Abstract
Exploration is a central component of human and animal behavior that has been studied in rodents for almost a century. The measures used by neuroscientists to characterize full-blown exploration are limited in exposing the dynamics of the exploratory process, leaving the morphogenesis of its structure and meaning hidden. By unfettering exploration from constraints imposed by hunger, thirst, coercion, and the confines of small cage and short session, using advanced computational tools, we reveal its meaning in the operational world of the mouse. Exploration consists of reiterated roundtrips of increasing amplitude and freedom, involving an increase in the number of independent dimensions along which the mouse moves (macro degrees of freedom). This measurable gradient can serve as a standard reference scale for the developmental dynamics of some aspects of the mouse's emotional-cognitive state and for the study of the interface between behavior and the neurophysiologic and genetic processes mediating it.
Keywords: dimensionality emergence assay (DIEM assay), dynamics of exploration, mouse open field behavior, neophobia
Exploration is the process by which animals and man familiarize themselves with a novel environment. The drive to explore is so fundamental that it overrides most of the other drives: Man enters life-threatening situations in his exploration of ever new territories on the planet and in outer space, and a dam rat placed in an unforeseen environment together with its pups, first explores the new territory extensively and only then attends to the pups. Exploratory behavior has been studied in rodents for almost a century in two main setups—in mazes (1, 2) and in the open field test (3). While mazes are most appropriate for testing formulated hypotheses because they impose a priori constraints on the path, the paucity of such constraints in the open field arena highlights intrinsic constraints, offering unexpected hypotheses (4–10). The open field is one of the most common tests in the study of navigation (11), curiosity (12), anxiety (13, 14), lesion-induced (15), drug-induced (16, 17), genetically engineered behavior (18, 19), and behavior of animal models of psychiatric diseases (20, 21). The measurements taken in it consist, however, of statistical summaries (22, 23) disregarding the dynamics of occupancy of a novel environment and the animal's moment-to-moment emotional and cognitive states. A dynamic representation of these processes and states is clearly indispensable for correlating the behavior with the neurophysiologic processes that mediate the behavior.
To reduce external constraints on the mouse's behavior, we extend the arena 10-fold in space and 100-fold in time (to 2.5-m diameter for 45 h). To increase the likelihood of novelty-seeking and inquisition rather than adjustment to novelty we replace the forced and stressful introduction of the mouse into the arena with free exploration from a home-shelter (8, 24–27), to reduce the likelihood of foraging we provide free supply of food and water in the home-cage (28), and to slow down the occupancy of the arena we study the inbred strain BALB/c, known for its neophobia (29, 30). The alleviation of all of these constraints, never before implemented simultaneously, creates a mouse-centered, as opposed to an experimenter-centered, setup. Allowing the mouse to manage actively the timing, amount, and type of perceptual input exposes a rule-governed measurable behavioral gradient whose dynamics highlights a progressive increase in the number of macro degrees of freedom (MDFs) that become available to the mouse, and a quantitative build up leading to the exhaustion and subsequent emergence of new MDFs. The growth process also involves approach-and-avoidance tendencies (8) that change with increasing familiarity with the environment and increase the mouse's freedom of movement within MDFs. We call the setup and measurement system we use to quantify this behavior the dimensionality emergence assay (DIEM assay). The gradient it yields provides a standard reference scale that sets the ground for the study of the allometry of behavior.
Results
Developmental Sequence.
In the free setup, the moment-to-moment developmental dynamics of the mouse-environment interaction consist of a gradual, stretched-out and well-ordered expansion of reiterated roundtrips performed from the home-cage. This growth process involves a progressive addition of types of motion to the mouse's repertoire in a recurring order, leading to a transition from restricted to free exploratory behavior. The emergence of a new type of motion is followed by a build up in its amplitude, frequency, and complexity.
As illustrated in the behavior of a selected mouse (Fig. 1), the sequence includes 12 types of motion that were performed in all of the mice (Fig. 2), where we further denote the first appearance of a type of motion as a landmark: First the mice (motion 1) Peep-and-hide from within the cage into the arena (part of body within the cage). Then they (motion 2) Cross-the-doorway-and-retreat backwards into the cage pelvis-on. Next, they fully enter the arena, (motion 3) Circle-in-place, and (motion 4) Depart-head-on into the home-cage. In the next stage, the path from entry to departure consists of borderline movement in which the mouse travels only along the wall. A (motion 5) simple Borderline-roundtrip is performed, consisting of a single outbound portion and a single return portion. At this stage, the mouse returns all of the way home and departs into the home-cage before starting a new roundtrip. Then, a new option appears, to reverse direction and proceed on a new outbound trip without going all of the way home. At this new stage the mouse may return all of the way to the doorway but skip a departure (cage skip), or return half-way, or even return an incipient homeward distance and then proceed with a new outbound trip. All of these interrupted returns are classified as (motion 6) Home-related-shuttles. The next motion breaks the asymmetry of the one-sided borderline roundtrips: (motion 7) Borderline-roundtrip-in-the-other-direction relative to the doorway. Movement along the wall finally culminates with a (motion 8) Full-circle around the arena. Movement into the center starts from somewhere along the wall with a (motion 9) simple Incursion into the center. It consists of a single center-bound and a single return portion back to the wall. As with the borderline roundtrips, restricted incursions including one approach (to center) and one avoidance (to wall) segment are followed by free ones, including an increasing number of (motion 10) Border-related-shuttles with several alternations between approach and avoidance on the return portion. The end of the third phase is marked by (motion 11) Reaching-the-center, the maximal distance from the wall. The mouse then commences with vertical movement by (motion 12) Jumping. Once a new type of motion emerges, it is performed repeatedly, conjointly with all of the other types of motion that preceded it in the sequence (for algorithmic definitions of the landmarks see SI Materials and Methods)
Fig. 1.
The moment-to-moment developmental sequence of free exploration. The developmental landmarks in a specific BALB/c mouse-session performed across a 3-h period. The spiral proceeding from top to bottom, first in the left and then in the right column, presents the time-series of 2-D locations on the path traced by the mouse. The enumerated figure-inserts show the 12 landmark motions described in the text, traced in red within the arena, and on the spiral. Blue dots indicate instances in which the mouse approached the cage doorway and did not enter the cage (cage-skips), or stopped short of returning all of the way home during a return (Home-related-shuttle). Absence of a blue dot implies departure into home-cage. Yellow path stands for the return portion within a Home-related-shuttle.
Fig. 2.
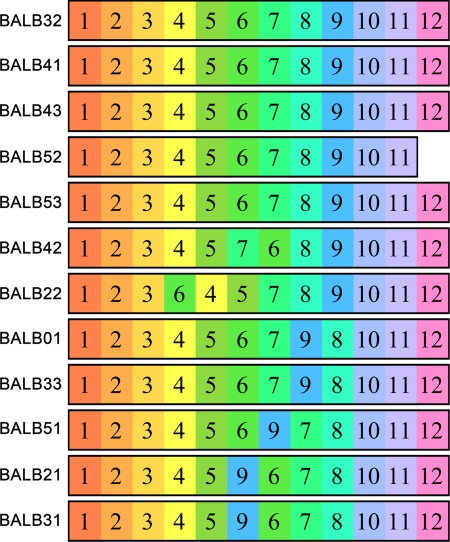
The generality of the developmental sequence in free BALB/c mice. Numerals and respective background colors represent the landmark motions that were described in Fig. 1 in the order of their emergence in each of the mice.
The types of motion are defined on the basis of their sequencing in all of the examined mice in this and other strains (see Materials and Methods and SI Materials and Methods). For example, circling in place (motion 3) and departing head on (motion 4) are not united into a single type because one BALB/c mouse (Fig. 2, mouse #7) as well as mice from other strains inserted a cage skip between them.
Generality of the Developmental Sequence.
Fig. 2 presents the actual genesis (31), i.e., the moment-to-moment developmental sequence, in which the path landmarks emerge and are appended to the repertoire in each of the tested BALB/c mice. Five out of the 12 mice share exactly the same order of the 12 landmarks (Fig. 2, mice #1–5) as the mouse described above. In three additional mice, a single swap between adjacent landmarks is needed to reproduce the sequential order (Fig. 2, mice #6, 8, and 9). In two mice, two swaps are needed (Fig. 2, mice #7 and 10), and in two mice, three swaps are needed (Fig. 2, mouse #11 and 12). An average of 33 swaps would be necessary to order a random sequence of 12 landmarks so as to fit a given order. Equivalently, whereas the average Spearman rank correlation for random sequences correlated with a given sequence is expected to be 0, here the correlation values range between 0.958 and 1, reflecting the strong similarity between the orders of the landmarks across mice. The sequence is thus extremely robust. It can be divided into four blocks: landmarks 1–4 represent “movement in place” (zero-dimension space); landmarks 5–8 represent movement along the border (one-dimension space); landmarks 9–11 represent radial movement (two-dimension space); and landmark 12 vertical movement (three-dimension space). The general order of the four blocks is fixed showing no overlap in the first six mice and little overlap of one to three swaps during the transitions from zero- to one- and from one- to two-dimension space.
Build Up in Amplitude, Frequency, and Geometrical Complexity of Motions.
Not only is the growth across stages from staying-in-place to movement with one, then with two, and then with three MDFs fixed, but it is also accompanied by a build up in amplitude and frequency of the motions within MDFs (for animation of build up in borderline and radial movements see Movie S1).
(i) The staying-in-place stage consists of a build up in amplitude of peeping and hiding leading to crossing and retreating, then to circling in place near the doorway and entering home-cage head on (Fig. 3A). This stage, which is essentially movement in place (zero-dimension space or zero MDF) is restricted spatially in BALB/c mice. It culminates with movement along the border (one-dimension space).
Fig. 3.
The build up of amplitude and complexity of movement in zero-, one-, and two-dimensions space in a free BALB/c mouse. (A) Build up in the portion of body area (in pixels) extending out of doorway during Peep-and-hide. (B1–4): The build up of angular positions (θ) across roundtrips (amplitude and complexity). Note change of time scale from (B1) through (B4). Black line, borderline movements. Blue data points, cage skips. Positive values designate right and negative values left borderline directions. Red lines, angular positions of doorway at ±360°. Graph lines between x-axis and red line represent full circles. All graphs start with the same initial roundtrip, progressively incorporating later roundtrips. (B5) Emergence and build up of radial movement away from wall (in green), superimposed on the θ plot (in black). Significant radial movements (incursions) are added only after 1.5 h.
(ii) Movement with one MDF builds up in maximal angular distance from home, θmax values, almost monotonically from one roundtrip to the next, keeping the radial distance from wall, ρ, close to zero (Fig. 3B). This increase in borderline roundtrip amplitude is joined next by the option not to return all of the way into the home-cage, as expressed by the emergence and subsequent proliferation of cage skips and home-related shuttles (blue dots in Fig. 1 and Fig. 3B). The simple borderline roundtrips turn in this way into complex ones including one to several home-related shuttles. The build up in the borderline roundtrips in the other direction, which follows the extended sequence of one-sided roundtrips (Fig. 1, top part of left spiral), is steep in comparison to the corresponding build up in the main direction (Figs. 3B and Fig. S1).
One to several full circles in one or both directions herald the end of the one-dimensional stage and the emergence of the two-dimension movement stage (for borderline and radial build up graphs of all BALB/c mice see Figs. S1 and S2).
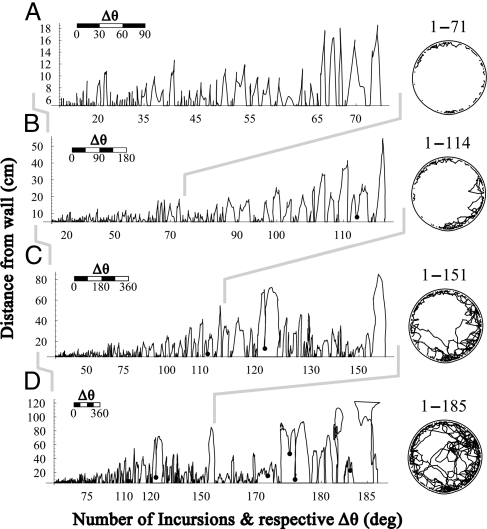
(iii) The simple incursions are short and linear, starting and ending in the same location along the wall. The build up within incursions includes both an increase in maximal distance from wall, ρmax values, and an increase in the wall section bounded between the start and end of the incursion. The increase in amplitude becomes associated with the option not to return all of the way to the wall. This is reflected in the emergence and subsequent proliferation of Border-related-shuttles (Fig. 4, black dots, and Fig. S3), turning simple incursions into complex ones, and simple roundtrips into complex ones, including one to several Border-related-shuttles. The invasion of the third, vertical, dimension space emerges much later.
Fig. 4.
The build up of amplitude and complexity of incursions. (A–D) Incursions are plotted side-by-side in the order of their performance, from the initial incursion to the incursion that reached the center, in a selected BALB/c mouse-session. For each incursion the x-axis demarcates the angular section (Δθ) it covers for that incursion along the wall, and the y-axis demarcates arena radius. Each of the graphs starts with the first Incursion and includes progressively later incursions. Black dots represent border-related shuttles. Cumulative paths of the respective incursions are plotted on the right of each graph. Numerals above circles represent the range of plotted incursions.
Developmental Sequence in Free C57BL/6 Mice.
To obtain a wider perspective on the generality of the sequence we tested in the same setup (see Materials and Methods) a group of C57BL/6 mice, the most common inbred strain used in biological research. Fig. 5 presents the sequences of each of the tested mice based on the same algorithms (SI Materials and Methods). As shown, both strains share the same types of motion (and therefore landmarks) except for Cross-the-doorway-and-retreat (landmark #2), which is absent in all but one C57BL/6 mouse, and Extended-garden-roundtrip (landmark 4′ and “garden” as defined in SI Materials and Methods), which is absent in the BALB/c mice (Fig. 2). In other words, the C57BL/6 mice largely do not retreat backward into the cage, and perform a few very short roundtrips away from the doorway and the wall (Fig. 5 and Fig. S4), thus extending the garden area, before commencing with the simple borderline roundtrips.
Fig. 5.
The generality of the developmental sequence in free C57BL/6 mice. Numerals and respective background colors represent the landmark motions that were described in Fig. 1 in the order of their emergence in each of the mice. Top two rows represent the prototype sequences of each of the two strains. Cross-the-doorway-and-retreat (Landmark 2) is skipped in all but one of the C57BL/6 mice; Extended-garden-roundtrips (4') are inserted in all C57BL/6 mice before or immediately after the onset of Borderline-roundtrips (6). Simple Incursions (9) typically emerge before the borderline dimension is exhausted (8) and early crossing of the center (11) occurs before Border-related-shuttles (10) in most of the mice.
Spearman's rank correlation coefficient between each sequence and the averaged rank sequence in that strain was used to assess the within-strain stability of the landmarks sequence. The correlation coefficients range between 0.944 and 0.993, with median of 0.975, a remarkable within-strain similarity. At most four swaps of neighbors are needed to reproduce the representative sequential order of the strain from any specific mouse sequence. When comparing the C57BL/6 to the BALB/c, there an addition of a landmark that brings about a spatially extended zero-dimension and a partial temporal overlap between zero- and one-dimension, a swap between landmarks 8 and 9, reflecting a temporal overlap between the one and two dimensions, and a swap between landmarks 10 and 11, reflecting early crossing of the center in some of the mice. As highlighted by the respective color groups, zero-, one-, two-, and three-dimension space unfold successively.
Discussion
Gradient.
Unfettering exploratory behavior from the constraints imposed by coercion, hunger, and thirst, and from the confines of a small cage and a short session exposes a stable rule-governed moment-to-moment developmental gradient. Using BALB/c mice exposes a gradient that is generated by (i) cyclic approach-and-avoidance roundtrips showing a stretched out progressive build up in amplitude and in geometrical complexity, involving (ii) a progressive increase in the number of MDFs manifested as independent spatial dimensions (zero-, one-, two-, and three-dimension space) along which the mouse moves, (iii) an exhaustion of each dimension before the emergence of the next dimension in the sequence, and (iv) a gradual increase in the freedom of movement within these dimensions. At a finer resolution, the increase in dimensionality and in freedom is marked by the sequential emergence of types of motion, where each initial performance of a type is termed a landmark. The landmarks provide a developmental reference scale. The superposition of all types of motion yields eventually an apparently unpredictable path.
The remarkable orderliness of the sequence raises many questions: How general are the four dimensions across strains, situations, and treatments? To what extent is the exhaustion of a dimension a prerequisite for the emergence of the next dimension in line? What are the variables that determine the rate of build up and the increase of freedom within dimensions? How distinct are the 12 landmarks, and to what extent can they be independently manipulated? Answering these questions will require extensive experimental manipulations and interstrain comparisons, yet, analyzing data obtained in two other studies, one performed under the same conditions on C57BL/6 in the free setup (see Materials and Methods) and another performed in similar, although not identical, conditions on BALB/c mice in two forced setups (32) (SI Materials and Methods), offers a direction.
Roundtrips.
The gradual occupancy of the arena through cyclic approach-and-avoidance roundtrips is preserved both in the free C57BL/6 mice (Fig. S4), and in the two forced setups with BALB/c, one with a walled arena and one with an arena surrounded by a cliff (Figs. S5–S7).
Emergent Dimensionality.
The primacy of zero-dimension space over one-dimension space, and of one-dimension space over two-dimension space is preserved (i) under coercion, (ii) with cliffs replacing walls, and (iii) with free C57BL/6 replacing free BALB/c mice (Fig. 5 and Figs. S4–S7). Because scanning movements direct the eyes and ears, and progressions carry the nose and whiskers to target locations in the environment (9), trajectory dynamics portray the dynamics of the mouse's operational space (33, 34). The regularity in which a MDF is managed and exhausted before passing to the next MDF implies record-keeping and therefore cognition-related skills. The primacy of attention to edges over attention to the spaces between them is characteristic of perceptual systems, regardless of the agent and the sensory channel used (35–38). The recent discovery of border cells in the rat's entorhinal cortex (39) supports the distinct status of borderline movement, while the sequence we report suggests a corresponding developmental dynamics in the brain. We predict the firing of a still undiscovered origin-of-axes class of neurons whose firing would encode zero-dimension movement and precede the firing of the border cells that encode borderline movement. Our descriptive model further predicts the existence of two additional classes of neurons, respectively encoding distance-from-edge and distance-from-floor, whose firing patterns would sequentially follow border cell firing in the process of arena occupancy.
Zero-Dimension Space.
Staying-in-place movements are spatially restricted in the free BALB/c mice (Figs. 1, 2, and 3A and Movie S1) and spatially extended in the free C57BL/6 mice (Fig. 5 and Fig. S4). In the forced setups, in the BALB/c mice, this stage is sometimes distorted by the coercive introduction of the mouse into the arena (Fig. S7). A retrospective examination of the free BALB/c mice behavior following the isolation of the extended garden roundtrips in the C57BL/6 mice suggests that even two of the free BALB/c mice show incipient motions of this type before commencing with the simple borderline roundtrips. In all of the setups and in both strains, zero-dimension space movements precede the systematic borderline movements, but in the BALB/c mice, zero-dimension space movements disappear or are diminished once borderline movements set in, whereas in the free C57BL/6 mice, there may be a greater overlap between the zero- and one-dimension movements (Fig. 5 and Fig. S4).
One and Two Dimensions.
In the free BALB/c mice, the radial movements commence immediately after circle closure (Movie S1 and Fig. S1). An overlap, if present, between the two dimensions, is small in the free and in the walled forced BALB/c mice (Fig. 2 and Figs. S5 and S7), larger in the BALB/c forced wall-less setup (Figs. S6 and S7), and much larger in the free C57BL/6 mice (Fig. 5 and Fig. S4). Regardless of this, there is always a build up in amplitude within each of the dimensions separately (Figs. 3B and Fig. S1 for borderline, and Fig. 4 and Fig. S2 for radial movement).
Three-Dimension Space.
Jumping on the wall follows the full coverage of the arena's floor, reflecting the conversion of the wall from a shelter to a limiting boundary. It is performed last in the sequence by all of the mice (Figs. 2, 5 and Fig. S8).
Management of Input.
Depriving mice of access to a familiar shelter results in the elevation of physiological and endocrinological correlates of anxiety and stress (24). The cutting-off of environmental input accomplished by periodic departures into the home-cage, the incremental growth of roundtrips, and the shuttling suggest that the mouse regulates the amount of novel input or the arousal associated with it (40, 41). Whereas during early borderline roundtrips, outbound trajectories are initiated only after a departure into the home-cage, later on departures are substituted increasingly more frequently by a visit to the garden, or by a small homebound segment. All of these variations suggest an attenuation of arousal to a level that allows subsequent resumption of an outbound trip involving a deeper penetration into unfamiliar terrain. The same dynamics repeat themselves during the invasion of the center. The gradual liberation of path dynamics from the compelling attraction of the home-cage or wall is expressed in the emergent freedom to reverse direction and resume outbound movement in the midst of return trips. Both home- and border-related shuttles are prevalent in all examined setups and strains. The assignment of specific cognitive and motivational significances to shuttling must await, however, validation through extensive environmental, genetic, and pharmacological manipulations. Quantification of the periodic departures, slow incremental growth, and shuttling should, however, provide an estimate of the management of arousal and anxiety.
Stability and Extendedness of Sequences.
Management of arousal is indispensable also for the stability and extendedness of the sequence. As illustrated in Figs. S5–S7, the orderliness of the sequence is reduced in the forced setups. The number of roundtrips preceding the closure of the borderline circle is smaller in the two forced, compared to the free BALB/c setup (P <0.00002). During forced exploration, BALB/c mice perform drastically abbreviated sequences (42) compared to the extended free exploration sequences described here. More generally, old ethological observations report that under disturbances or coercion, low-intensity patterns are skipped, and the otherwise stable sequences of innate patterns are drastically abbreviated (31, 43, 44). The inability to manage arousal in coercive forced exploration thus disrupts the otherwise stable sequence, explaining perhaps the previously held view that open field behavior is largely unpredictable.
A comparison of the stability of the sequences across the two free strains without the two nonshared landmarks yields a Spearman rank correlation coefficient of 0.988, implying a remarkable stability [BALB/c having the most common landmarks sequence: (1,3,4,4′,5,6,7,8,9,10,11,12) with a min = 0.958, median = 0.993, and max = 1, and C57BL/6 with the most common landmarks sequence: (1,3,4,4′,5,6,7,9,8,11,10,12) with a min = 0.944, median = 0.975, and max = 0.993].
An Autocentric Mobility Gradient.
A developmental gradient involving a progressive addition of MDFs in the animal's autocentric space has also been established at the level of interlimb coordination (45, 46). This gradient unfolds in psychoactive drug-induced behavior (47) and social interactions (48), allowing quantification of the animal's position on the freedom-of-movement scale.
Standard Reference Scale.
The developmental sequence of exploration is vulnerable to stress. Its full-blown version reveals the generative rules of mature exploratory behavior, and the landmarks on its freedom-of-movement scale specify positions estimating the mouse's momentary cognitive and emotional state. Because the free BALB/c sequence is the fullest and most stretched out sequence exposed so far, it is qualified, with the addition of extended garden roundtrips (motion 4′), for serving as a standard reference scale for deciphering other sequences in other strains and preparations. Equivalent positions denoting the onset, build up, and offset of the same states in different strain sequences can now be aligned vis-à-vis each other, highlighting similarities and differences in developmental dynamics. Enhancing, or reducing of build up processes, skipping of landmarks (Fig. 5), expanding processes, and even inserting motion types (Fig. 5) can all be identified, measured, and compared, thus setting the ground for an allometry of behavior. The freedom-of-movement metric of this gradient fulfils a need for a quantitative representation of behavior that will allow the study of the moment-to-moment connectivity between behavior and neurophysiologic processes that mediate it.
Materials and Methods
Animals.
The BALB/c mice (n = 12 males, 11 weeks of age; Harlan Laboratories) were kept in a 12:12-h light cycle (Light: 6:00 AM to 6:00 PM), singly housed due to their known inter-male aggressiveness for 2 weeks before testing, at 22 °C room temperature with water and food ad libitum, maintained in facilities fully accredited by the National Institutes of Health (NIH) Animal Welfare Assurance Number A5010–01 (TAU). The studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals provided by the NIH, “Principles of Laboratory Animal Care” (NIH publication no. 86–23, 1996). To rule out the possibility that behavioral asymmetry reflected an absence of corpus callosum in some of the BALB/c mice (49), all mice were screened by fMRI and found to have a normal structure. To obtain a wider perspective on the sequential order of landmarks, we videotaped in the free setup and examined both C57BL/6 and wild-caught mice (n = 10 per group). The C57BL/6 mice whose sequence is presented in Fig. 5 (n = 10 males, 11 weeks of age; Harlan Laboratories) were kept in a 12:12-h light cycle (Light: 06:00 AM to 6:00 PM), housed three per cage, for 2 weeks before testing. Their behavior was recorded 2 years after the free BALB/c mice.
Experimental Setup.
The DIEM assay setup consists of a 250-cm-diameter circular arena having a nonporous gray floor illuminated with an IR projector (880-nm) and dim white light (<1 Lux) placed on the ceiling above arena center, simulating moonlight. The arena is surrounded by a 60-cm-high, primer gray continuous wall with a single 4 × 5 cm′ doorway leading to an infra-red lit Plexiglas home-cage (30 × 40 × 50 cm′) containing wood- or paper-shavings from the original home-cage and food and water ad lib. A small Plexiglas box attached to the home-cage doorway on its inner side forces the mouse to pass through it on its way into the arena without carrying along shavings that might distract the tracking system (Fig. S9). Arena floor and Plexiglas-box floor are leveled. The vertical home-cage wall is firmly attached to the vertical arena wall securing an immediate passage between the arena and the home-cage interior (no corridor). Four heavy curtains separate the arena from the rest of the room. The arena was thoroughly rinsed with water and soap and then dried, and the home-cage was replaced by a clean home-cage, at the end of each mouse-session.
Testing Protocol and Analysis.
The mouse was housed in the replaced home-cage, which included shavings from the original home-cage, for a 24-h adjustment period. To increase the likelihood that the mouse's activity was elicited by the exposure to the open space rather than by the diurnal cycle, the session commenced 4 h after the onset of the light cycle, which is the nonactive part of the cycle of mice, at 10:00 AM, when the doorway barrier was gently removed and kept open throughout the session. The infra red and dim light were switched on when the mouse was introduced into the home-cage (24 h before door removal). The BALB/c mice session extended over a 45-h period, and the C57BL/6 mice session extended over a 24-h period. The animals' location was tracked using EthoVision (50) (25 frames per s, 1 pixel = 1 cm), and smoothed (51) and segmented (6) using SEE, a software-based Strategy for Exploring Exploration (52) available at http://www.tau.ac.il/∼ilan99/see/help. Further analysis was done using Mathematica (53).
Landmark Algorithms.
The algorithms used to identify and quantify the types of motion are available in SI Materials and Methods.
Public Database.
All data used in the present study are publicly available (see SI Materials and Methods for details and access instructions).
Supplementary Material
Acknowledgments.
We thank Noldus Information Technology for the use of their EthoVision system, Dr. Anna Dvorkin for her forced BALB/c data, and Mr. David Checkroun for his free C57BL/6 data. This work was supported by the Israel Science Foundation of the Israeli Academy of Science Grant 915/05 (to I.G. and Y.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812513106/DCSupplemental.
References
- 1.Olton DS, Collison C, Werz MA. Spatial memory and radial arm maze performance of rats. Learn Motiv. 1977;8:289–314. [Google Scholar]
- 2.Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. J Neurosci. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CS. Emotional behavior in the rat: I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- 4.Eilam D, Golani I, Szechtman H. D2-agonist quinpirole induces perseveration of routes and hyperactivity but no perseveration of movements. Brain Res. 1989;490:255–267. doi: 10.1016/0006-8993(89)90243-6. [DOI] [PubMed] [Google Scholar]
- 5.Horev G, Benjamini Y, Sakov A, Golani I. Estimating wall guidance and attraction in mouse free locomotor behavior. Genes Brain Behav. 2007;6:30–41. doi: 10.1111/j.1601-183X.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 6.Golani I, Benjamini Y, Eilam D. Stopping behavior: Constraints on exploration in rats (Rattus norvegicus) Behav Brain Res. 1993;53:21–33. doi: 10.1016/s0166-4328(05)80263-3. [DOI] [PubMed] [Google Scholar]
- 7.Lipkind D, Sakov A, Kafkafi N, Elmer GI. New replicable anxiety-related measures of wall versus center behavior of mice in the Open Field. J Appl Physiol. 2004;97:347–359. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- 8.Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berline D. Motivational problems raised by exploratory and epistemic behavior, in Psychology: A Study of a Science. In: Koch S, editor. Vol 5. New York, NY: McGraw-Hill; 1963. pp. 284–364. [Google Scholar]
- 10.Dvorkin A, Benjamini Y, Golani I. Mouse cognition-related behavior in the open-field: Emergence of places of attraction. PLoS Comput Biol. 2008;4:e1000027. doi: 10.1371/journal.pcbi.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 12.Berline D. Conflict, Arousal, and Curiosity. New York, NY: McGraw Hill; 1960. [Google Scholar]
- 13.Archer J. Tests for emotionality in rats and mice: A review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 14.Walsh RN, Cummins RA. The open-field test: A critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 15.Luhmann HJ, Huston JP, Hasenohrl RU. Contralateral increase in thigmotactic scanning following unilateral barrel-cortex lesion in mice. Behav Brain Res. 2005;157:39–43. doi: 10.1016/j.bbr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 17.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 18.Bolivar V, Cook M, Flaherty L. List of transgenic and knockout mice: Behavioral profiles. Mamm Genome. 2000;11:260–274. doi: 10.1007/s003350010051. [DOI] [PubMed] [Google Scholar]
- 19.Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol. 2001;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 20.Crawley JN. What's Wrong With My Mouse? Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- 21.Ohl F, Keck ME. Behavioural screening in mutagenised mice—in search for novel animal models of psychiatric disorders. Eur J Pharmacol. 2003;480:219–228. doi: 10.1016/j.ejphar.2003.08.108. [DOI] [PubMed] [Google Scholar]
- 22.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 23.Kafkafi N, Benjamini Y, Sakov A, Elmer GI, Golani I. Genotype-environment interactions in mouse behavior: A way out of the problem. Proc Natl Acad Sci USA. 2005;102:4619–4624. doi: 10.1073/pnas.0409554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misslin R, Cigrang M. Does neophobia necessarily imply fear or anxiety? Behav Processes. 1986;12:45–50. doi: 10.1016/0376-6357(86)90069-0. [DOI] [PubMed] [Google Scholar]
- 25.Welker WI. ‘Free’ versus ‘forced’ exploration of a novel situation by rats. Psychol Rep. 1957;3:95–108. [Google Scholar]
- 26.Halliday MS. Exploration and fear in the rat. Symp Zool Soc Lond. 1966;18:45–59. [Google Scholar]
- 27.Renner MJ. Neglected aspects of exploratory and investigatory behavior. Psychobiology. 1990;18:16–22. [Google Scholar]
- 28.Hughes RN. Intrinsic exploration in animals: Motives and measurement. Behav Processes. 1997;41:213–226. doi: 10.1016/s0376-6357(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 29.Beuzen A, Belzung C. Link between emotional memory and anxiety states: A study by principal component analysis. Physiol Behav. 1995;58:111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 30.Griebel G, Belzung C, Misslin R, Vogel E. The free-exploratory paradigm: An effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol. 1993;4:637–644. [PubMed] [Google Scholar]
- 31.Lorenz KZ. The Foundations of Ethology. New York, NY: Springer-Verlag; 1978. [Google Scholar]
- 32.Dvorkin A. Tel Aviv, Israel: Tel Aviv University; 2004. Open field behavior of BALB/c mice in a walled versus an elevated arena. M.Sc. Thesis in Zoology. [Google Scholar]
- 33.Hassler R. Brain mechanisms of intention and attention with introductory remarks on other volitional processes. Prog Brain Res. 1980;54:585–614. doi: 10.1016/S0079-6123(08)61680-5. [DOI] [PubMed] [Google Scholar]
- 34.Uexkull von J. A stroll through the worlds of animals and men. In: Schiller C, editor. Instinctive Behavior. New York, NY: International Universities Press; 1934. 1957. [Google Scholar]
- 35.Lettvin JY, Maturana HR, McCulloch WS, Pitts WH. What the frog's eye tells the frog's brain. Proc Inst Radio Eng. 1959;47:1940–1951. [Google Scholar]
- 36.Marr D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. San Francisco, CA: W.H. Freeman; 1982. [Google Scholar]
- 37.Leppanen PK, Ewalds-Kvist SB, Selander RK. Mice selectively bred for open-field thigmotaxis: Life span and stability of the selection trait. J Gen Psychol. 2005;132:187–204. doi: 10.3200/GENP.132.2.187-204. [DOI] [PubMed] [Google Scholar]
- 38.Avni R, Tzvaigrach Y, Eilam D. Exploration and navigation in the blind mole rat (Spalax ehrenbergi): Global calibration as a primer of spatial representation. J Exp Biol. 2008;211:2817–2826. doi: 10.1242/jeb.019927. [DOI] [PubMed] [Google Scholar]
- 39.Solstad T, Boccara CN, Kropff E, Moser MB, Moser E. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 40.Chance MRA. Attention structure as the basis of primate rank orders. Man (New Series) 1967;2:503–518. [Google Scholar]
- 41.Hebb DO. Drives and the CNS (conceptual nervous system) Psychol Rev. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- 42.Drai D, Kafkafi N, Benjamini Y, Elmer G, Golani I. Rats and mice share common ethologically relevant parameters of exploratory behavior. Behav Brain Res. 2001;125:133–140. doi: 10.1016/s0166-4328(01)00290-x. [DOI] [PubMed] [Google Scholar]
- 43.Seitz A. Die Paarbildung bei einige Cichliden I. Z Tierpsychol. 1940;4:40–84. [Google Scholar]
- 44.Kalueff AV, Aldridge JW, LaPorte JL, Murphy DI, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- 45.Eilam D, Golani I. The ontogeny of exploratory behavior in the house rat (Rattus rattus): The mobility gradient. Dev Psychobiol. 1988;21:679–710. doi: 10.1002/dev.420210707. [DOI] [PubMed] [Google Scholar]
- 46.Golani I. A mobility gradient in the organization of vertebrate movement: The perception of movement through symbolic language. Behav Brain Sci. 1992;15:249–308. [Google Scholar]
- 47.Szechtman H, Ornstein K, Teitelbaum P, Golani I. The morphogenesis of stereotyped behavior induced by the dopamine receptor agonist apomorphine in the laboratory rat. Neuroscience. 1985;14:783–798. doi: 10.1016/0306-4522(85)90143-5. [DOI] [PubMed] [Google Scholar]
- 48.Yaniv Y, Golani I. Superiority and inferiority—a morphological analysis of free and stimulus bound behavior in honey badger (Mellivora capensis) interactions. Ethology. 1987;74:89–116. [Google Scholar]
- 49.Wahlsten D, Crabbe JC, Dudek BC. Behavioural testing of standard inbred and 5HT(1B) knockout mice: Implications of absent corpus callosum. Behav Brain Res. 2001;125:23–32. doi: 10.1016/s0166-4328(01)00283-2. [DOI] [PubMed] [Google Scholar]
- 50.Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33:398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- 51.Hen I, Sakov A, Kafkafi N, Golani I, Benjamini Y. The dynamics of spatial behavior: How can robust smoothing techniques help? J Neurosci Methods. 2004;133:161–172. doi: 10.1016/j.jneumeth.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 52.Drai D, Golani I. SEE: A tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev. 2001;25:409–426. doi: 10.1016/s0149-7634(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 53.Wolfram SWR. Mathematica Edition: Version 5.2. Champaign, IL: Wolfram Media; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.