Abstract
Oxytocin, a peptide that functions as both a hormone and neurotransmitter, has broad influences on social and emotional processing throughout the body and the brain. In this study, we tested how a polymorphism (rs53576) of the oxytocin receptor relates to two key social processes related to oxytocin: empathy and stress reactivity. Compared with individuals homozygous for the G allele of rs53576 (GG), individuals with one or two copies of the A allele (AG/AA) exhibited lower behavioral and dispositional empathy, as measured by the “Reading the Mind in the Eyes” Test and an other-oriented empathy scale. Furthermore, AA/AG individuals displayed higher physiological and dispositional stress reactivity than GG individuals, as determined by heart rate response during a startle anticipation task and an affective reactivity scale. Our results provide evidence of how a naturally occurring genetic variation of the oxytocin receptor relates to both empathy and stress profiles.
Keywords: emotional, polymorphism, social
Oxytocin is a peptide of nine amino acids that is produced in the hypothalamus and released into both the brain and bloodstream. Functioning as both a neurotransmitter and hormone, oxytocin's targets are widespread and include the hypothalamus, amygdala, hippocampus, brainstem, heart, uterus, and regions of the spinal cord that regulate the autonomic nervous system, especially the parasympathetic branch (1, 2). Oxytocin's role in reproductive functions is well known, and its contribution to pair-bond formation has been systematically studied (3, 4).
Oxytocin also appears to modulate broad profiles of social and emotional behaviors in both males and females (5, 6). One hypothesis is that oxytocin supports affiliative behavior. Indeed, injections of oxytocin increase prosocial behaviors in a variety of species, including primates, voles, rats, and sheep (7). In humans, intranasal administration of oxytocin increases generosity (8), trust (9), eye gaze (10), and the ability to infer the affective mental states of others (11). Furthermore, assessments of plasma oxytocin in humans find that oxytocin levels relate to parent–child bonding behaviors (12), feelings of romantic love and trust (13), and empathy and subsequent generosity toward strangers (14).
A second hypothesis is that oxytocin interacts with the hypothalamo-pituitary-adrenal axis to attenuate the stress response (6, 15), which has pervasive influences throughout the body and the brain (16, 17). Notably, oxytocin has been shown to induce potent physiological anxiolytic effects, by decreasing cortisol levels (18, 19), inhibiting cardiovascular responses to stress (19), and attenuating amygdala responsivity to emotional stimuli (20).
Given these literature findings, we derived two a priori hypotheses about the social-emotional functions of oxytocin. We examined individual differences at a polymorphic site in the oxytocin receptor (OXTR) gene, which is localized in single copy to chromosome 3 of the human genome (4). Interestingly, OXTR knockout mice display a variety of aberrant social and emotional behaviors, including increased aggression and deficits in nurturing and social memory (21, 22), that are in keeping with the two hypothesized functions of oxytocin tested here. In humans, a single-nucleotide polymorphism (SNP) of an adenine (A) or guanine (G) within intron 3 of the OXTR gene (rs53576) has been associated with autism (23), a disorder characterized by impairments in social interactions and communication. This genetic variation has also been associated with the degree of warm and empathic parenting displayed toward offspring (24). Thus, individuals with one or two copies of the A allele, when compared to those homozygous for the G allele, have an increased likelihood of an autism diagnosis (23) and display less parental sensitivity (24).
These studies suggest that genetic variation of the OXTR could systematically explain variation in two basic socioemotional processes in humans—empathy and stress reactivity. We hypothesized that rs53576 variations would be related to both behavioral and self-report measures of empathy and to both physiological and self-report measures of stress reactivity. More specifically, we predicted that individuals homozygous for the G allele (GG) would have higher levels of empathy and lower levels of stress reactivity than individuals with one or two copies of the A allele (AA and AG).
Results
As it has been proposed that oxytocin evolved to influence social and stress profiles of males and females differently (25), we verified that there were no significant interactions of rs53576 with gender in our analyses and display our findings for males and females separately for illustrative purposes.
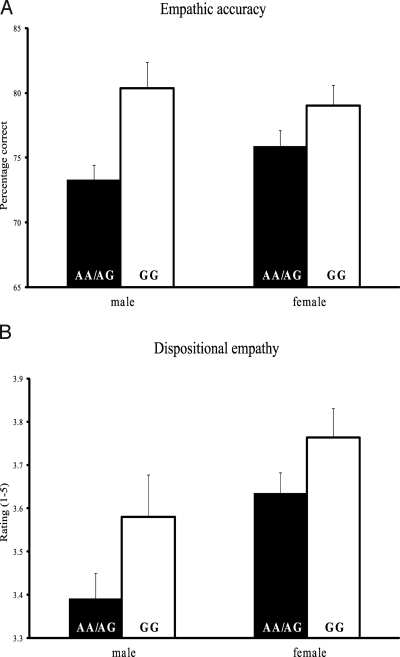
To assess individual variation in a behavioral measure of empathic accuracy, participants completed the “Reading the Mind in the Eyes” Test (RMET), which tests for the empathic ability to infer the emotional states of others (11, 26–29). Previous studies have documented that impaired performance on the RMET predicts higher scores on a measure of autistic traits in both clinical and control groups (27, 29). Moreover, in a healthy population, intranasal administration of oxytocin was shown to improve empathic accuracy scores (11). As predicted, we found that GG individuals performed significantly better on this behavioral measure of empathy (Fig. 1A), being 22.7% less likely to make a mistake on the RMET than AA/AG individuals [F (1, 177) = 8.18, P = 0.005, Cohen's d = 0.49, GG (M = 20.55% incorrect, standard error [SE] = 1.43), AA/AG (M = 25.22% incorrect, SE = 0.86)].
Fig. 1.
OXTR rs53576 polymorphism relates to behavioral and self-report measures of empathy. (A) Individuals with the GG genotype perform better on an empathic accuracy task than individuals with the AA/AG genotypes, as measured by the “Reading the Mind in the Eyes” Test. (B) Individuals with the GG genotype report higher dispositional empathy than individuals with the AA/AG genotypes, as measured by other-oriented subscales of the Davis Interpersonal Reactivity Index. Error bars represent standard error of the mean.
Dispositional empathy was assessed with a well-validated self-report scale that reflects the core facets of other-oriented empathic behavior (30). Consistent with the findings from the behavioral measure of empathy, GG individuals reported higher levels of dispositional empathy (Fig. 1B) than AA/AG individuals [F (1, 183) = 5.11, P = 0.025, Cohen's d = 0.37, GG (M = 3.69, SE = 0.06), AA/AG (M = 3.53, SE = 0.04)].
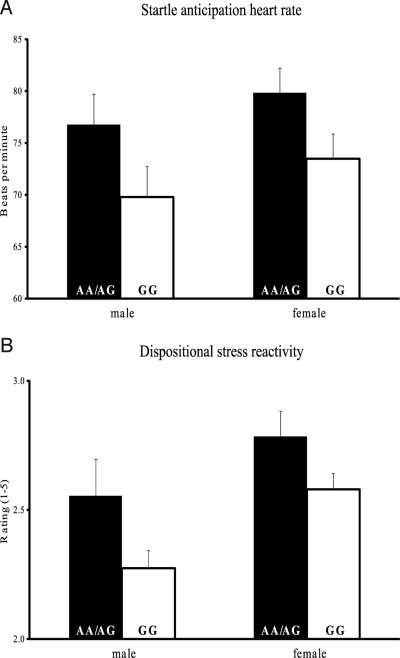
The modulation of the acoustic startle response has been validated as one of the best indexes of physiological stress reactivity, because potentiated startle reactivity engages basic fear-related activation in the central nervous system (31, 32). To measure stress reactivity, participants were presented initially with an unanticipated white-noise burst startle stimulus. After this, to potentiate stress reactivity, the participants were told that they were going to hear the loud noise two more times at the end of a 20-second countdown. During this last anticipation period, we computed an index of heart rate (HR) reactivity by averaging the HR of each participant during the final poststartle countdown, controlling for baseline HR taken at the beginning of the experimental session. As predicted, GG individuals scored significantly lower on HR reactivity to the startle anticipation than AG/AA individuals (Fig. 2A): HR [F (1, 144) = 4.97, P = 0.027, Cohen's d = −0.52, GG (M = 72.1, SE = 0.54), AA/AG (M = 78.4, SE = 1.19)].
Fig. 2.
OXTR rs53576 polymorphism relates to physiological and self-report measures of stress reactivity. (A) Individuals with the GG genotype display lower heart-rate reactivity than individuals with the AA/AG genotypes during an anticipation segment of a startle laboratory task. (B) Individuals with the GG genotype report lower dispositional stress reactivity than individuals with the AA/AG genotypes. Error bars represent standard error of the mean.
Dispositional levels of stress reactivity were assessed with a 12-item scale that captures affective reactivity to stressful situations, emergencies, and crises, rather than baseline negative affect. Consistent with the physiological startle reactivity findings, GG individuals reported lower levels of dispositional stress reactivity (Fig. 2B) [F (1, 184) = 5.61, P = 0.019, Cohen's d = −0.39, GG (M = 2.47, SE = 0.08), AA/AG (M = 2.69, SE = 0.05)].
Finally, we tested whether the rs53576 variation would relate to self-report measures of attachment style and received parental care. These data are important to rule out potential self-report biases as an alternative explanation of our self-report findings (e.g., GG individuals might simply report more positive outcomes on any self-report measure). This analysis also provided an initial test of the alternative hypothesis that early relationships with parents account for these effects. Replicating past research, we found no link between rs53576 variations and attachment style (33), nor did we find an association with received parental care.
Discriminant Validity.
Attachment style.
Consistent with previous findings, we found no association between rs53576 variations and attachment style. GG individuals did not differ from AG/AA individuals on either attachment avoidance [F (1, 104) = 1.02, P = 0.315, G/G (M = 2.48, SE = 0.11), A/A or A/G (M = 2.35, SE = 0.07)] or attachment anxiety [F (1, 104) = 1.20, G/G (M = 2.58, SE = 0.13), A/A or A/G (M = 2.75, SE = 0.08)]. These findings delineate an important boundary condition of the impact of this OXTR polymorphism on interpersonal functioning.
Consistent with the attachment findings, GG individuals did not differ from AG/AA individuals on received paternal [F (1, 176) = 0.15, P = 0.699, G/G (M = 3.45, SE = 0.13), A/A or A/G (M = 3.51, SE = 0.08)] or maternal caring [F (1, 176) = .22, P = 0.643, Cohen's d = 0.08, G/G (M = 4.01, SE = 0.13), A/A or A/G (M = 3.94, SE = 0.08)]. Again, this result helps us begin to rule out positive report bias for OXTR genotype and gives some evidence that our results are not due to positive early relationships with parents.
Discussion
Our results are consistent with past research relying on intranasal administrations or plasma assays of oxytocin showing that empathy and stress responsivity are both influenced by oxytocin (6, 11, 18, 34). Although of the functionality of the rs53576 OXTR polymorphism is unknown and its position within an intron suggests that it is unlikely to confer any distinctive molecular function, future work is needed to determine whether it is related to oxytocin sensitivity and OXTR signaling pathways. Nevertheless, our results and the findings of other groups (23, 24) suggest that probing for variations at this site may be meritorious in mixed-population gene-association studies of individual differences in social and emotional processing. Given the limitations of single-gene approaches and that all socio-emotional behaviors are influenced by multiple genes, future research should determine how the OXTR gene may interact with others involved in social and emotional processing.
Without respect to genotype, empathy and stress reactivity measures in this study are slightly negatively correlated with each other (average correlation: r = −0.021), but such correlations do not approach statistical significance. Therefore, the OXTR variant analyzed in this study reveals a unique dissociation. The empathy and stress reactivity used in this study vary systematically in healthy human populations in other studies (27, 30, 32, 35). Interestingly, previous work has shown that autistic individuals display lower scores in both the behavioral (RMET) and dispositional empathy measures (27, 29, 36) and that intranasal oxytocin improves both RMET performance and prosocial behaviors (8, 9, 11, 37). Furthermore, intranasal oxytocin reduces amygdala activation and cortisol increases to emotional stimuli (20, 37). These data converge with the findings from the present study and lend credence to the claim that this genetic variation of the oxytocin system influences emotional processing and other-oriented behavior in tandem. Future work is needed to more fully characterize the relationship between empathy and stress reactivity, as influenced by oxytocin, in both clinical and nonclinical populations.
This study demonstrates a link between OXTR variations and individual differences in empathy and stress-related profiles. In the present research, those individuals who are homozygous for the G allele, compared to those with one or two copies of the A allele, proved to be more adept at inferring the mental states of others and also reported experiencing tonically higher levels of empathy. Complementing these findings, those with an A allele showed greater cardiovascular reactivity to an imminent startle probe and also reported higher levels of reactivity across a variety of stressful contexts. These findings are in keeping with two central hypotheses about the functions of oxytocin—that it enables social affiliation and the reduction of stress. These findings have important implications for understanding how naturally occurring variations in the oxytocin system, which can influence neural and physiological processing (19, 20, 25, 38), affect both social and emotional systems.
Materials and Methods
College students at the University of California, Berkeley (n = 192, 59% female) with a mean age of 20.2 years (SE = 0.20 years) and of mixed ethnicity (35% Caucasian, 41% Asian, and 24% other or multiple ethnicities) participated in the study for partial course credit. Initial analyses confirmed that OXTR variations did not significantly interact with gender or ethnicity for any of the dependent variables. This research was authorized by the university's Committee for Protection of Human Subjects, and all participants gave informed consent before beginning each portion of the study. Participants completed the self-report measures and the behavioral empathy task at least 1 week before taking part in the laboratory-based experimental session.
Genotyping.
DNA was collected from saliva using Oragene kits (DNA Genotek, Kanata, Ontario, Canada), which allow for long-term preservation and storage of DNA at room temperature. All DNA samples were labeled with an anonymous code designed to protect the privacy of participants and were purified by Creative Genomics (Port Jefferson Station, NY). First, DNA samples were heat-treated to maximize DNA yield and to ensure that nucleases are permanently inactivated. Next, DNA samples were mixed and ice-incubated with Oragene DNA Purifier to remove impurities. After this, each sample was centrifuged at room temperature and the clear supernatant was transferred to a new tube. Tubes were centrifuged after 100% ethanol was added to precipitate the DNA. DNA samples were then washed with ethanol, rehydrated, assayed for concentration, and sent to the University of California, San Francisco Genomics Core to conduct the genotyping assay. The SNP marker for rs53576 was genotyped using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) functionally tested by Applied Biosystems and available on demand. TaqMan polymerase chain reaction (PCR) reactions were done with Universal Master Mix Amperase UNG, 0.083 μl Taqman 40X probe mix, and 1.417 μl of water, 1 μl of DNA normalized to 10 ng/μl, for a 5 μl total volume. The PCR conditions for the TaqMan SNP Genotype Assays were as follows: one enzyme activation step at 95.0 °C for 10 min, and 50 alternating cycles of denaturation at 95.0 °C for 15 s and reannealing and extension at 60.0 °C for 1 min. All PCR reactions and allelic discrimination reactions were performed on an ABI 7900HT Real-Time PCR System (Applied Biosystems) and analyzed using SDS 2.3 software (Applied Biosystems).
Genotype distribution (n = 44 A/A, n = 88 A/G, n = 47 G/G) aligns with previous reports for this variation according to the ethnic background of our participants (23, 39). No sex differences could be detected (χ2 = 2.16; df = 2, P = 0.34). Data quality was assessed by duplicating a subset of random DNA samples and genotype data reproducibility was 100%. In all but one of our measures, the AA and AG groups did not differ in statistically significant ways; we therefore combined them in our comparative analyses to GG, as done previously by other researchers (24).
Empathy: Behavioral Task.
As an objective measure of empathy, we used the Reading the Mind in the Eyes Task (11, 26–29), which assesses individual differences in the ability to infer the affective mental states of strangers. In this standardized multiple-choice test, participants are shown 36 black-and-white photos of the eye region of different individuals. The individual in each photo displays a particular emotional or cognitive state. Each photo is paired with four affective-state adjectives as response options (e.g., “terrified,” “upset,” “arrogant,” and “annoyed”). Participants select the adjective that in their judgment best describes what the individual in the photo is feeling or thinking.
Empathy: Self-Report.
The most commonly used self-report measure of dispositional empathy (30) consists of three core facets of other-oriented empathic behavior, namely perspective taking (e.g., “I sometimes try to understand my friends better by imagining how things look from their perspective”), empathic concern (e.g., “I often have tender, concerned feelings for people less fortunate than me”), and fantasy (e.g., “I really get involved with the feelings of the characters in a novel.”), all of which are positively intercorrelated, capture the broader concept of other-oriented empathy, and have been shown to predict helping for altruistic reasons (40). Participants rated each item from 1 (strongly disagree) to 5 (strongly agree). This 21-item composite measure of dispositional empathy had a Cronbach's alpha reliability of 0.81.
Stress Reactivity: Physiological Task.
Throughout the laboratory session, participants' heart rate was recorded. As an index of stress reactivity, we assessed the average heart rate of each participant during a startle anticipation task and controlled for the average baseline heart rate during a 2-minute paced breathing task. To make these measurements, participants were connected to a physiological measurement system (P150, Biopac Systems Inc., Santa Barbara, CA) using disposable snap electrodes (Biopac, EL503) with LEAD110 series electrode leads. Heart rate data were stored and analyzed using Acknowledge software (Biopac Systems Inc.). If any artifacts in the data were observed, they were repaired, if possible, or discarded from analyses. Data averages that were more than three standard deviations below or above the mean (outliers) were discarded.
In the laboratory, participants were hooked up to physiological equipment and were given a 15-min period to rest while seated in a comfortable chair. Next, participants were asked to relax and then engaged in a 2-minute paced-breathing task, which guided participants through controlled breathing with their eyes closed. The task limits the variability in respiration across participants and allows for the analysis of heart rate under controlled conditions (41).
Participants were then seated directly in front of a 17“ flat-screen LCD monitor. The auditory startle probes were administered via computer-based instructions on the screen. Instructions guided participants to put on calibrated headphones (Sennheiser HD-280) and to fixate on a large ”X“ presented in the center of the screen. Fifteen seconds later, an unanticipated loud noise was presented through the headphones. The stimulus was a standard white-noise burst used in previous startle experiments (500 ms, 120 dB) (42). After this first unanticipated startle probe, the computer screen notified the participants that after each 20-s countdown, loud noises would be presented: ”In this part of the experiment, you will know exactly when the loud noises will occur. You will see a countdown from 9 to 0 on the video screen. When you see “0” the loud noise will happen. Before beginning the countdown, please relax.“ To measure stress reactivity, we averaged the participants' heart rate during the second (final) countdown, thus capturing the maximum effect of fear-potentiated anticipation, and compared this with baseline heart rate during the paced breathing task.
Stress-Reactivity: Self-Report.
Stress reactivity was assessed with a 12-item scale (Cronbach's alpha = 0.86). Following Gross et al. (35), we used items from the broad Neuroticism personality domain that measure negative-emotion reactivity in stressful situations, emergencies, and crises, rather than baseline negative affect. This unidimensional scale measures a general factor of stress reactivity. On the highly reactive end, item examples include feeling apprehensive and ill at ease in emergencies, and becoming very emotional when under a great deal of stress; on the low-reactive end, examples include remaining calm in tense situations and being relaxed and handling stress well. Participants rated their level of agreement from 1 (disagree strongly) to 5 (agree strongly).
Discriminant Validity.
To test the boundary conditions of the impact of the rs53576 OXTR polymorphism, we examined two possible confounds, i.e., that OXTR variations are related to positive self-report bias or that they are linked to differential early-life experiences.
Attachment Style.
Some participants completed a shortened version of the Experiences in Close Relationships Inventory (43), which measures the two major dimensions of attachment functioning in adults. Attachment avoidance is measured with items such as “I have not felt comfortable opening up to my partner,” and “I want to get close to my partner, but I find myself pulling back” (alpha = 0.81). Attachment anxiety is measured with items such as “I have often worried that my partner won't care about me as much as I care about him/her,” and “I have often wanted to merge completely with my partner, and this sometimes scared him/her away” (alpha = 0.80). Participants rated how much they agreed that each item was true of their own experiences in romantic relationships, using a scale from 1 (strongly disagree) to 5 (strongly agree).
Received Paternal and Maternal Caring.
Using the caring scale of the Parental Bonding Instrument (44), participants rated their father's and their mother's caregiving behavior before the participants' age of 16; the same 12 items are worded once for rating one's father and once for rating one's mother, using a five-point response scale from 1 (strongly disagree) to 5 (strongly agree). Items include “He/She could make me feel better when I was upset” and “He/She was affectionate to me.” Cronbach's alpha was 0.94 for the paternal caring scale and 0.95 for the maternal caring scale.
Acknowledgments.
We gratefully acknowledge all of the wonderful research assistants and honors students involved in this project. This research was supported by grants from the Metanexus Institute, the Greater Good Science Center, and the University of California, Berkeley Swan Research Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Rev. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 2.Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 3.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Progress Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 4.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 6.Neumann ID. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo-pituitary-adrenal axis. Progress Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 7.Insel TR, Young LJ. The neurobiology of attachment. Nature Rev. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 8.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PloS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 10.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzaga GC, Turner RA, Keltner D, Campos B, Altemus M. Romantic love and sexual desire in close relationships. Emotion. 2006;6:163–179. doi: 10.1037/1528-3542.6.2.163. [DOI] [PubMed] [Google Scholar]
- 14.Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann N Y Acad Sci. 2009;1167:182–189. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartz JA, Hollander E. The neuroscience of affiliation: Forging links between basic and clinical research on neuropeptides and social behavior. Hormones Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues SM, Sapolsky RM. Disruption of fear memory through dual-hormone gene therapy. Biol Psychiatry. 2009;65:441–444. doi: 10.1016/j.biopsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 19.Knox SS, Uvnas-Moberg K. Social isolation and cardiovascular disease: An atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 20.Domes G, et al. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Nishimori K, et al. New aspects of oxytocin receptor function revealed by knockout mice: Sociosexual behaviour and control of energy balance. Progress Brain Res. 2008;170:79–90. doi: 10.1016/S0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor SE, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 26.Richell RA, et al. Theory of mind and psychopathy: Can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia. 2003;41:523–526. doi: 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 27.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry Allied Disc. 2001;42:241–251. [PubMed] [Google Scholar]
- 28.Pardini M, Nichelli PF. Age-related decline in mentalizing skills across adult life span. Exp Aging Res. 2009;35:98–106. doi: 10.1080/03610730802545259. [DOI] [PubMed] [Google Scholar]
- 29.Baron-Cohen S, Wheelwright S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- 30.Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Personal Social Psychol. 1983;44:113–126. [Google Scholar]
- 31.Davis M. Neural systems involved in fear-potentiated startle. Ann N Y Acad Sci. 1989;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- 32.Lang PJ, Davis M, Ohman A. Fear and anxiety: Animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 33.Gillath O, Shaver PR, Baek JM, Chun DS. Genetic correlates of adult attachment style. Personal Soc Psychol Bull. 2008;34:1396–1405. doi: 10.1177/0146167208321484. [DOI] [PubMed] [Google Scholar]
- 34.Meyer-Lindenberg A. Impact of prosocial neuropeptides on human brain function. Prog Brain Res. 2008;170:463–470. doi: 10.1016/S0079-6123(08)00436-6. [DOI] [PubMed] [Google Scholar]
- 35.Gross JJ, Sutton SK, Ketelaar T. Relations between affect and personality: Support for the affect-level and affective-reactivity views. Personal Soc Psychol Bull. 1998;24:279–288. [Google Scholar]
- 36.Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PloS One. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ditzen B, et al. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- 39.Jacob S, et al. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberg N, et al. Relation of sympathy and personal distress to prosocial behavior: A multimethod study. J Personal Soc Psychol. 1989;57:55–66. doi: 10.1037//0022-3514.57.1.55. [DOI] [PubMed] [Google Scholar]
- 41.Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 42.Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? J Abnorm Psychol. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- 43.Brennan KA, Clark CL, Shaver PR. Self-report measurement of adult attachment: An integrative overview. In: Simpson JA, Rholes WS, editors. Attachment Theory and Close Relationships. New York: Guilford Press; 1998. pp. 46–76. [Google Scholar]
- 44.Parker G. The Parental Bonding Instrument. A decade of research. Soc Psychiatry Psychiatr Epidemiol. 1990;25:281–282. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]