Abstract
Heat shock protein 90-α (Hsp90α) is an intracellular molecular chaperone. However, it can also be secreted with the underlying regulatory mechanism remaining far from clear. Here we show that the secreted Hsp90α is a C-terminal truncated form and its secretion is regulated by the C-terminal EEVD motif via interacting with proteins containing tetratricopeptide repeat domains. We also demonstrate that secretion of Hsp90α is determined by the phosphorylation status at residue Thr-90, regulated by protein kinase A and protein phosphatase 5. We further demonstrate that the secretion of Hsp90α is a prerequisite for its proinvasiveness function and blocking the secreted Hsp90α results in significant inhibition of tumor metastasis. Meanwhile, the level of plasma Hsp90α is positively correlated with tumor malignancy in clinical cancer patients. In sum, our results reveal the regulatory mechanism of Hsp90α secretion, and its function in tumor invasiveness, indicating it can be a promising diagnostic marker for tumor malignancy in clinical application.
Keywords: heat shock protein 90-α, extracellular, nonconventional protein secretion, MMP-2, tumor marker
The eukaryotic heat shock protein 90-α (Hsp90α) is an essential and ubiquitous molecular chaperone with remarkably versatile functions involved in homeostatic control under both normal and stress conditions (1, 2). Noticeably, over 100 Hsp90α client proteins identified so far are typically associated with the cellular signal transduction pathways (3–5). Due to its key roles in modulating the signal transduction, especially in tumor cells, Hsp90α has become a novel therapeutic target in cancer therapy. The inhibitors of Hsp90α, notably Geldanamycine and its derivatives, exhibit very potent antitumor effect (6–8).
As one of the most abundant cellular proteins (approximately 1% of total proteins), Hsp90α mainly resides in the cytosol, and previous studies about Hsp90α have mostly focused on its cytosolic functions. Also, although less studied, Hsp90α can be released to the extracellular space (9–11). The existence of extracellular Hsp90α has long been observed and its secretion is considered to be in an unconventional way due to the lack of N-terminal classic secretion signal peptide (12, 13). However, the regulatory mechanism of Hsp90α secretion, such as what signaling cascades and direct regulators are involved in this process, is still poorly understood. More recently, it was discovered that Hsp90α secretion was enhanced under stress conditions such as hypoxia and oxidative stress (13–15). The question then arises of how those different stimuli converge to stimulate Hsp90α secretion.
On the other hand, it was reported recently that secreted Hsp90α by fibrosarcoma tumor cells could interact with matrix metalloproteinase-2 (MMP-2) and thus facilitate the maturation of MMP-2, promoting tumor invasiveness (10). MMP-2 is a member of the matrix metalloproteinases, dysregulation of which has been linked with diseases such as tumors (16). However, it is still unclear whether the proinvasive function of Hsp90α is mainly attributed by the secreted Hsp90α; whether or not the secreted Hsp90α mainly relies on MMP-2 to exert its pro-invasive function; and whether the secreted Hsp90α is linked with tumor malignancy.
To answer these questions, we investigated the secretion of Hsp90α by tumor cells and the underlying regulatory mechanism of this process. We also explored the relationship between Hsp90α and MMP-2, as well as the function of secreted Hsp90α in tumor invasiveness and the correlation of its level in plasma with tumor malignancy.
Results
The C-Terminal EEVD Motif Interacts with Proteins Containing Tetratricopeptide Repeat (TPR) Domains to Regulate Hsp90α Secretion.
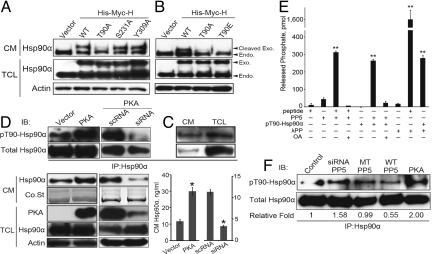
To elucidate the regulatory mechanism of Hsp90α secretion, we took the strategy of studying the secretion behavior of exogenously overexpressed Hsp90α. The full-length human Hsp90α was constructed with N-terminal Myc and C-terminal Myc-His tags (hereafter referred to as Myc-H) for detection (Fig. S1). Very unexpectedly, the secreted exogenous Hsp90α in the conditioned media (CM) could only be detected with the anti-Myc antibody but not the anti-His antibody, while the exogenous Hsp90α in the total cell lysate (TCL) could be detected by both (Fig. 1A, lanes 1–2, 4–5), which implies that the secreted Hsp90α is cleaved somewhere in the C terminus. To demonstrate this hypothesis, we added another His tag to the N terminus of the Myc-H, hereafter referred as His-Myc-H (Wild Type, WT-Hsp90α) (Fig. S1), and found that both Myc and His tags could be detected in the secreted exogenous Hsp90α (Fig. 1A, lanes 3 and 6). We also observed that in the CM, the difference in the molecular weights between the exogenous and endogenous Hsp90α was much smaller than that in the TCL (Fig. 1A, Lower), which indicates that the secreted Hsp90α is truncated. To determine the location of this cleavage event, we constructed a plasmid expressing an Hsp90α-GST fusion protein, hereafter referred as H-GST (Fig. S1), and found that the GST cleaved from the intact fusion protein could only be detected in the TCL (Fig. S2A), which suggests that this cleavage occurs intracellularly. To further elucidate the cleavage mechanism, we made several C-terminal deletion mutants of Hsp90α and found that mutants lacking the C-terminal 25, 12, 8, or 4 residues could be secreted directly without the cleavage of the C-terminal tags (Fig. 1B and S2B). We then performed point mutations at the last 4 residues of EEVD (EE->AA, VD->AA, EEVD->All Ala) and found that they were all secreted without the cleavage (Fig. 1B) while mutations ahead of this motif had no such effect. These observations demonstrate that the cleavage is mediated by the last 4 residues, namely the EEVD motif. We then made a specific polyclonal antibody against MEEVD and found that it could only recognize the TCL Hsp90α but not the CM Hsp90α, which suggests that this motif is deleted or at least partially deleted in the secreted Hsp90α (Fig. S2C).
Fig. 1.
The C-terminal EEVD motif regulates Hsp90α secretion. (A) Secretion of Hsp90α in MCF-7: samples were separated with 7.5% SDS/PAGE and were blotted with anti-Myc, anti-His, and anti-Hsp90α antibodies, respectively. CM: conditioned media; TCL: total cell lysate. Exo.: exogenous Hsp90α; Cleaved Exo.: secreted exogenous Hsp90α in the cleaved form; Endo.: endogenous Hsp90α. (B) Secretion of the EEVD motif mutants of Hsp90α in MCF-7. (C) Secretion of Hsp90α upon siRNA treatment of indicated genes in MCF-7 detected by ELISA (Upper) and Western blotting (Lower). Co.St.: Coomassie Blue staining as a loading control for CM. (D) Secretion of Hsp90α upon PP5 siRNA treatment in MCF-7 with 3 independent siRNA duplexes detected by ELISA (Upper) and Western blotting (Lower). (E) Secretion of Hsp90α upon overexpression of the WT or indicated mutants of PP5 detected by ELISA (Upper) and Western blotting (Lower). (F) Secretion of Hsp90α upon overexpression of the TPR domain or full length of PP5 in MCF-7 detected by ELISA (Upper) and Western blotting (Lower). FL: full length. Error bars represent SD (n = 3); P value: Student's t test; *, P < 0.05; #, P > 0.05.
The EEVD motif of Hsp90α can specifically interact with a family of proteins containing the TPR domains (17). The interactions of these proteins with Hsp90α have been studied extensively, but none of them was reported to be linked with Hsp90α secretion. Since we found that the EEVD motif was involved in regulating Hsp90α secretion, we then speculated that whether these TPR-domain-containing proteins were also involved in this process. To test this hypothesis, we chose 4 of these proteins—CHIP, cyclophilin 40 (Cyp 40), protein phosphatase 5 (PP5), and FK506-binding protein 52 (FKBP52)—as representative proteins due to their different functions with Hsp90α (17–20). We found that knocking down any of these genes expression using siRNA resulted in increased Hsp90α secretion to different extents (Fig. 1C and Fig. S2H). The efficiency of siRNA was examined by qRT-PCR and Western blotting (Fig. S2 D–H). This result indicates that the regulation of Hsp90α secretion by these proteins is a generic mechanism. Since knocking down the expression of PP5 resulted in the most profound increase of Hsp90α secretion (Fig. 1C), we next chose PP5 to confirm this TPR-EEVD inhibitory effect. Treatment of 3 independent and effective siRNAs of PP5 on 3 different cell lines all resulted in increased Hsp90α secretion (Fig. 1D and Fig. S2H). Consistently, overexpression of the WT PP5 inhibited Hsp90α secretion, while overexpression of the PP5 mutant K32A/R101A, which could not interact with Hsp90α (Fig. S3) (21), showed no inhibitory effect (Fig. 1E). Another enzymatically inactive mutant of PP5-H304A (22), whose interaction with Hsp90α was not affected, behaved the same as the WT PP5 (Fig. 1E and Fig. S3). Moreover, overexpression of the PP5 TPR domain alone also dramatically attenuated Hsp90α secretion (Fig. 1F). Collectively, we propose that EEVD acts as an intrinsic docking motif recognized by the TPR-containing proteins, trapping Hsp90α inside the cell (namely EEVD-TPR occupancy) and making it unavailable for secretion. Secretion of Hsp90α requires a presently unidentified mechanism to remove this EEVD motif.
Phosphorylation at Residue Thr-90 Regulates Hsp90α Secretion.
Secretion of Hsp90α is believed to be a highly regulated process (12–15). Indeed, we observed that both the cell membrane Hsp90α and secreted Hsp90α were significantly decreased in starved cells compared with proliferating cells (Fig. S4 A–C), and this could be restimulated (i) by the treatment of cytokines such as VEGF, SDF and PDGF (Fig. S4A) and (ii) under stress conditions such as hypoxia (Fig. S4B). Since the prosurvival or stress conditions normally result in the activation of signaling cascades, especially kinases, we then wondered whether the phosphorylation of Hsp90α could regulate its secretion. We chose Scansite (23) to predict the phosphorylation sites of Hsp90α, and also referenced previous reports (24, 25). Then we used the construct of WT Hsp90α to make the mutants T90A, S231A, and Y309A to mimic loss of function. We found that the T90A mutant could not be secreted, while the secretion of S231A and Y309A mutants behaved the same as the WT Hsp90α (Fig. 2A). We then examined the secretion behavior of a phosphorylation mimic mutant T90E and found that this mutant could not be secreted either (Fig. 2B). This suggests that the mutation of threonine to glutamic acid residue may not efficiently mimic the conformational change induced by the naturally added phosphate group. The fact that the T90E mutant cannot be secreted further strengthens the specific role of phosphorylation at Thr-90 in determining Hsp90α secretion. Besides, a double-mutant (CΔ12&T90A) could not be secreted either, even in the absence of the EEVD motif (Fig. S5A), which indicates that phosphorylation at residue Thr-90 is a prerequisite for Hsp90α secretion. This is also consistent with the previous report that phosphorylation at Thr-90 regulates the translocation of Hsp90α to the cell membrane (25). More importantly, using an antibody which can specifically recognize the phosphorylation status of Thr-90 at Hsp90α (25), we found that the secreted Hsp90α in the CM was indeed phosphorylated at Thr-90 (indicated as pT90-Hsp90α) (Fig. 2C).
Fig. 2.
Phosphorylation at residue Thr-90 regulates Hsp90α secretion. (A) Secretion of the WT Hsp90α and phosphorylation-site mutated constructs: T90A, S231A and Y309A. (B) Secretion of the Hsp90α phosphorylation-site mutated constructs: T90A and T90E. Exo.: exogenous Hsp90α; Cleaved Exo.: secreted exogenous Hsp90α in the cleaved form; Endo.: endogenous Hsp90α. (C) Thr-90-phosphorylation status of Hsp90α in the CM and TCL. pT90-Hsp90α was detected using anti-phospho-(Ser/Thr) PKA substrate antibody. (D) Secretion and Thr-90-phosphorylation status of Hsp90α upon overexpression of Flag-tagged PKA (Left) or upon siRNA treatment (Right) detected by Western blotting and ELISA. PKA was detected by the anti-Flag antibody in the left panel and by the PKA antibody in the right panel. Error bars represent SD (n = 3); P value: Student's t test; *, P < 0.05. (E) Phosphatase activity assay of PP5 in vitro. peptide: RRA(pT)VA, a standard substrate for the Ser/Thr-phosphatases, was used to verify the activity of PP5; λPP: non-specific protein phosphatase as positive control; OA: Okadaic Acid (1 μM); error bars represent SD (n = 3); P value: Student's t test; **, P < 0.01. (F) Thr-90-phosphorylation status of Hsp90α upon overexpression of the WT or MT PP5, or knocking-down of PP5 using RNA interference. MT PP5: K32A/R101A PP5; PKA served as a positive control.
Since phosphorylation of Hsp90α at Thr-90 is catalyzed by protein kinase A (PKA) (Fig. S5B) (25), we next examined the effect of PKA in regulating Hsp90α secretion by 2 means. Firstly, we found that overexpression of PKA resulted in a significant increase of the cytosolic level of pT90-Hsp90α compared with the control group and consistently resulted in an increased Hsp90α secretion in the CM (Fig. 2D). Secondly, we found that siRNA-mediated specific PKA knocking-down could significantly decrease the cytosolic level of pT90-Hsp90α compared with the scRNA control group, and consequently, a decreased Hsp90α secretion in the CM was observed (Fig. 2D). Treatment of H-89, a specific inhibitor of PKA, also led to the similar results (Fig. S4D). These observations together strongly support PKA as direct positive regulator of Hsp90α secretion acting at the Thr-90 phosphorylation site. This also explains the aforementioned observations that cytokines or hypoxia stress could stimulate Hsp90α secretion (Fig. S4) since those stimuli normally result in the activation of PKA (26, 27).
Protein phosphorylation is a reversible process regulated by kinases and protein phosphatases. We have demonstrated PKA is involved in Thr-90 phosphorylation. We next wondered which phosphatase was responsible for the dephosphorylation. Since the overexpression of TPR domain could inhibit the secretion of Hsp90α just like WT PP5 (Fig. 1F), the increased TPR-EEVD interaction caused by the overexpression of the enzymatically inactive PP5 mutant H304A is enough to fully achieve the similar inhibitory effect. Therefore, although overexpression of H304A can inhibit Hsp90α secretion (Fig. 1E), we cannot exclude the possibility that the intrinsic phosphatase activity of PP5 might be also involved in the regulation of Hsp90α secretion. To test whether PP5 is responsible for the dephosphorylation of pT90-Hsp90α, we examined the phosphatase activity of PP5 toward pT90-Hsp90α in vitro and in vivo. First, we found that incubation of PP5 with the in vitro phosphorylated pT90-Hsp90α resulted in an obvious release of phosphate (Fig. 2E), which could be inhibited by 1 μM okadaic acid—an inhibitor of the Ser/Thr-phosphatases (19)—indicating that PP5 can specifically dephosphorylate pT90-Hsp90α in vitro. Meanwhile, the nonspecific λ-protein phosphatase also led to increased release of phosphate, which served as a positive control (Fig. 2E). Secondly, we found that overexpression of the WT PP5 could significantly decrease the cytosolic level of pT90-Hsp90α compared with the control group, while the PP5 mutant K32A/R101A, which could not interact with Hsp90α (Fig. S3), had no such effect (Fig. 2F). On the other hand, knocking down the endogenous PP5 expression resulted in the cytosolic level of pT90-Hsp90α increased to almost 1.6-fold compared with the control group (Fig. 2F). These results suggest that PP5 can directly dephosphorylate Hsp90α at Thr-90 in vivo.
Next, we asked whether there is any relationship between the Thr-90 phosphorylation-dependent Hsp90α secretion and the inhibitory effect of PP5 through TPR/EEVD interaction. To address this issue, we compared the interaction of PP5 with WT Hsp90α and the Thr-90 (non)phosphomimic mutants, T90A and T90E. Their interactions were examined using reciprocal coimmunoprecipitations in MCF-7 cells cotransfected with PP5 and WT, T90A, or T90E mutants of Hsp90α, respectively (Fig. S6A). Interestingly, the nonphosphomimic mutant T90A showed a remarkably decreased while the phosphomimic mutant T90E showed a dramatically increased interaction with PP5, compared with the WT Hsp90α (Fig. S6 B and C). Clearly, PP5 shows a preference to Thr-90-phosphorylated Hsp90α, which gives a direct link between the TPR/EEVD interaction of PP5 with Hsp90α and the Thr-90-phosphorylation status. In sum, these results provide strong evidences that PP5 is an endogenous negative regulator of Hsp90α secretion and it has dual roles in inhibiting this process.
Secreted Hsp90α Promotes Tumor Invasiveness via MMP-2 and Blockade of the Secreted Hsp90α Inhibits Tumor Invasiveness.
Secreted Hsp90α has been linked to tumor invasiveness (10, 28). However, the underlying mechanism is not clear so far. We next systematically examined the level of secreted Hsp90α, and its biological function in tumors. We first examined 5 breast epithelial tumor cell lines with different malignancies (29) and found that the level of secreted Hsp90α in more malignant tumor cell lines was much higher than that of the less malignant ones, while the cytosolic level of Hsp90α was almost the same (Fig. S7). More interestingly, the expression level of PP5 was reversely correlated with the level of secreted Hsp90α (Fig. S7). This is consistent with our conclusion that PP5 is an endogenous negative regulator of Hsp90α secretion (Fig. 1–2).
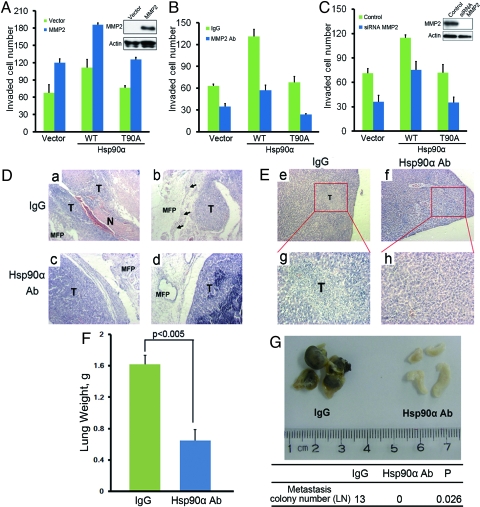
To further explore the relationship of secreted Hsp90α with tumor invasiveness, we then tested the in vivo effect of 2 constructs, WT and T90A mutant of Hsp90α. We found that overexpression of the WT Hsp90α stimulated the invasiveness of MDA-MB-231 cells in a Matrigel invasion assay, while the T90A mutant, which could not be secreted, showed no such stimulatory effect (Fig. 3A). Secreted Hsp90α by tumor cells can interact with and facilitate the activation of MMP-2, thus promoting tumor invasiveness (10). We next examined whether the proinvasiveness function of secreted Hsp90α relied on MMP-2. We found that cotransfection of WT Hsp90α, but not the T90A mutant, with MMP-2 showed a synergistic effect on stimulating tumor cell invasion (Fig. 3A). In contrast, the treatment of MMP-2 antibody or knocking down MMP2 using siRNA could abolish the proinvasiveness effect of WT Hsp90α overexpression (Fig. 3 B–C). These observations demonstrate that the secretion of Hsp90α is a prerequisite for this protein to exert its proinvasiveness function which is dependent on MMP-2.
Fig. 3.
Secreted Hsp90α promotes tumor invasiveness: in vitro and in vivo. (A–C) Matrigel invasion assay of MDA-MB-231 cells transfected with WT Hsp90α or T90A mutant: cotransfected with MMP2 or control vector (A); treated with IgG or MMP2 antibody (B); treated with MMP2 siRNA or control scRNA (C). Error bars represent SEM (n = 3). (D–G) The effect of Hsp90α antibody on inhibiting tumor invasiveness in vivo. (D–E) The orthotopic breast tumor model (n = 6 mice): H&E-stained sections of primary mammary tumors. Arrows indicate muscular invasion and stromal invasion. (D) MFP: mammary fat pad; N: normal tissue; T: tumor. (E) H&E-stained sections of livers from the tumor-bearing mice, indicating liver metastasis (magnification, a–f: ×100; g–h: ×400). (F–G) The B16/F10 melanoma experimental metastasis model (n = 5 mice): the lung weight of mice treated with IgG or Hsp90α antibody; error bars represent SD (n = 5) (F). (G) Representative images of lymph nodes from the B16/F10 melanoma mice bearing lung metastasis, treated with IgG or Hsp90α antibody. LN: lymph node; P value: Student's t test.
The proinvasiveness function of secreted Hsp90α prompted us to test whether blocking the secreted Hsp90α would result in the inhibition of tumor invasiveness. We prepared a monoclonal antibody (mAb) against Hsp90α to functionally block the secreted Hsp90α. We found that the invasiveness but not the proliferation of MDA-MB-231 cells treated with this mAb was dramatically inhibited in a Matrigel invasion assay compared with the IgG treated control group (Fig. S8 A and B). Then we examined the in vivo proinvasiveness function of secreted Hsp90α. Firstly, we tested this on an orthotopic breast tumor mouse model of MDA-MB-231 cells, in which the breast tumor cells were inoculated into the mammary fat pad of nude mice. The animals were then treated with mAb of Hsp90α or control IgG. After the treatment, the primary tumors and metastasis sites were analyzed. Consistent with the in vitro results, no obvious primary tumor growth difference was observed in the 2 groups (Fig. S8C). However, we found that Hsp90α mAb treatment could potently prevent the stromal invasion in the primary tumors as the primary tumors from Hsp90α mAb treated group showed clear boarders without obvious stromal invasion to the surrounding tissues, while tumors from the IgG treated group showed significant stromal invasion as indicated by the invasion of tumor cells into the surrounding muscles and mammary fat pad (Fig. 3D). Besides, lymph node metastasis (Fig. S8D) and distant metastasis such as liver metastasis (Fig. 3E) were also inhibited in Hsp90α mAb treated group.
Secondly, we tested this on B16/F10 melanoma experimental metastasis mouse model. Similarly, the treatment of Hsp90α mAb significantly inhibited the lung metastasis (Fig. 3F and Fig. S8F). Noticeably, the metastasized tumors in the lungs from Hsp90α mAb treated group were also shown to have clear boarders with the surrounding normal lung tissues, while tumors from the IgG treated group infiltrated into the surrounding normal lung tissues without clear boarders, indicating severe invasion (Fig. S8G). More strikingly, when we examined the lymph nodes of the mice, we found a total of 13 metastatic colonies in the 5 mice treated with IgG, while none was found in the Hsp90α mAb treated group (Fig. 3G and Fig. S8E). These results demonstrate that the secretion of Hsp90α is essential for its proinvasiveness function, and the blockage of secreted Hsp90α can efficiently suppress tumor invasiveness.
Plasma Level of Hsp90α Is Positively Correlated with Tumor Malignancy in Clinic.
The proinvasiveness function of secreted Hsp90α suggests it may serve as an indicator of tumor malignancy. To investigate its clinical relevance, initially, we wondered whether secreted Hsp90α could be examined in the plasma. We found that Hsp90α could be detected exclusively in the plasma of tumor bearing mice, but not normal mice (Fig. 4A). Importantly, the Hsp90α detected in plasma is secreted by tumor cells but not a product of immune cells in circulation (Fig. S8H). Then we examined the plasma from 6 liver cancer patients and found that the levels of plasma Hsp90α were all significantly elevated compared with that from normal people (Fig. 4B). We then established an ELISA to accurately quantify the level of plasma Hsp90α in clinic. We first detected the plasma Hsp90α by ELISA with the anti-EEVD antibody to test whether the plasma Hsp90α was in an EEVD truncated form. The anti-EEVD antibody did not give a distinctive signal between the normal and cancer patient groups, while the anti-Hsp90α antibody detection showed a higher signal in the cancer patient group (Fig. S8J). The fact that elevated plasma Hsp90α of cancer patient cannot be detected by the anti-EEVD antibody confirms that the plasma Hsp90α is also truncated at the C-terminal EEVD motif. Then we used anti-Hsp90α ELISA to quantify and compare the amount of plasma Hsp90α in normal people and cancer patients. We chose 50 ng/ml of plasma Hsp90α as the defining threshold concentration because it was the upper limit of plasma Hsp90α level in normal people examined. We found that the levels of plasma Hsp90α in most cancer patients were statistically higher than the threshold (Fig. 4C), while the levels of plasma Hsp90α in patients with benign tumors were more or less the same with that in normal people (Fig. 4C). These results demonstrate that the increased level of plasma Hsp90α is specific for malignant tumors and is a generic indicator of tumor malignancy. More importantly, by dividing the tumor patients into metastasis and metastasis-free groups, we observed that the levels of plasma Hsp90α in liver or breast tumor patients with metastasis were much higher than that of patients without metastasis (Fig. 4 D and E), further proving that secreted Hsp90α is highly associated with tumor malignancy, especially metastasis. These observations demonstrate that the level of plasma Hsp90α is positively correlated with tumor malignancy and it may be a potential diagnostic and prognostic marker in clinic.
Fig. 4.
Plasma level of Hsp90α is positively correlated with tumor malignancy in clinic. (A) Western blotting of plasma Hsp90α from tumor bearing mice. (B) Western blotting of plasma Hsp90α in normal people and liver cancer patients. (C) The level of plasma Hsp90α in normal people, patients with benign tumors (breast and uterus) and patients with malignant tumors (breast, lung, pancreas, and liver) detected by ELISA. (D–E) The levels of plasma Hsp90α in liver cancer (D) or breast cancer (E) patients with or without metastasis. Patients with confirmed pathology verifications about metastasis were counted for analysis. Error bars represent SD; P value: Student's t test.
Discussion
As the well-known and abundant intracellular chaperone, Hsp90α has been found in the extracellular space for 2 decades (9, 11), but its regulatory mechanism remains largely uninvestigated. In the present study we found that the secretion of Hsp90α is exclusively dependent on the phosphorylation status at residue Thr-90 but not other sites studied here (Ser-231, Tyr-309), and PKA was proven to be the direct regulator. Activation of PKA has been well-accepted to be related with cell's proliferative state (30), and some cytokines and hypoxia stress have been shown to induce the activation of PKA signaling pathway either directly or indirectly (26, 27). More importantly, the observation that the generic inhibitory effect of H-89 on Hsp90α secretion, as well as the decreased Thr-90 phosphorylation and Hsp90α secretion upon PKA knockdown firmly supports the hypothesis that PKA is a critical and direct modulator of Hsp90α secretion. Phosphorylation normally results in conformational change of proteins. The residue Thr-90 of Hsp90α was reported to be partially buried within a local structure (31). We propose that upon phosphorylation, the change of the local charge may lead to the exposure of the local conformational change, which may then be recognized by other yet to be discovered cofactors to initiate the downstream translocation process.
Interestingly, we also found that the C-terminal EEVD motif functions as a docking motif which signals to keep Hsp90α residing in the cytosol, and the secretion of Hsp90α requires removing this motif. It is still unclear what factors are involved in the cleavage process and how the phosphorylated Hsp90α is recognized if conformational change indeed occurs. Recently, Keller et al. (32) found that the unconventional secretion of some proteins (especially those related with inflammation) is actively regulated by caspase-1. This raises the question of whether there are other proteases involved in regulating the unconventional protein secretion and whether this could be generic machinery. Future studies on the identification of these proteases and cofactors will be of great value for better and thorough understanding of Hsp90α secretion.
On the other hand, the EEVD is very unique to Hsp90α, because it acts as an adaptor motif for Hsp90α to interact with a family of proteins containing TPR domains, such as FKBP52, Cyp40, CHIP, and PP5 (17–20). Although the interactions and functions of these TPR-domain containing proteins with Hsp90α have been well documented, none of them has been related to Hsp90α secretion (17–20). In this study, we demonstrate that TPR-EEVD interaction suppresses the Hsp90α secretion. As these TPR-domain containing proteins reside in different microcompartments in the cytosol, we propose that the EEVD docking signal of Hsp90α is recognized by these proteins; therefore, the EEVD-TPR interaction traps Hsp90α in a bound complex form at different locations in the cytosol. The Hsp90α in this complex may not be accessible for other cofactors, thus it becomes unavailable for secretion and stays in cytosol. Nevertheless, more evidences, such as the identification of these cofactors, are needed to prove this proposed model.
Meanwhile, the inhibitory function of PP5 on Hsp90α secretion is investigated in a more detailed way. We demonstrate that PP5, in addition to occupy Hsp90α via its TPR domain, can directly dephosphorylate pT90-Hsp90α thereafter reverse the secretion process stimulated by PKA. More importantly, PP5 shows preference to bind with pT90-Hsp90α, suggesting a link between the TPR-EEVD occupancy and Thr-90 phosphorylation. Although the interaction between EEVD and TPR domain has been well acknowledged, the regulatory mechanism of how these diversified TPR-containing proteins interact with Hsp90α and the resulted physiological consequences are not well documented. To our knowledge, this is the first time to show that the Thr-90-phosphorylation status of Hsp90α affects the interaction of Hsp90α with PP5 and the subsequent biological relevance.
Recently, the presence of Hsp90α on cell surface has been shown to correlate with melanoma progression (33). In our studies, the levels of Hsp90α secreted by breast tumor cell lines are found to be positively correlated with the increased tumor malignancy. Furthermore, we found that the level of plasma Hsp90α in cancer patients is highly correlated with tumor malignancy, especially the metastasis. The association of plasma Hsp90α level with the age, tumor volume, estrogen receptor (ER), and progesterone receptor (PR) of the breast cancer patients were also assessed, and no correlation was found (Fig. S8I), which indicates that Hsp90α is an independent marker for tumor diagnosis and prognosis. Collectively, the level of plasma Hsp90α shows wide-spectrum (in all 4 tumor types examined), tumor type specific (variations among the tumor types), and high correlation (75% in average) with the tumor malignancy, suggesting its promising application for tumor diagnosis.
Inhibition of cell-surface Hsp90α with antibodies or cell-impermeable Hsp90α inhibitors blocks cell motility and invasion in vitro (28, 34). In this study, Hsp90α mAb shows inhibitory effect on primary tumor invasion, tumor metastasis but feeble effect on tumor growth. These data further strengthen the role of secreted Hsp90α in proinvasiveness and also imply that the secreted Hsp90α involves in both regional and distance metastasis, which is mediated by the lymphatic and blood systems (35, 36). Therefore, the regulatory mechanism of secreted Hsp90α on stromal invasion, lymphangiogenesis, angiogenesis, and the association of tumor cells with the vasculatures, which is also called intravasation and extravasation, all merit further investigation.
In summary, our study presented here for the first time elucidates the regulatory mechanism of Hsp90α secretion, and open a new avenue for future studies of Hsp90α secretion. Meanwhile, the proinvasiveness function of the secreted Hsp90α suggests it be an effective target in cancer therapeutics especially in preventing tumor metastasis. The correlation between the levels of plasma Hsp90α with tumor malignancy indicates that it may not only be a promising marker for diagnosis of malignant tumors but also a potential index for prognosis of metastasis.
Methods
Construction of human Hsp90α is described in Fig. S1 and SI Materials and Methods. Site-directed mutagenesis was performed using QuikChange site-directed mutagenesis kit according to the manufacturer's instructions (Stratagene). All constructs and mutants were confirmed by sequencing (Invitrogen). Cells and transfections are described in SI Materials and Methods. siRNA sequences are described in Table S1. Immunoprecipitation, Western blotting, and immunofluoresence analyses were performed in accordance with standard protocols. All animal studies were performed with the approval of the Scientific Investigation Board of Tsinghua University, Beijing, China. The plasma samples of tumor patients were obtained from Beijing Cancer Hospital, Beijing, China, and The First Hospital of Xiamen (Xiamen, China) along with confirmed pathology verifications.
Supplementary Material
Acknowledgments.
We greatly thank the members of the Luo lab for insightful discussion and comments on the manuscript. We also greatly thank Bipo Sun for her contribution as the lab manager. We also thank Jiuyong Xie (University of Manitoba, Winnipeg, Canada) and Duanqing Pei (Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China) for kindly providing the plasmid of PKA and MMP-2 respectively. This study was supported in part by the General Programs of National Natural Science Foundation of China (Grants 30670419 and 30771083), the Major Program of National Natural Science Foundation of China (Grant 30490171), the National High Technology Research and Development Program of China (Grant 2007AA02Z155), and the State Key Development Program for Basic Research of China (Grant 2006CB910305).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908151106/DCSupplemental.
References
- 1.Pearl LH, Prodromou C. Structure and in vivo function of Hsp90. Curr Opin Struct Biol. 2000;10:46–51. doi: 10.1016/s0959-440x(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 2.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 3.McClellan AJ, et al. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 5.Richter K, Buchner J. Hsp90: Chaperoning signal transduction. J Cell Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 6.Clarke PA, et al. Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene. 2000;19:4125–4133. doi: 10.1038/sj.onc.1203753. [DOI] [PubMed] [Google Scholar]
- 7.Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003–4009. [PubMed] [Google Scholar]
- 8.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8(Suppl 4):S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 9.Erkeller-Yuksel FM, Isenberg DA, Dhillon VB, Latchman DS, Lydyard PM. Surface expression of heat shock protein 90 by blood mononuclear cells from patients with systemic lupus erythematosus. J Autoimmun. 1992;5:803–814. doi: 10.1016/0896-8411(92)90194-u. [DOI] [PubMed] [Google Scholar]
- 10.Eustace BK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich SJ, Robinson EA, Appella E. Characterization of a chemically homogeneous tumor antigen from a methylcholanthrene-induced sarcoma, Meth A. Mol Immunol. 1986;23:545–555. doi: 10.1016/0161-5890(86)90118-5. [DOI] [PubMed] [Google Scholar]
- 12.Cheng C-F, et al. Transforming growth factor {alpha} (TGF{alpha})-stimulated secretion of HSP90{alpha}: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGF{beta}-rich environment during wound healing. Mol Cell Biol. 2008;28:3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, et al. Extracellular heat shock protein-90alpha: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007;26:1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao DF, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 15.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 16.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: Biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 17.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 18.Connell P, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 19.Dean DA, et al. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2001;2:6. doi: 10.1186/1471-2121-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–3686. doi: 10.1074/jbc.273.6.3679. [DOI] [PubMed] [Google Scholar]
- 21.Russell LC, Whitt SR, Chen MS, Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem. 1999;274:20060–20063. doi: 10.1074/jbc.274.29.20060. [DOI] [PubMed] [Google Scholar]
- 22.von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W, Dhillon AS. Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat Cell Biol. 2006;8:1011–1016. doi: 10.1038/ncb1465. [DOI] [PubMed] [Google Scholar]
- 23.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lees-Miller SP, Anderson CW. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem. 1989;264:2431–2437. [PubMed] [Google Scholar]
- 25.Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007;282:9364–9371. doi: 10.1074/jbc.M608985200. [DOI] [PubMed] [Google Scholar]
- 26.deBlaquiere J, Walker F, Michelangeli VP, Fabri L, Burgess AW. Platelet-derived growth factor stimulates the release of protein kinase A from the cell membrane. J Biol Chem. 1994;269:4812–4818. [PubMed] [Google Scholar]
- 27.Takagi H, King GL, Aiello LP. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes. 1998;47:1480–1488. doi: 10.2337/diabetes.47.9.1480. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsumi S, et al. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27:2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 30.Walsh DA, Van Patten SM. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J. 1994;8:1227–1236. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- 31.Stebbins CE, et al. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 32.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 33.Becker, et al. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol. 2004;13:27–32. doi: 10.1111/j.0906-6705.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- 34.Stellas D, Karameris A, Patsavoudi E. Monoclonal antibody 4C5 immunostains human melanomas and inhibits melanoma cell invasion and metastasis. Clin Cancer Res. 2007;13:1831–1838. doi: 10.1158/1078-0432.CCR-06-1585. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 36.Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Partridge JJ, et al. Functional analysis of matrix metalloproteinases and tissue inhibitors of metalloproteinases differentially expressed by variants of human HT-1080 fibrosarcoma exhibiting high and low levels of intravasation and metastasis. J Biol Chem. 2007;282:35964–35977. doi: 10.1074/jbc.M705993200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.