere. A majority of WT GPVI platelets that rolled along the collagen surface successfully adhered, with an adhesive efficiency of 0.65 ± 0.11, whereas only a small number of vector control GPVI-deficient platelets were able to transition from rolling to firm adhesion, with an efficiency of 0.09 ± 0.04 (Fig. 3A). PD GPVI-expressing platelets exhibited a markedly reduced adhesive efficiency of 0.31 ± 0.15, demonstrating that, compared to WT GPVI, approximately half as many PD GPVI platelets were able to transition from the rolling phase to the firmly adherent phase (see Fig. 3A).
Fig. 3.
The GPVI PRD is required for efficient platelet adhesion to collagen under flow. (A) Adhesive efficiency to collagen under flow of WT or PD GPVI-expressing platelets or vector control platelets. (B) Rolling times on collagen for WT or PD GPVI platelets. The time each adherent platelet spent in the rolling phase was calculated based on the number of frames during which each platelet changed position. (i) Rolling times are shown divided into terciles (1st, shortest third of rolling times in each group; 2nd, middle third of rolling times in each group; 3rd, longest third of rolling times in each group). (ii) Percentage rolling times: <350 ms, 350 to 700 ms, and >700 ms. The same adherent platelets were analyzed in (i) and (ii). **, P < 0.01; error bars indicate mean ± SD; three to five movies using three to four animals for each GPVI variant or vector control were analyzed in a blinded fashion; n = total number of adherent platelets analyzed.
A second measure of how efficiently platelets adhere to collagen is the time required for each adherent platelet to transition from a rolling state to one of firm adhesion (i.e., the time each adherent platelet spends in the rolling phase). Of the firmly adherent platelets, WT GPVI platelets spent a shorter time in the rolling phase compared to PD GPVI platelets (mean rolling time 711 ± 525 ms for WT GPVI vs. 972 ± 674 ms for PD GPVI; P < 0.0005) (Fig. 3B), a finding consistent with the slower kinetics of PD GPVI signaling, which results in a longer time to activate platelet integrins and mediate firm adhesion to collagen. These findings demonstrate that the GPVI PRD is required for efficient platelet adhesion to collagen under flow, the ex vivo response that most closely models the physiologic role of GPVI signaling in platelets in vivo.
GPVI PRD Preferentially Binds the SH3 Domains of Lyn and Hck.
To identify all of the SH3 domain-containing proteins that might bind the GPVI PRD, we expressed WT GPVI and PD GPVI cytoplasmic tails as recombinant GST (GST)-fusion proteins in Escherichia coli (Fig. S4 A and B). These recombinant proteins were used to screen a human SH3 proteome phage-display library to identify the preferred SH3 domain binding partners for a number of proteins containing PRDs (17). The WT GPVI fusion protein served as an excellent SH3 domain target in this assay, binding ≈1,000-fold more SH3 domain-expressing phage than did the PD GPVI fusion protein or GST alone (Fig. S4C). Of 48 SH3 domains bound by GST-WT GPVI proteins, 40 encoded the SH3 domains of SFKs (Table S1). Remarkably, 35 out of 40 encoded the SH3 domain of either Lyn (16 out of 40) or Hck (19 out of 40) (see Table S1). These results suggest that the SH3 domains of the SFKs Lyn and Hck are specific binding partners of the GPVI PRD.
Lyn coimmunoprecipitated with endogenous platelet GPVI both under resting conditions and following receptor stimulation with convulxin (Fig. S4D). In contrast, Fyn was observed to associate with GPVI receptors following receptor stimulation but to have little or no association with GPVI in the resting platelet (see Fig. S4D). The Fyn SH3 domain was identified in the phage display screen, although as a rare binding partner compared to Lyn or Hck (see Table S1). Src and Burton's tyrosine kinase (Btk) did not coimmunoprecipitate with GPVI (see Fig. S4D). Hck is not expressed in platelets (12), suggesting that Hck is not a physiologically relevant GPVI binding partner. These studies identify Lyn as the constitutive GPVI PRD binding partner in platelets.
GPVI PRD Binding Directly Activates the Lyn and Hck Kinases.
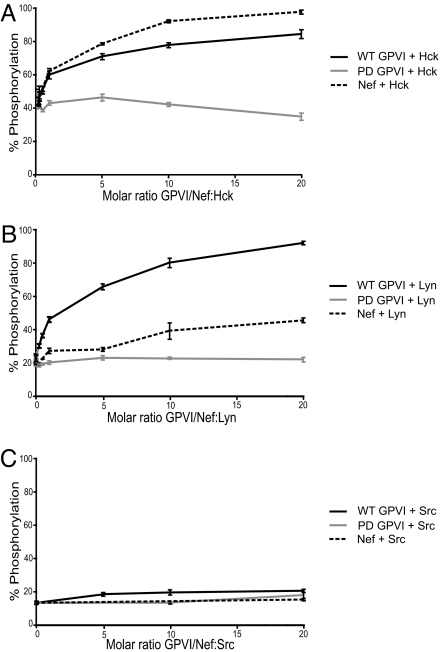
Constitutive recruitment of Lyn to GPVI via an SH3-domain/PRD interaction could accelerate GPVI signaling merely by placing the kinase in close proximity to its substrate, the FcRγ ITAM. Alternatively, because SFKs can be activated via SH3-domain displacement (18), GPVI PRD-Lyn SH3 domain binding could directly regulate the kinase activity of Lyn. Hck and Lyn are efficiently activated through SH3 binding by the HIV-1 protein, Nef (18, 19), and Hck and Lyn have been identified as the preferred binding partners for the Nef SH3 domain using the same SH3 phage display screen as that described above (17). We therefore reasoned that the GPVI PRD might activate Hck and Lyn through a mechanism similar to that of Nef.
To determine if the GPVI PRD can directly activate Hck and Lyn, we used an in vitro kinase assay that has previously demonstrated direct Nef activation of these kinases (19). Purified kinase activity was then assayed in vitro with a peptide substrate, either alone or in the presence of increasing concentrations of purified recombinant GST-WT GPVI, GST-PD GPVI, or Nef protein. WT GPVI fusion protein activated Hck to a level comparable to that of Nef at similar molar ratios (Fig. 4A). WT GPVI also induced strong activation of Lyn, even at low GPVI:Lyn molar ratios, to a significantly greater extent than did Nef under the same conditions (Fig. 4B). PD GPVI fusion proteins were unable to activate Hck or Lyn even at high molar excess, demonstrating the requirement for the GPVI PRD to mediate kinase activation. Consistent with the binding studies described above and with previous studies (19), neither WT GPVI nor Nef were capable of inducing c-Src activation in vitro (Fig. 4C). These findings demonstrate that GPVI functions as a direct activator of Hck and Lyn kinase activity, presumably via displacement of the intramolecular negative regulatory SH3/linker interaction within these kinases by the GPVI PRD.
Fig. 4.
GPVI directly activates Lyn and Hck in a PRD-dependent manner. Recombinant Hck (A), Lyn (B), and c-Src (C) were purified from Sf9 insect cells in an inactive state and assayed for kinase activity with a peptide substrate in vitro in the presence of increasing concentrations of purified GST-WT GPVI, GST-PD GPVI, or recombinant HIV-1 Nef. Each condition was repeated in quadruplicate, and the extent of phosphorylation is expressed as mean percent-phosphorylation relative to a control phosphopeptide ± SD. The results shown are representative of two independent experiments.
GPVI-Bound Lyn Is Held in the Active State in Resting Platelets.
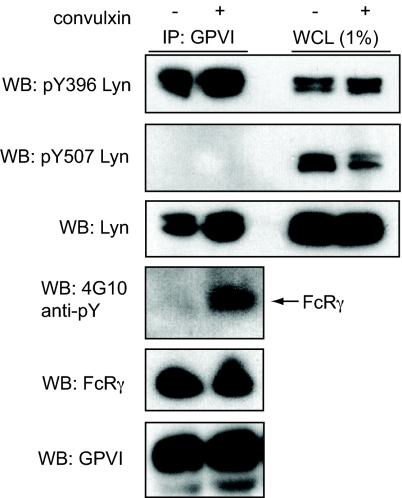
To determine if GPVI-bound Lyn is found in an activated state in vivo, we immunoprecipitated GPVI from human platelets under resting and stimulated conditions and used phospho-specific antibodies to assess the kinase activation state. The SFK that coimmunoprecipitated with GPVI under both resting and stimulated conditions was phosphorylated at the activation loop, indicating that it was in the active state (Fig. 5). More strikingly, none of the GPVI-bound Lyn was found to be phosphorylated on its negative regulatory tail, despite a large amount of cellular Lyn that was found in this inactive state (see Fig. 5). To ensure that the detection of activated Lyn associated with GPVI in resting platelets was not caused by low-level platelet activation during cell harvest, we assessed the phosphorylation state of the FcRγ ITAM tyrosines. Robust FcRγ tyrosine phosphorylation was observed following 15 s of convulxin stimulation but none was observed in resting platelets (see Fig. 5), although small amounts of basal FcRγ phosphorylation have been reported previously (20). These findings demonstrate that the Lyn associated with GPVI receptors in resting platelets is held in the active state but that the FcRγ ITAM is not phosphorylated in the absence of receptor ligand.
Fig. 5.
GPVI-bound Lyn is held in the active state in unstimulated platelets. Washed human platelets were left unstimulated or stimulated with 3-nM convulxin for 15 s, lysed, and proteins immunoprecipitated (IP) with GPVI were immunoblotted (WB) with phosphospecific antibodies for Lyn. pY396 indicates Lyn phosphorylated at the activation loop, and pY507 indicates Lyn phosphorylated at the negative regulatory site. Phospho-Lyn Y396 antibody reacts with other SFKs phosphorylated at their activation loop, but the characteristic doublet at 53 kDa and 56 kDa identifies Lyn as the predominant species. Probing with 4G10 was used to detect phosphorylated FcRγ chain. An immunoblot of whole cell lysate (WCL), loaded at 1% of GPVI immunoprecipitation input, is also shown. Blots depicted are representative of three independent experiments.
Lyn-Deficient Platelets Exhibit the Delayed GPVI Receptor Signaling and Defective Adhesion to Collagen Under Flow Characteristic of PD GPVI Platelets.
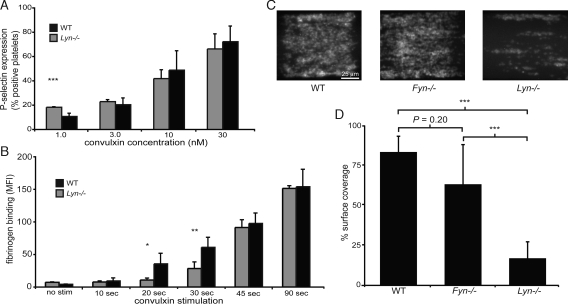
The studies described above suggest that the rapid early signal transduction conferred by the GPVI PRD is dependent upon association with active Lyn. To test the dependence of this mechanism on Lyn, we examined GPVI signaling and collagen adhesion under flow in mouse platelets lacking Lyn or Fyn. As observed for PD GPVI platelets (see Fig. 1A), the dose-response of Lyn-deficient platelets stimulated with convulxin for 10 min was similar to that of wild-type platelets (Fig. 6A), although at the lowest dose of convulxin stimulation a gain of function was observed in Lyn-deficient platelets consistent with previous findings (21). However, when platelet activation responses were examined at short time-points following GPVI receptor stimulation, a delay in the activation of Lyn-deficient platelets similar to that of PD GPVI platelets was observed (Fig. 6B). In contrast, Fyn-deficient platelets did not demonstrate a significant delay in GPVI-induced platelet activation at short time points (Fig. S5). However, Fyn-deficient platelets exhibited a defect in activation following convulxin stimulation for 10 min, as previously reported (see Fig. S5) (21). Like PD GPVI platelets, Lyn-deficient, but not Fyn-deficient, platelets exhibited a significant defect in adhesion to collagen under flow (Fig. 6 C and D). These studies reveal that rapid GPVI signals require both the GPVI PRD and Lyn kinase. Similar to loss of the GPVI PRD, loss of Lyn confers delayed GPVI receptor signaling and defective adhesion to collagen under flow.
Fig. 6.
Lyn-deficient platelets exhibit delayed GPVI-induced platelet activation and defective adhesion to collagen under flow. (A) Platelets from Lyn−/− mice or wild-type littermate controls (WT) were stimulated with the indicated concentrations of convulxin for 10 min, and P-selectin expression was determined by flow ctyometry. (B) Time-course of platelet fibrinogen binding following convulxin stimulation. Platelets were stimulated with 10-nM convulxin in the presence of Alexa-Fluor 647-conjugated fibrinogen for times indicated, fixed, and analyzed by flow cytometry. *, P < 0.05; **, P < 0.01; ***, P < 0.005; error bars indicate mean ± SD; n = 4 to 6 experiments using three mice of each genotype for each concentration of convulxin or time point. (C) Lyn-deficient platelets exhibit defective adhesion to collagen under flow. Blood from Lyn−/−, Fyn−/−, or wild-type control animals was flowed over a patterned type-I collagen strip at a shear rate of 1,000 s−1. Images depict adhesion of fluorescently labeled platelets after 5 min of flow. The direction of flow is from left to right. (D) Quantitation of percent of surface area coverage following 5 min of flow. ***, P < 0.005 for surface area coverage between WT and Lyn−/− platelets and between Fyn−/− and Lyn−/− platelets; error bars indicate mean ± SD; n = 6 to 8 experiments using four mice of each genotype.
Discussion
Immune-type receptors are expressed by nonimmune cells where they have adopted new functions, but the molecular adaptations that have fostered such novel roles remain unknown. In the present study, we identify the binding and direct activation of Lyn by the PRD of the GPVI receptor as a molecular mechanism by which platelets accelerate immune receptor signaling to adapt it to hemostasis, a biological response that must take place within seconds. Previous studies have demonstrated that the GPVI PRD binds SFKs and contributes to GPVI signaling in cell lines (11, 12), but the molecular consequences of GPVI PRD interaction with SFKs and the functional role of this interaction in platelet responses was unknown. Our findings demonstrate that the PRD is not essential for GPVI signaling, but instead primes the receptor for rapid platelet activation required for efficient adhesion to collagen in the setting of blood flow by binding and directly activating Lyn.
This study provides biological insight into the molecular regulation of SFKs through identification of an unexpected mechanism by which the GPVI PRD accelerates immune receptor-induced cellular activation. The GPVI PRD selectively binds the SH3 domains of Lyn and Hck and directly activates these kinases in vitro. Studies of Hck activation by Nef support a model in which binding of Nef to the SH3 domain of Hck displaces the SH3 domain from an intramolecular association with a polyproline II helix in the SFK linker that holds the kinase in an inactive state (22). Although studies of Nef have demonstrated SH3 domain displacement to be a potent mechanism of SFK activation that likely contributes to HIV virulence (18, 19), the extent and purpose to which endogenous proteins activate SFKs by this mechanism is not clear. Our studies suggest that GPVI activation of Lyn occurs through a Nef-type SH3 displacement mechanism, although structural studies comparing SFK activation by these two proline-rich polypeptides are needed to determine whether or not they are molecularly identical.
While both Lyn and Fyn are required for optimal GPVI signaling (21), our studies suggest Lyn, but not Fyn, drives the explosive GPVI signaling required for adhesion to collagen under flow. The preference of the GPVI PRD for binding Lyn over Fyn and the observation that Lyn, but not Fyn, binds GPVI receptors in resting platelets support a division of labor in which PRD-bound Lyn drives initial GPVI-induced platelet activation and Fyn participates in more sustained GPVI signaling. This model of SFK signaling downstream of GPVI is supported by biochemical studies demonstrating a delay in FcRγ, Syk, and PLCγ2 phosphorylation following GPVI stimulation of Lyn-deficient platelets. Consistent with this model, PD GPVI receptors exhibit delayed Syk phosphorylation and calcium signaling in RBL-2H3 cells. A final question raised by these findings is whether GPVI-bound activated Lyn affects other Lyn substrates in the platelet. There are roughly 1,500 GPVI receptors expressed on the platelet surface (23), an amount that is likely to be considerably lower than the total amount of intracellular Lyn. We therefore speculate that GPVI-induced activation of Lyn is unlikely to significantly affect the phosphorylation of other Lyn substrates because of both physical sequestration of the activated kinase and the relatively small effect on total Lyn activation state.
Our studies also provide unexpected insight into ITAM activation, a primary step in all immune receptor signal transduction. An apparent paradox raised by our findings is how the GPVI receptor can constitutively associate with activated Lyn kinase yet not phosphorylate the ITAM of its coreceptor FcRγ and activate the resting platelet. A recent study of ITAM phosphorylation by the T-cell receptor has revealed that the CD3ε ITAM is embedded in the lipid bi-layer of the cell membrane and sequestered from activating SFKs in unstimulated cells (24). Through a yet undefined mechanism, T-cell receptor ligation and clustering result in release of the ITAM from the membrane, exposure to SFKs, and initiation of downstream receptor signaling (24). These studies suggest that a similar sequestration of the FcRγ ITAM in the cell membrane may allow activated Lyn to be held by GPVI in the immediate proximity of the FcRγ subunit without initiating signal transduction before receptor-ligand interaction (Fig. S6). Future studies of GPVI-FcRγ signaling in platelets, such as mutation of the acidic membrane proximal residues in FcRγ proposed to mediate lipid interaction and separation from the GPVI receptor tail (24), may be informative regarding both the basis of signal acceleration by the GPVI PRD and ITAM signaling by immune receptors.
It is intriguing to consider the appearance of the GPVI PRD in the context of platelet and immune receptor evolution. Adaptive immunity and ITAM signaling receptors emerged over 500 million years ago with jawed vertebrates (25), while platelets emerged more recently in mammals 300 million years ago (26). The GPVI receptor is encoded by a gene in the leukocyte receptor complex, a region of the mammalian genome that underwent rapid evolution between egg-laying mammals, such as the platypus, and placental and marsupial mammals (27, 28). Consistent with its location within the reorganized mammalian leukocyte receptor complex, homology-based searches reveal clear GP6 orthologues in the genome of placental and marsupial mammals but not in the platypus or avian genomes. Thus, the GPVI receptor appears to have evolved with other leukocyte receptor complex immune receptors after platelets had already evolved in mammals. Alignment of the predicted opossum and human GPVI receptor amino acid sequences reveals the presence of a highly conserved intracellular PRD that is present in all known GPVI receptors (see Fig. S1A). These observations suggest that acceleration of immune receptor signaling by the PRD may have been instrumental in the evolution of GPVI as a platelet collagen receptor.
Materials and Methods
Platelet Stimulation Assays.
In dose-response assays, platelets were stimulated in the presence of anti-murine P-selectin antibody (BD Biosciences). In time course assays, platelets were stimulated with 10 nM convulxin (Alexis) or 10 μg/ml CRP (obtained from R. Farndale) in the presence of 100 μg/ml Alexa-Fluor 647-conjugated human fibrinogen (Invitrogen) (14).
In Vitro Kinase Assays.
Production of recombinant HIV-1 Nef and tyrosine kinase assays were performed in 384-well plates using the Z'-Lyte kinase assay system and the Tyr-2 peptide substrate (Invitrogen) (19).
For additional materials and methods, see the SI Text.
Supplementary Material
Acknowledgments.
This work was supported by The University of Pennsylvania School of Medicine Department of Medicine, National Institute of Health training Grant T32HL07439–30 (to A.A.S.) and Grant CA81398 (to T.E.S.), and National Heart Lung and Blood Institute training Grant T32HL007971 (to S.F.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906436106/DCSupplemental.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 3.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112:3555–3562. doi: 10.1182/blood-2008-04-144758. [DOI] [PubMed] [Google Scholar]
- 4.Clemetson JM, Polgar J, Magnenat E, Wells TN, Clemetson KJ. The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcalphaR and the natural killer receptors. J Biol Chem. 1999;274:29019–29024. doi: 10.1074/jbc.274.41.29019. [DOI] [PubMed] [Google Scholar]
- 5.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 6.Nieswandt B, Watson SP. Platelet-collagen interaction: Is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 7.Nieswandt B, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith MA, Weiss A. Early signal transduction by the antigen receptor without commitment to T cell activation. Science. 1988;240:1029–1031. doi: 10.1126/science.3259335. [DOI] [PubMed] [Google Scholar]
- 9.Locke D, Liu C, Peng X, Chen H, Kahn ML. Fc Rg-independent signaling by the platelet collagen receptor glycoprotein VI. J Biol Chem. 2003;278:15441–15448. doi: 10.1074/jbc.M212338200. [DOI] [PubMed] [Google Scholar]
- 10.Rickles RJ, et al. Identification of Src, Fyn, Lyn, PI3K and Abl SH3 domain ligands using phage display libraries. EMBO J. 1994;13:5598–5604. doi: 10.1002/j.1460-2075.1994.tb06897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki-Inoue K, et al. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. J Biol Chem. 2002;277:21561–21566. doi: 10.1074/jbc.M201012200. [DOI] [PubMed] [Google Scholar]
- 12.Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J Exp Med. 1998;188:267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bori-Sanz T, Inoue KS, Berndt MC, Watson SP, Tulasne D. Delineation of the region in the glycoprotein VI tail required for association with the Fc receptor gamma-chain. J Biol Chem. 2003;278:35914–35922. doi: 10.1074/jbc.M301826200. [DOI] [PubMed] [Google Scholar]
- 14.Zou Z, Chen H, Schmaier AA, Hynes RO, Kahn ML. Structure-function analysis reveals discrete beta3 integrin inside-out and outside-in signaling pathways in platelets. Blood. 2007;109:3284–3290. doi: 10.1182/blood-2006-10-051664. [DOI] [PubMed] [Google Scholar]
- 15.Asselin J, et al. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C gamma2 in platelets independent of the integrin alpha2beta1. Blood. 1997;89:1235–1242. [PubMed] [Google Scholar]
- 16.Sarratt KL, et al. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106:1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karkkainen S, et al. Identification of preferred protein interactions by phage-display of the human Src homology-3 proteome. EMBO Rep. 2006;7:186–191. doi: 10.1038/sj.embor.7400596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moarefi I, et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 19.Trible RP, Emert-Sedlak L, Smithgall TE. HIV-1 Nef selectively activates Src family kinases Hck, Lyn, and c-Src through direct SH3 domain interaction. J Biol Chem. 2006;281:27029–27038. doi: 10.1074/jbc.M601128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori J, et al. G6b-B inhibits constitutive and agonist-induced signaling by glycoprotein VI and CLEC-2. J Biol Chem. 2008;283:35419–35427. doi: 10.1074/jbc.M806895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quek LS, et al. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–4253. [PubMed] [Google Scholar]
- 22.Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001;105:115–126. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Locke D, Liu Y, Liu C, Kahn ML. The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J Biol Chem. 2002;277:3011–3019. doi: 10.1074/jbc.M109714200. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litman GW, Cannon JP, Dishaw LJ. Reconstructing immune phylogeny: new perspectives. Nat Rev Immunol. 2005;5:866–879. doi: 10.1038/nri1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelson AD. Platelets. New York: Elsevier; 2006. p. 1376. [Google Scholar]
- 27.Laun K, et al. The leukocyte receptor complex in chicken is characterized by massive expansion and diversification of immunoglobulin-like Loci. PLoS Genet. 2006;2:e73. doi: 10.1371/journal.pgen.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren WC, et al. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.