Abstract
Purpose
To determine the impact of the antiangiogenic factor α1(IV)NC1 on vascular endothelial growth factor mediated proangiogenic activity in mouse retinal endothelial cell (MLEC).
Methods
Primary culture of mouse retinal endothelial cells were established as previously described and used to determine the effects of α1(IV)NC1 on proangiogenic activity of VEGF. Cell proliferation was evaluated using [H3] thymidine incorporation and 3,(4,5-dimethylthiazol-2-yl)-2,5- diphenyl-tetrazolium bromide colorimetric assays. Cell migration was determined using modified Boyden chamber and scratch wound assays and tube formation was assessed on Matrigel. The intracellular signaling events Bcl-2/Bcl-xL and caspase-3/poly (ADP-ribose) polymerase (PARP) activities were evaluated in cells stimulated with VEGF and plated on type IV collagen coated dishes. Apoptosis was assessed by measuring different caspases activity as well as quantitative fluorescence analysis using fluorescence-activated cell sorting assay. Subcutaneously injected VEGF induced in-vivo neovascularization was studied using Matrigel plug assay.
Results
VEGF induced sub-confluent MREC proliferation, migration, and tube formation was significantly inhibited by α1(IV)NC1 at 1.0µM (P<0.001). α1(IV)NC1 induced MREC apoptosis mediating through by inhibition of Bcl-2 and Bcl-xL expressions and activation of caspase-3/PARP through FAK/p38-MAPK signaling. In addition, α1(IV)NC1 dose dependently inhibited VEGF-mediated neovascularization in-vivo.
Conclusions
α1(IV)NC1 inhibited VEGF-mediated angiogenesis by promoting apoptosis, caspase-3/PARP activation and negatively impacting FAK/p38-MAPK phosphorylation, Bcl-2 and Bcl-xL expressions leading to MREC death. The endothelial specific inhibitory actions of recombinant α1(IV)NC1 may be of benefit in the treatment of a variety of eye diseases with a neovascular component.
Introduction
Angiogenesis, the formation of new blood vessels from preexisting capillaries, is a very tightly regulated process and normally does not occur, except during developmental and wound repair processes1. This strict regulation is manifested by a balanced production of pro-and anti-angiogenic factors, which keep angiogenesis in check1. However, this balance between pro- and anti-angiogenic factors abrogates under many pathologic conditions, including cancer, diabetes, age-related macular degeneration (AMD), and retinopathy of prematurity (ROP), resulting in the growth of abnormal new blood vessels2–4.
Vascular endothelial growth factor (VEGF) is a major proangiogenic factor, which promotes growth of new blood vessels under ischemic conditions. VEGF stimulates endothelial cells proliferation, migration, and survival. When retinal pigment epithelial (RPE) cells begin to wither due to lack of nutrition (ischemia), VEGF goes into action to create neovascularization, and it acts as a restorative function in other parts of the body. In the retina, these vessels do not form properly and leak, ultimately resulting in retinal detachment and loss of vision. Recently several antiangiogenic compounds have been shown to inhibit neovascularization and prevent bleeding into the vitreous by inhibiting VEGF’s binding to its receptors on endothelial cell5, 6. Therefore, there is significant interest in the development and identification of molecules that can inhibit growth of new blood vessels.
Vascular basement membranes (VBM) constitutes an important component of blood vessels7. Remodeling of VBM can provide crucial pro- and anti-angiogenic molecules to control formation of new capillaries8–10. Type IV collagen is a major component of VBM and plays a crucial role in regulation of angiogenesis7. Proteolytic degradation of type IV collagen in the VBM generates antiangiogenic molecules11. One such antiangiogenic molecule derived from α1 chain of type IV collagen non-collagenous (NC1) domain, α1(IV)NC1 (arresten), has been tested in mice against a variety of cancers12, 13. However, the molecular and cellular mechanisms responsible for inhibition of angiogenesis require further delineation. The in-vitro and in-vivo studies have demonstrated that α1(IV)NC1 can directly affect endothelial cell migration and impact their proliferation and sprouting13. However, the effects of α1(IV)NC1 on retinal endothelial cell function and vascularization have not been previously studied.
In the present study, we demonstrate that α1(IV)NC1 is a potent inhibitor of mouse retinal endothelial cell (MREC) proliferation, migration and tube formation in-vitro and angiogenesis in-vivo. We demonstrate that α1(IV)NC1 promotes apoptosis via activation of caspase-3 and PARP cleavage, presumably by inhibiting FAK/p38-MAPK/Bcl-2 and Bcl-xL signaling cascade in MREC without affecting mouse retinal pigment epithelial (MRPE) cells. These findings contribute significantly towards understanding the apoptotic signaling mechanism and therapeutic potential of α1(IV)NC1 molecule in retinal diseases with a neovascular component.
Methods
Preparation and culture of primary mouse retinal endothelial and retinal pigment epithelial cells
Primary MREC were prepared and maintained as describe previously14. MREC were maintained in 40% HAM´s F-12, 40% DME-Low Glucose, 20% FCS supplemented with heparin (50mg/l), endothelial mitogen (50mg/l), L-glutamine (2mM), penicillin/streptomycin (100 units/ml each), Na Pyruvate (2.5 mM), NEAA (1X), 5µg/L of murine INF-γ and cultured on 0.8% gelatin coated plates at 33°C with 5% CO2. MRPE cells were maintained in DMEM containing 10% FCS and 100-units/ml antibiotic and antimycotic solutions at 37°C with 5% CO2.
Proliferation assay
A suspension of 40×103 MREC/well was used in proliferation assay. 500µl/well of MREC medium was added to 24-well plates pre-coated with 10µg/ml of type IV collagen. After 24h, medium was replaced with MREC medium containing 10ng/ml of VEGF and different concentrations of baculovirus expressed recombinant α1(IV)NC1 or α3(IV)NC1 (tumstatin) (0.5 and 1.0µM) and after 24h, 1µI of [3H]-thymidine was added into each well. After 24h, [3H]-thymidine incorporation was measured using a scintillation counter13.
Cell viability assay
MREC and MRPE cell viability were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide assay (MTT) following the manufacturer's protocols. Over night serum starved MREC or MRPE (7.0×103 cells/well) were plated on a 96-well plate and stimulated with VEGF (10ng/ml) containing medium. Next day different concentration of α1(IV)NC1 (0.25 to 1.0µM) was added and incubated for 24h. Apoptosis was monitored by trypan blue exclusion using the cell death detection ELISA kit15.
Migration assay
MREC (1.0×104 cells/well) were seeded in no serum medium with and without recombinant α1(IV)NC1. Medium containing 10ng/ml of VEGF was placed into the bottom wells of the Boyden chamber and incubated for 24h at 37°C with 5% CO2. The numbers of MREC that migrated and attached to the bottom side of the membrane were counted13, 16.
Tube formation assay
Briefly, Matrigel matrix, approximately 250µl, was added to each well of a 24-well plate and allowed to harden for 30 min at 37°C. A suspension of 50×103 MREC in medium without antibiotic was plated on top of the Matrigel matrix coated wells. The MREC were stimulated with VEGF for tube stabilization and incubated with and without α1(IV)NC1 for 24h at 37°C and viewed using a CK2 Olympus microscope17, 18.
Scratch wound assays
Briefly, MREC wound assays were conducted in type IV collagen coated 6 well tissue culture plates. MREC were cultured to sub-confluence and wounds were created at the center of each plate by scraping the monolayer with a sterile pipette tip19. Only in experimental wells α1(IV)NC1 (1µM) containing medium was added and migrating cells into the wound area were monitored microscopically upto 36h. Photographs ware taken at different time points and migrated cells were counted in control as well as α1(IV)NC1 treated wells.
Caspase-3, -8 and -9 activity assay
MREC and MRPE cells were grown to sub-confluence; cells detached and plated at 0.5×106 cells/well in a 6 well plate (pre-coated with type IV collagen 10µg/ml) in 10% FCS supplemented medium overnight. The following day, serum containing medium was replaced with serum free medium and incubated overnight at 37°C. Cells were then stimulated with VEGF (10ng/ml) in MREC medium containing α1(IV)NC1 (1µM) and incubated for 24h. TNF-α (80ng/ml) was used as a positive control for caspase-3 dependent apoptosis, whereas, staurosporine (50nM) was used as a positive control for mitochondrial caspase-dependent apoptosis. A specific inhibitor of caspase-3, DEVD-fmk (20µM), was used to confirm the specificity of the assay, similarly caspase-8 and -9 inhibitors z-IEPD-fmk, z-LEHD-fmk (20µM) were used. After 24h, the floating cells were collected, combined with the adherent cells and washed three times with sterile PBS. Equal number of cells in each condition were re-suspended and mixed in appropriate peptide substrate reaction buffers containing 100mM HEPES pH 7.0 (for caspase-3 and-8) and 100mM MES pH 6.5 (for caspase-9) in 30µl of PBS and transferred to a 96 well plate for fluorometric analysis in a microplate reader. Caspase-3, -8 and -9 activities were determined fluorometrically by cleavage of cellular substrate DEVD-AMC, IEPD-AMC or LEHD-AFC as described20.
FACS analysis of MREC incubated with α1(IV)NC1
Apoptotic and viable MREC were measured by quantitative fluorescence analysis performed using fluorescence-activated cell sorting (FACS). Briefly, MREC were incubated with and without α1(IV)NC1 (1µM) for 15 min at room temperature and plated on type IV collagen coated 10 cm tissue culture plates containing VEGF (10ng/ml). The culture plates were incubated at 37°C with 5% CO2 for 24h and used for FACS analysis. Floating MREC were collected, combined with adherent cells removed by trypsinization (0.05% [wt/vol] trypsin in 0.02% [wt/vol] EDTA), incubated for 5 min at 37°C, washed twice with PBS, and the cell pellet was re-suspended in 1X binding buffer (10mM HEPES/NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2). In each condition, MREC were suspended in binding buffer containing fluorescein isothiocyanate (FITC) conjugated annexin-V and propidium iodide (PI) and incubated for 20 min at room temperature in dark. Additional binding buffer was added and 10 thousand events were acquired and analyzed by FACS21.
Cell signaling experiments
For FAK and p38-MAPK signaling experiments, approximately 1.0×106 serum-starved MREC or MRPE cells were pre-incubated 10min with α1(IV)NC1 (1µM), followed by VEGF (10ng/ml) stimulation and seeded into 10cm2 dishes coated with type IV collagen (10mg/ml) for different time intervels. Cells were lysed in RIPA lysis buffer and extracts were analyzed by SDS-PAGE and immunoblotting using antibodies specific to phosphorylated and total FAK or p38-MAPK13, 18. For measuring caspase-3 activation, MREC were incubated with α1(IV)NC1 for 6–24h at 37°C. Floating and adherent cells were pooled and lysed, and cytosolic extracts were immunoblotted. Cleaved activated caspase-3 was identified by specific monoclonal antibodies and using ECL detection kit.
Effect of α1(IV)NC1 on p38-MAPK dependent Bcl-2/Bcl-xL regulation and caspase-3/PARP activation
About 1×106 serum starved MREC were collected and suspended in serum free medium containing VEGF (10ng/ml). These cells were pretreated with α1(IV)NC1 (1µM) or p38-MAPK specific inhibitor SB203580 (20µM) or TNF-α (80 ng/ml) for about 10min and seeded on type IV collagen pre-coated 10cm2 dishes for 24h. Plates were washed once with cold PBS and cells were lysed on ice in RIPA lysis buffer and centrifuged at 4°C for 30min at 13000rpm. About 30µg of cytosolic extract/lane was separated using 10% SDS-PAGE followed by immunoblotting with anti-Bcl-xL, Bcl-2, caspase-3 and PARP antibodies. Immunoreactivity of Bcl-xL, Bcl-2 proteins and cleaved caspase-3 and PARP were visualized using ECL detection kit.
Matrigel matrix plug assay
Twelve-week-old 15 male 129/Sv mice were used for this study. For each condition 3 mice or 6 Matrigels were used. All animal studies were reviewed and approved by the animal care and use committee of Boys Town National Research Hospital. Matrigel was injected subcutaneously into the right and left sides of mice lateral to the abdominal midline. Control groups received only heparin in the Matrigel, where as positive control groups received heparin and VEGF (100ng/ml) with and without type IV collagen (10µg/ml). The experimental group received two different doses of α1(IV)NC1 (0.5 and 1µM) along with heparin and VEGF in the Matrigel22. After 10 days, mice were sacrificed and half of the Matrigel plugs were fixed in 4% paraformaldehyde, sectioned, and stained with hematoxylin and eosin. The numbers of blood vessels from 12 high power fields were counted. The other half of the Matrigel plugs were dispersed in sterile PBS and incubated at 4°C overnight and hemoglobin levels were determined with Drabkin's18.
Statistical analysis
Each experiment was performed three times and values are expressed as mean±SEM. Statistical analysis based on the student’s t test (unilateral and unpaired) was scored to identify significant differences in multiple comparisons. A level of p<0.05 was considered statistically significant.
Results
Distinct anti-angiogenic activities of α1(IV)NC1 on MREC
α1(IV)NC1 was discovered as an anti-angiogenic molecule with significant anti-tumor activities13. α1(IV)NC1 is liberated from the non-collagenous (NC1) domain of α1 chain of type IV collagen by matrix metalloproteinases-9 (MMP-9)23. The present studies were aimed at understanding the molecular mechanisms underlying angiogenesis inhibition by α1(IV)NC1 and its implications in the treatment of cancer. Our studies attempted to determine the antiangiogenic actions of α1(IV)NC1 in MREC.
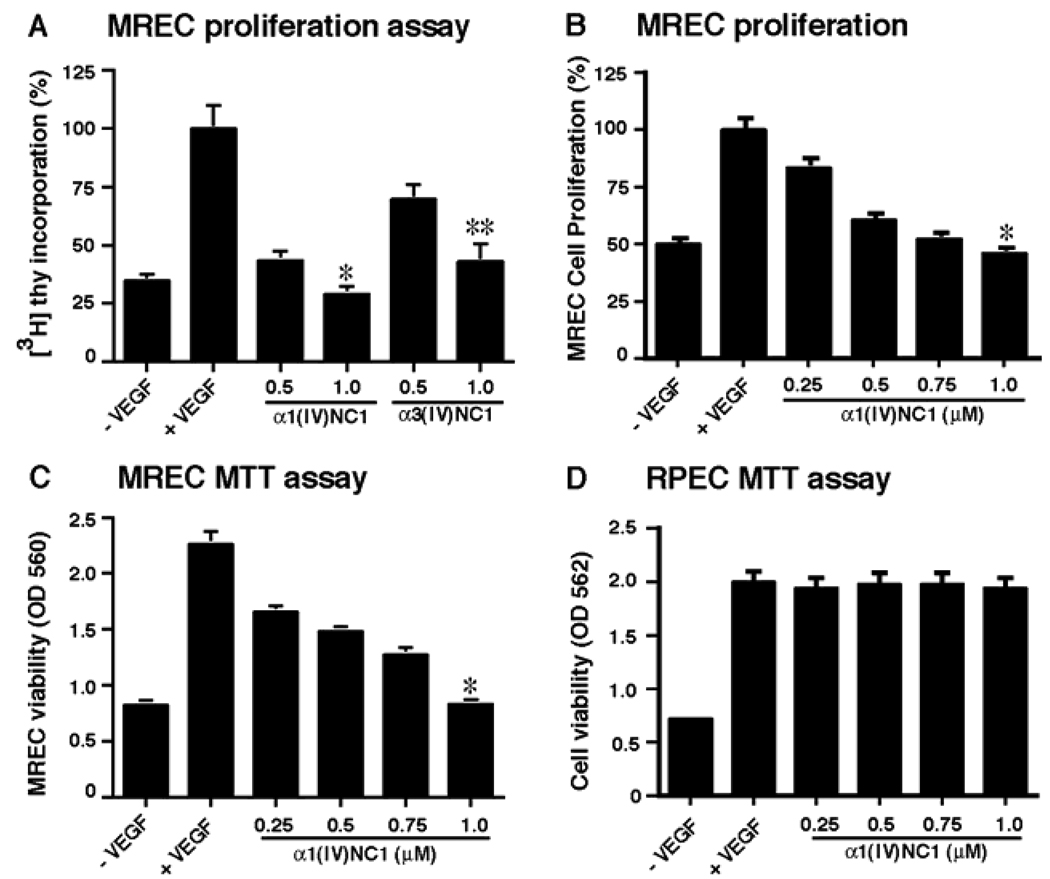
A series of angiogenesis experiments were conducted to determine the antiangiogenic activity of α1(IV)NC1 using MREC and MRPE cells. VEGF is a major proangiogenic factor, which is responsible for the majority of ocular angiogenesis driven by ischemia. We first determined VEGF stimulated antiangiogenic activity of α1(IV)NC1 by measuring MREC proliferation. The anti-proliferative effect of α1(IV)NC1 was examined using [3H]-thymidine incorporation. VEGF-stimulated proliferation of MREC was significantly inhibited by α1(IV)NC1 in a concentration dependent manner compared to α3(IV)NC1. The graph summarizes relative [3H]-thymidine incorporation in MREC incubated with α1(IV)NC1 and α3(IV)NC1 (α1(IV)NC1; 70.49% compared to α3(IV)NC1 at 50.49% at 1.0µm) (Figure 1A). We also confirmed that MREC proliferation was inhibited dose dependently by α1(IV)NC1 using methylene blue staining (Figure 1B). Such proliferation inhibition ([3H]-thymidine incorporation or methylene blue staining) was not observed in MRPE cells incubated with α1(IV)NC1 (data not shown). Further we tested the antiangiogenic activity at different doses of α1(IV)NC1 (0, 0.25, 0.5, 0.75 and 1.0µM) by MTT cell viability assay after VEGF stimulation in MREC. The results showed that MREC proliferation increased significantly by VEGF stimulation which was inhibited by α1(IV)NC1 dose dependently after 24h (Figure 1C). Interestingly, α1(IV)NC1 had no significant effect on MRPE proliferation in presence of VEGF, indicating endothelial cell specific action of α1(IV)NC1 (Figure 1D).
Figure 1.
(A) MREC proliferation assays. Graph summarizes relative [3H]-thymidine incorporation inhibition in MREC upon treatment with different concentrations of α1(IV)NC1 and α3(IV)NC1 compared with and without VEGF as controls. (B) MREC proliferation assays ware also performed with methylene blue staining method, treating cells with different concentrations of α1(IV)NC1 compared with and without VEGF as controls. All groups represent triplicate samples. (C and D) MREC and MRPE cell viability. MTT assay performed to evaluate MREC and MRPE viability after treatment with various concentrations of α1(IV)NC1. MREC and MRPE grown with and without VEGF showed as negative and positive controls. Results shown in panels A–C were significant; mean ± the standard error of the mean [SEM] of three independent experiments is shown. *P<0.001 and **P<0.005 compared to VEGF treatment.
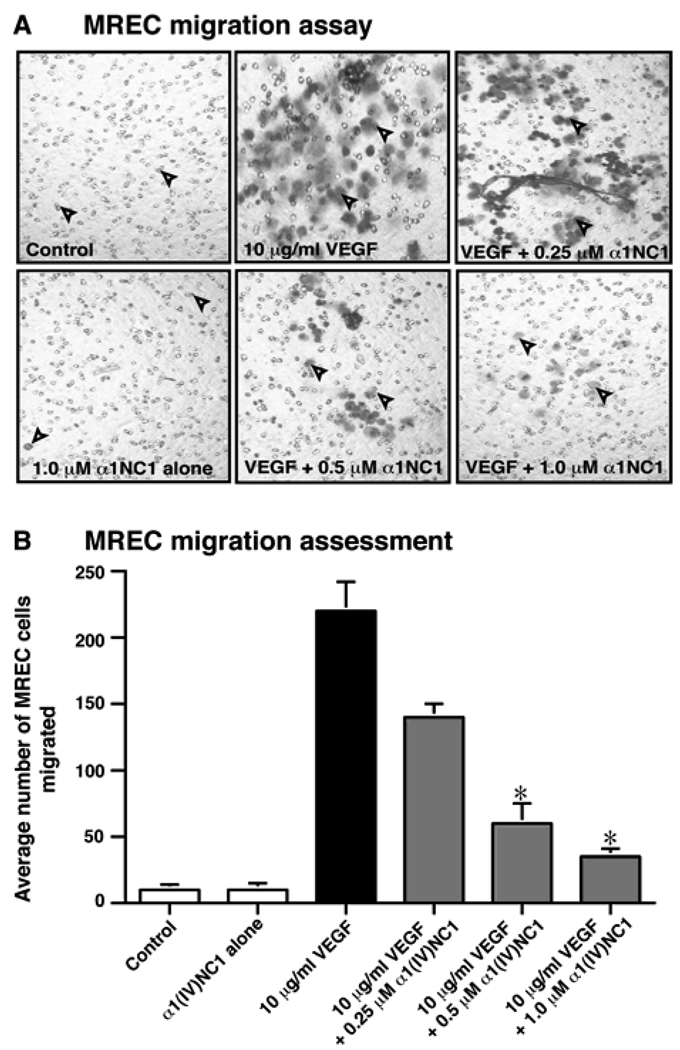
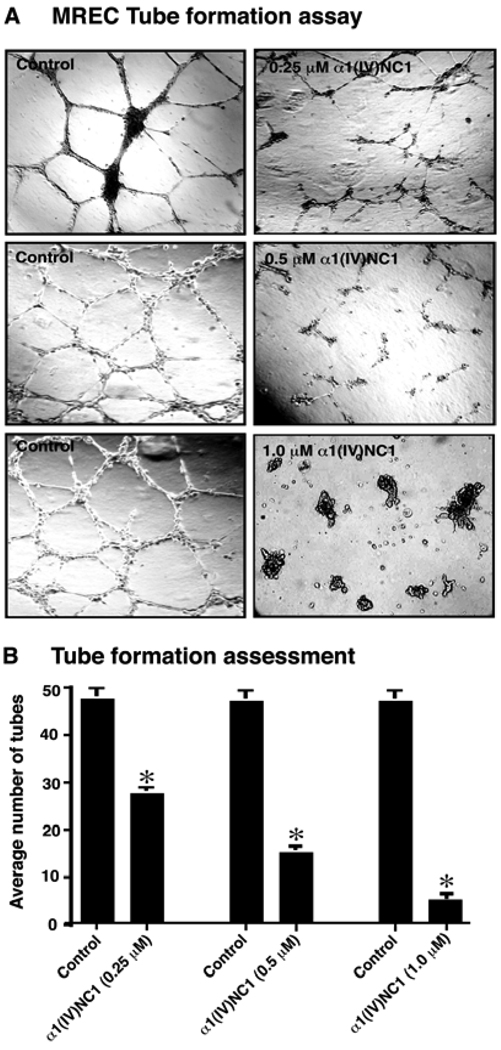
Migration of endothelial cells is fundamentally important during angiogenesis13, 24. Migration of MREC across a collagen type IV coated membrane towards VEGF in a Boyden chamber was significantly inhibited by α1(IV)NC1 in a dose dependent manner (0, 0.25, 0.5 and 1.0µM; Figures 2 A and B). We further confirmed the antiangiogenic activity of α1(IV)NC1 by a functional assay of VEGF mediated stabilized MREC tube formation17. Tube formation on Matrigel is associated with endothelial cell migration and survival25. Incubation of MREC with α1(IV)NC1 inhibited VEGF stabilized tube formation on Matrigel matrix in a dose dependent manner (Figure 3A α1(IV)NC1). Our previous report states that the anti-angiogenic activity of α1(IV)NC1 is mediated through α1β1 integrin13. Interestingly, we observed that MREC when incubated with α1(IV)NC1 (1.0µM) becomes rounded (apoptosis-like) and detach from the Matrigel matrix forming clumps of cells (Figure 3A, lower right corner panel). This is perhaps due to activation of the apoptosis in MREC incubated with α1(IV)NC1. The number of tubes formed after 36h in presence of different doses of α1(IV)NC1 from three independent experiments was shown in graph (Figure 3B).
Figure 2.
(A) MREC migration assays. Number of MREC migrated in VEGF with and without α1(IV)NC1 using a light microscope and representative fields (100x magnification) are shown. Photographs of MREC on the underside of Boyden chamber membrane are shown. (B) MREC cells migration assessment. Graph displays the average number of MREC migrated in 4 different wells in each condition (n=4); mean±the standard error of the mean [SEM] of three independent experiments is shown. *P<0.001 compared to VEGF treatments.
Figure 3.
(A) Tube formation assay. MREC were plated on Matrigel coated plates in retinal endothelial cell medium as control or with 0.25–1.0µM α1(IV)NC1 protein. Tube formation was evaluated using a light microscope, and representative fields at 100x magnification are shown. (B) Tube formation assessments. Graph displays the average number of tubes in 2 different wells in each condition and mean±standard error of the mean [SEM] of three independent experiments (n=6). *P<0.001 compared to control.
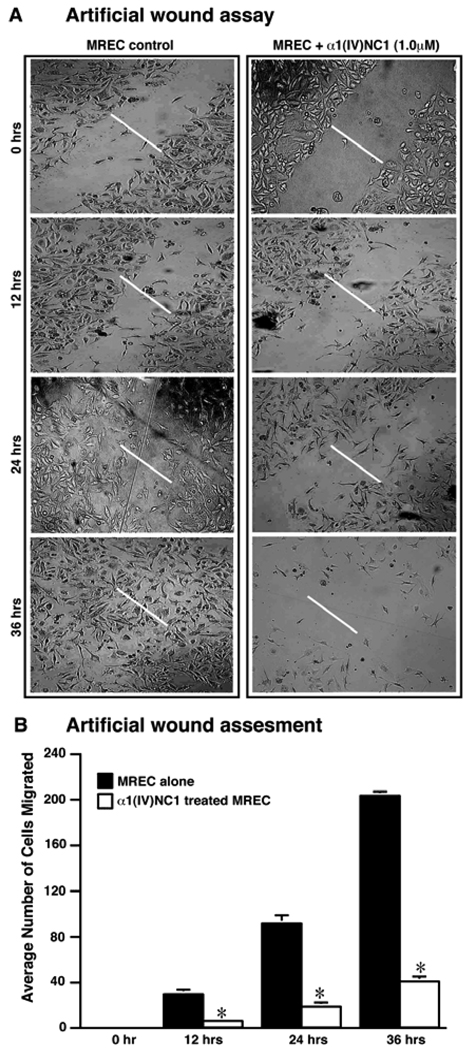
The decrease in proliferation of MREC incubated with α1(IV)NC1 was associated with a significant reduction in the migration of these cells. Consistent with reduced proliferation and migration of MREC incubated with α1(IV)NC1 on type IV collagen, MREC also showed attenuation of migration in scratch wound assays (Figure 4A; α1(IV)NC1). In addition we also observed apoptotic MREC when incubated with α1(IV)NC1. Prolonged incubation of MREC with α1(IV)NC1 resulted in their rounding and detachment from the matrix, indicating onset of apoptotic events. Moreover, the number of adherent MREC decreased and the floating cells increased when compared to control cells suggesting that the detachment of cells is due to the presence of α1(IV)NC1. The average number of migrated MREC into the scratch wound at different times with and without α1(IV)NC1 treatment in three independent experiments was shown in the graph (Figure 4B).
Figure 4.
Impaired migration and proliferation of MREC incubated with α1(IV)NC1 in a scratch wound assay. (A) MREC were cultured to 80% confluence in 24-well plates in serum containing medium. Wounds were created in the MREC monolayer using a sterile pipette tip. Photographs were taken immediately and later at indicated time intervals after creating the wounds. Data is from one representative experiment. Similar results were also obtained in two independent experiments. (B) Bar diagram represents quantification of the wound-healing assessment from duplicate wells of three independent experiments (n=6). *P<0.001 for control Vs. α1(IV)NC1 treated MREC.
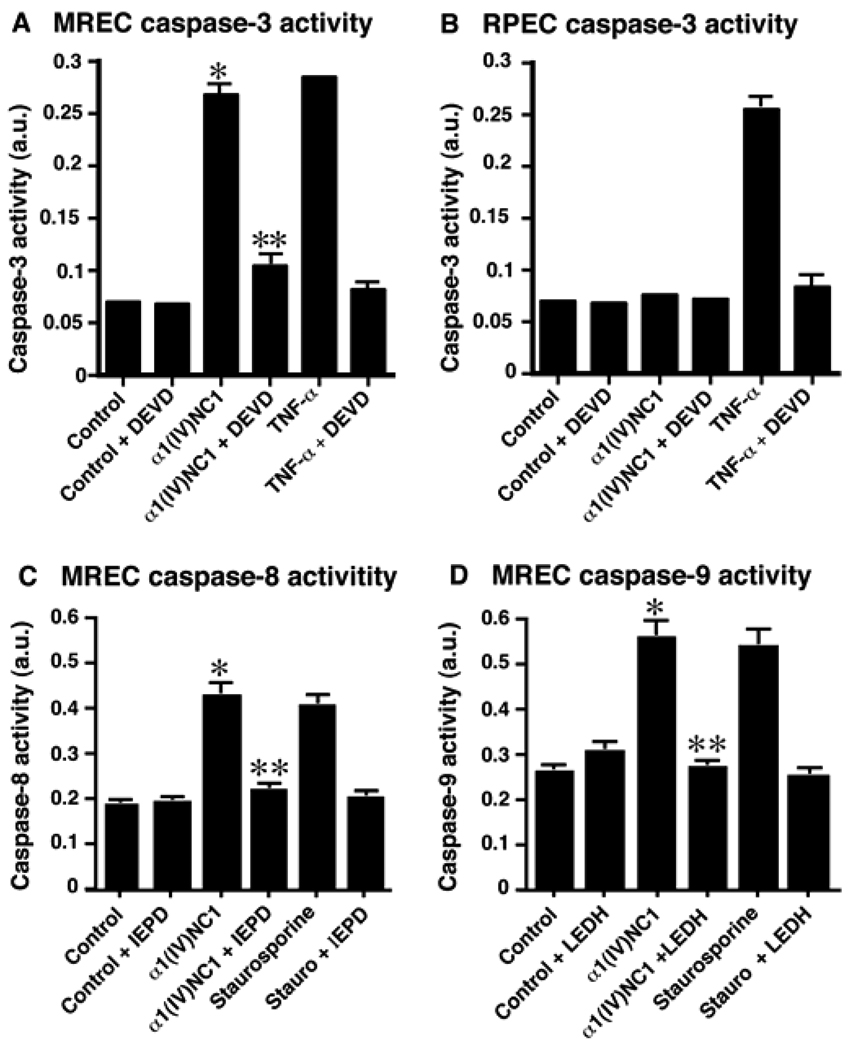
Impairment of in-vitro apoptotic activity of α1(IV)NC1 by caspase inhibitors
In tracing the signaling mechanisms involved in rapid impairment of cell proliferation which precedes loss of MREC viability and irreversible commitment to cell death upon incubation with α1(IV)NC1, we identified that α1(IV)NC1 induces apoptosis in MREC (Figure 5A). To study whether caspase-3 could be activated by α1(IV)NC1, we incubated MREC with α1(IV)NC1 and observed activation of caspase-3 (Figure 5A). Caspase-3 is a pivotal molecule mediating cellular apoptosis26. If this activation of caspase-3 by α1(IV)NC1 is necessary for cellular apoptosis, a caspase-3 specific inhibitor should abolish α1(IV)NC1 induced apoptosis in MREC. Incubation of MREC with z-DEVD (a specific caspase-3 inhibitor) showed complete suppression α1(IV)NC1 induced cellular apoptosis and inhibition of caspase-3 activity (Figure 5A; α1(IV)NC1+DEVD). Whereas similar apoptotic caspase-3 activation was not observed in MRPE cells incubated with α1(IV)NC1 (Figure 5B, α1(IV)NC1). These results suggest pro-apoptotic action of α1(IV)NC1 through activation of caspase-3 is specific to MREC.
Figure 5.
Caspase-3 activation. (A and B) MREC and MRPE cells incubated with and without α1(IV)NC1, and cytosolic extracts were analyzed for caspase-3 activity. DEVD-fmk and TNF-α was used as a positive control. Results shown in panels A and B were significant; mean±standard errors of the mean [SEM] of three independent experiments are shown. *P< 0.001 compared to control and **P<0.001 compared to α1(IV)NC1 treatment. (C and D) Activation of caspase-8 and -9 by α1(IV)NC1 at 24h as measured by cleavage of specific substrate IEDP-AMC and LEDH-AFC. Staurosporine used as a positive control. The results are shown as mean ± SEM from three independent experiments. *P< 0.005 compared to control and **P<0.005 compared to α1(IV)NC1 treatment.
We further evaluated whether α1(IV)NC1 induces activation of upstream caspases such as caspase-8 and -9. We treated MREC with α1(IV)NC1 and observed activation of caspase-8 and -9 (Figure 5C and D). If this activation of caspase-8 and -9 by α1(IV)NC1 is playing role in cellular apoptosis, specific inhibitors should abolish α1(IV)NC1 induced caspase-8 and -9 activities in MREC. Incubation of MREC with z-IEPD-fmk, z-LEHD-fmk (a specific caspase-8 and-9 inhibitors) showed suppression of α1(IV)NC1 induced cellular apoptosis and inhibition of caspase-8 and-9 activity (Figure 5C and D; α1(IV)NC1+IEPD and α1(IV)NC1+LEDH).
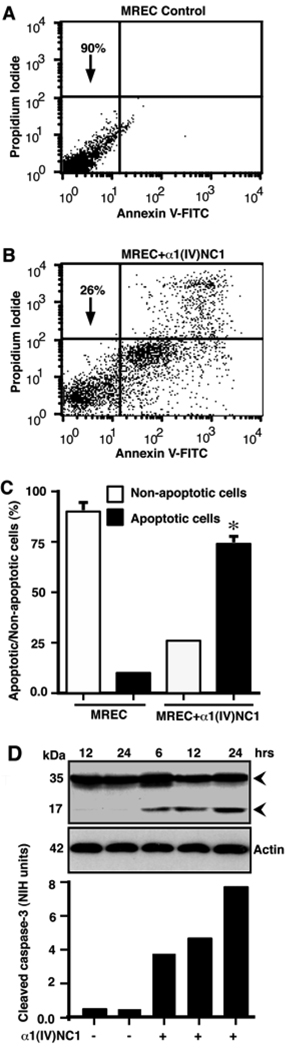
FACS analysis of MREC apoptosis by α1(IV)NC1
We further investigat the activation of apoptosis in MREC incubated with and without α1(IV)NC1 using a fluorescence-activated cell sorting (FACS) analysis. We quantified cellular apoptosis by measuring propidium iodide staining vs. annexin V-FITC content in MREC. MREC incubated with control medium containing VEGF showed more than 90% cell viability while apoptosis was observed in less than 10% of total cell population (Figure 6A). Addition of α1(IV)NC1 under similar conditions decreased MREC viability to 26% indicating about 74% of cells undergoing apoptosis (Figure 6B). A comprehensive statistics of the observed apoptotic and non-apoptotic MREC (incubated with and without α1(IV)NC1) is summarized in figure 6C. Among the known caspases, caspase-3 is an important effector molecule for most cellular apoptosis26. To study whether caspase-3 could be activated by α1(IV)NC1, we incubated MREC with α1(IV)NC1 for different time periods and observed activation of caspase-3 in a time dependent manner (Figure 6D, lower blot). The time dependent activation of caspase-3 band intensities is shown in figure 6D (upper graph).
Figure 6.
MREC were treated with and without α1(IV)NC1 and stained with annexin V-FITC and PI, and analyzed by flow cytometry. (A and B) Dot blots showing with and without α1(IV)NC1 treated MREC survival that was measured by annexin V and PI negative cell populations as indicated by arrows. (C) Bar graph indicates % annexin V and PI negative cells after growing on type IV collagen coated tissue cultures plates for 24h. Similar results were also obtained in two other independent experiments conducted in triplicates. *P<0.005 α1(IV)NC1 treated apoptotic Vs non apoptotic MREC. (D) Control and α1(IV)NC1 treated MREC were lysed, and caspase-3 activity was determined with caspase-3 specific antibody in top panel (upper and lower arrows in the immunoblot indicates pro and active caspase-3). Bottom panel in D, graphical representation of relative densities of pixels and area of activated caspase-3 bands were determined with NIH image software.
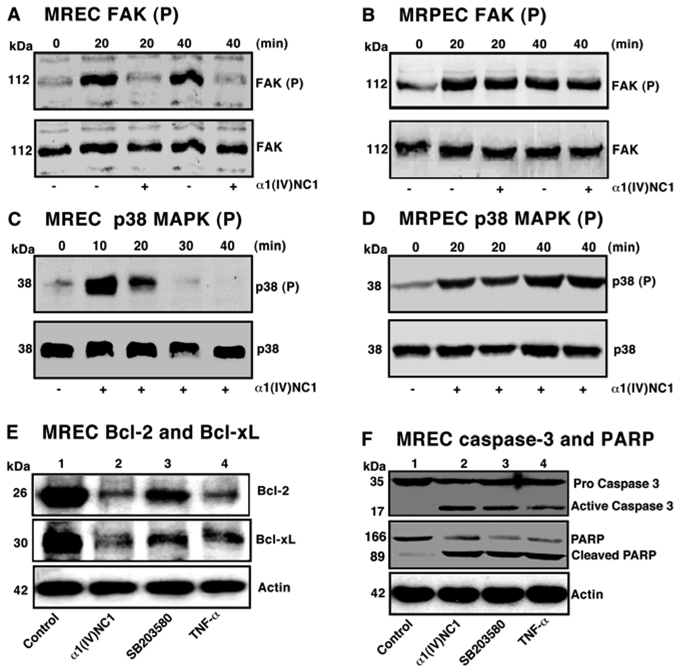
Specific apoptotic effects of α1(IV)NC1 on p38-MAPK signaling in regulation of Bcl-2/Bcl-xL and caspase-3/PARP activation in MREC
In this study we investigated whether α1(IV)NC1 is playing a role in activation of caspase-3 mediated MREC apoptosis via inactivation of p38-MAPK pathway? p38-MAPK pathway is an important downstream target of FAK that is critical for the regulation of endothelial cell migration27, 28. Mice deficient in FAK display severe impaired vasculogenesis and cell migration, while over expression significantly increases the migratory capacity of cells via activation of the MAPK pathway29, 30. These findings suggest that type IV collagen integrins/FAK/p38-MAPK pathway may play a major role in regulation of matrix-mediated migration. Attachment of VEGF treated MREC cells to type IV collagen activated FAK/p38 pathway (data not shown). Pretreatment of MREC with α1(IV)NC1 did not inhibit expression of FAK and p38-MAPK, but significantly blocked sustained phosphorylation of FAK and p38-MAPK (Figure 7A and C, top panels). In endothelial cells, VEGF induced migration and cytoskeletal reorganization is mediated by ERKs-MAPK, whereas, the expression of integrins and proteases is regulated by p38-MAPK31, 32. In contrast, incubation of MRPE cells with α1(IV)NC1 had no effect on phosphorylation of FAK and p38MAPK on type IV collagen matrix, suggesting that α1(IV)NC1 induced signaling is specific to endothelial cell (Figure 7B and D, upper panel).
Figure 7.
(A and B) FAK phosphorylation in MREC and MRPE. Immunoblots for phospho-FAK indicate that VEGF mediated phosphorylation of FAK (p-FAK) was inhibited in MREC incubated with α1(IV)NC1 but not in MRPE (A–B, top blot) and total FAK proteins (A and B, lower blots). (C and D) p38-MAPK phosphorylation in MREC and MRPE. Immunoblots for phospho-p38 indicate that VEGF mediated sustained phosphorylation of p38 (p-p38) was inhibited by incubation with α1(IV)NC1 in MREC but not in MRPE (C and D, upper blot) and total p38-MAPK (C and D, lower blot). (E and F) Effect of α1(IV)NC1 on p38 MAPK dependent regulation of Bcl-2/Bcl-xL and activation of Caspase-3/PARP. VEGF stimulated MREC treated with PBS (lane 1, control), 1µM α1(IV)NC1 (lane 2), 20 µM SB203580, a specific p38-MAPK inhibitor (lane 3) and 80ng/ml TNF-α̣ (lane 4) for 24h. Total cells were collected, lysed for 30 min in ice-cold RIPA lysis buffer and 25 µg of total cytosolic extract per lane was separated and immunoblotted with primary antibodies against signaling molecules in the mitochondrial apoptotic pathway.
To understand whether p38-MAPK pathway is involved in the pro-apoptotic activity of α1(IV)NC1, we have examined changes in the expression of pro-apoptotic (Bax) and anti-apoptotic (Bcl-2, Bcl-xL) proteins in MREC treated with α1(IV)NC1. α1(IV)NC1 treated MREC did not show any effect on Bax expression (data not shown), where as expression of Bcl-xL and Bcl-2 were significantly inhibited at 24h (Figure 7E, lane 2 Vs. 1). Earlier we reported that α1(IV)NC1 regulates HIF-1α expressioṇ dependent on p38-MAPK13. Here, we evaluated whether α1(IV)NC1 regulated Bcl-2 and Bcl-xL expressions were mediating through p38-MAPK dependent. A specific p38-MAPK inhibitor (SB203580) should inhibit Bcl-2 and Bcl-xL expressions, treatment of MREC with SB203580 showed inhibition of Bcl-2 and Bcl-xL conforming, expression of these anti-apoptotic molecules in MREC by α1(IV)NC1 is dependent on p38-MAPK (Figure 7E, lane 3). This is consistence with p38-MAPK mediated endothelial cell apoptosis via down regulation of Bcl-xL and Bcl-233. Here, we also identified that activation of caspase-3 and PARP cleavage down stream to Bcl-2/Bcl-xL in MREC treated with α1(IV)NC1 was p38-MAPK dependent (Figure F, lane 2 Vs.1). In addition treatment of MREC with p38-MAPK inhibitor (SB203580) showed activation of caspase-3 and PARP cleavage further conforming that inactivation of p38-MAPK pathway by α1(IV)NC1 impacts MREC apoptosis (Figure 7E, lane 3).
Regulation of VEGF induced neovascularization by α1(IV)NC1
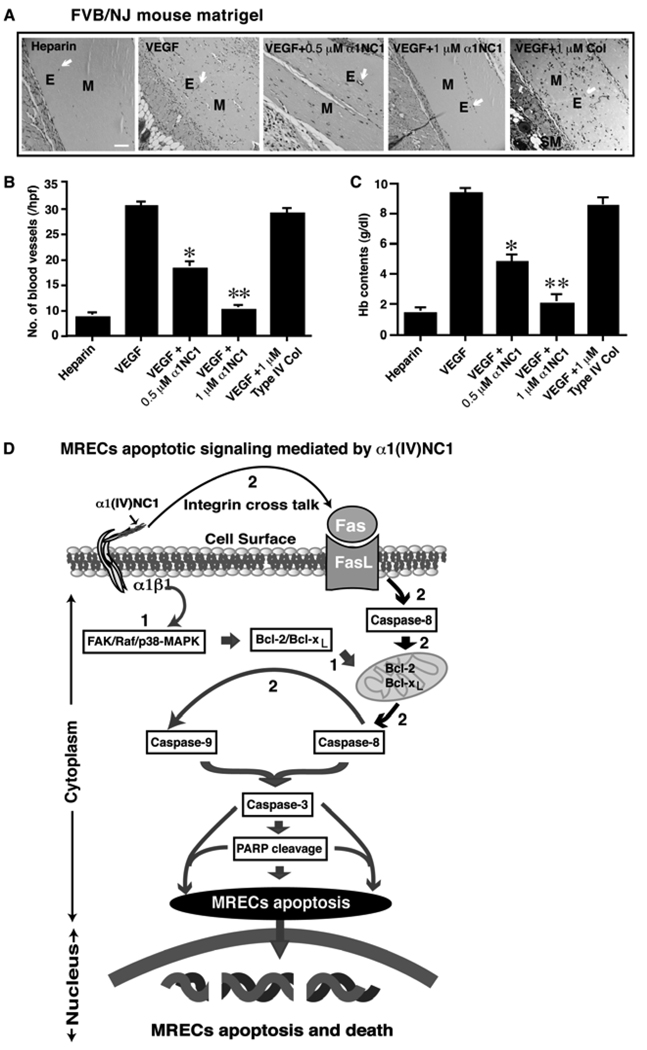
We further evaluated the effects of baculovirus expressed recombinant human α1(IV)NC1 on VEGF-mediated angiogenesis in-vivo using the Matrigel plug assay in FVB/NJ mice. Matrigel plugs containing VEGF were used to assess the role of different doses of α1(IV)NC1 (0.5 to 1.0µM) in inhibition of VEGF induced neovascularization. At 1.0µM concentration of α1(IVNC1 significantly inhibited (nearly 88%) VEGF induced neovascularization in the Matrigel plugs (Figure 8A). The number of blood vessels in different Matrigel plugs are as follows: VEGF+α1(IV)NC1 (0.5µM), 17.45±0.28; VEGF+α1(IV)NC1 (0.5µM), 11.05±0.15; and controls, VEGF with and without Collagen type IV or, 30.0±2.5 (Figure 8B). In contrast, the hemoglobin contents in different Matrigel plugs are as follows: VEGF+α1(IV)NC1 (0.5µM) treated was 4.15±0.25g/dl (n=6) or VEGF+α1(IV)NC1 (1.0µM) treated was 2.75±0.16g/dl (n=6); and hemoglobin content in VEGF with and without type IV collagen controls was 10.0±1.2g/dl (n=6) (Figure 8C). These results suggest that administration of recombinant α1(IV)NC1 inhibits VEGF mediated neovascularization in-vivo. A schematic illustration of distinct apoptotic signaling induced by α1(IV)NC1 in MREC apoptosis presumably through inhibition of FAK/p38-MAPK/Bcl-2/Bcl-xL and caspase-3 activation and PARP cleavage was shown (Figure 8).
Figure 8.
Regulation of VEGF induced neovascularization of Matrigel matrix implants in mice. (A) FVB/NJ mice Matrigel. From left to right different conditions of Matrigel are shown. Arrows point to the blood vessels. E, M, and SM represent endothelial cells, Matrigel and smooth muscle cells. Scale bar: 50µm. (B and C) Number of blood vessels and hemoglobin (Hb) content quantification from A. The mean ± SEM are shown. *p<0.01 compared VEGF with and without α1(IV)NC1 (0.5µM). **p<0.005 compared VEGF with and without α1(IV)NC1 (1µM). The number of blood vessels in the Matrigel plug was counted in 10 fields at x200 magnification (n=6). (D) Schematic illustration of distinct apoptotic signaling induced by α1(IV)NC1 in MREC. α1(IV)NC1 binds to α1β1 integrin and initiates two apoptotic pathways, involving activation of caspase-9 and -8, leading to activation of caspase-3 and PARP cleavage. (1) α1(IV)NC1 activates caspase-3 directly through inhibition of FAK/p38-MAPK/Bcl-2/Bcl-xL and activation of caspase-9; (2) Integrin cross talk with Fas through mitochondrial pathway and lead to activations caspase-8 and-3.
Discussion
The NC1 domain generated from type IV collagen α1 chain α1(IV)NC1 was identified as an antiangiogenic molecule and its activity is mediated through α1β1 integrin, specifically in proliferating endothelial cells13, 34. However, it is critical to examine α1(IV)NC1 effects in several well defined relevant in-vitro model culture systems before assuming that this NC1 domain is universally antiangiogenic. In this study, we examined the effects of baculovirus expressed human α1(IV)NC1 protein in in-vitro and in-vivo model of neovascularization. We demonstrate, for the first time, that α1(IV)NC1 inhibits VEGF induced MREC proliferation, migration, tube formation via FAK/p38-MAPK pathway. In addition we discovered pro-apoptotic effect of α1(IV)NC1 mediating through regulation of anti-apoptotic Bcl-xL/Bcl-2 expression and activation of caspase-3/PARP cleavage through inhibition of p38-MAPK signaling. This is consistent with the previous studies demonstrating that antiangiogenic activity of α1(IV)NC1 is mediated through α1β1 integrin and inhibition of hypoxia mediated signaling and apoptosis13, 35. α1(IV)NC1 inhibited phosphorylation of FAK and p38-MAPK when MREC were plated on type IV collagen matrix. Similarly, we previously reported other collagen NC1 domain α3(IV)NC1, inhibits phosphorylation of FAK on fibronectin matrix18. Understanding the mechanism(s) of action of these molecules is crucial for their therapeutic development and use.
To further support angiogenesis inhibition observed in MREC incubated with α1(IV)NC1 in culture, we assessed its activity in vivo. The intravenous administration of α1(IV)NC1 caused selective inhibition of endothelial cells growth in the VEGF stimulated Matrigel matrix resulting in suppression of neovascularization. Thus, α1(IV)NC1, which has previously been shown to suppress tumor angiogenesis in different mouse models, is also a strong antiangiogenic agent in MREC and may be an effective therapeutic candidate for treatment of neovascular diseases in the eye.
Current evidence indicates that VEGF is a major angiogenic factor and plays a prominent role in ocular neovascularization36. VEGF is massively up regulated by hypoxia and its levels are increased in the retina and vitreous of patients, as well as in animal models of ischemic retinopathies37–39. VEGF is responsible for the growth of new blood vessels by stimulating endothelial cells proliferation and migration36. Increased expression of VEGF in transgenic mice stimulates neovascularization within the retina and VEGF receptor antagonists inhibit retinal and choroidal neovascularization in many animal models40, 41. Compared to VEGF expression, there is less evidence implicating basic fibroblast growth factor (FGF-2) in the development of ocular neovascularization42.
Recently many researchers noticed that several antiangiogenic drugs [Macugen (pegaptanib sodium), Lucentis (formerly RhuFab V2), tryptophanyl-tRNA synthetase (TrpRS), VEGF-TRAP, AdPEDF, AG-013958, Avastin (bevacizumab), JSM6427 etc] inhibit ocular neovascularization and prevent leakiness of retinal blood vessels in-vivo by preventing binding of VEGF to its receptors on endothelial cells6, 43–48. A known endogenous angiogenesis inhibitor α2(IV)NC1 (canstatin), was recently reported to inhibit laser induced CNV by inducing apoptosis of endothelial cells49. Our findings suggest that α1(IV)NC1 may also be effective in inhibition of choroidal neovascularization. This is further supported by earlier findings that type IV collagen derived NC1 domains regulate angiogenesis in a number of in-vitro and in-vivo models11. Thus, this work not only supports our efforts in development of α1(IV)NC1 as a potential candidate for treatment of ocular diseases with a neovascular component, but also support our ongoing oncology development efforts.
ACKNOWLEDGEMENTS
This study was supported by Flight Attendant Medical Research Institute Young Clinical Scientist Award Grant FAMRI# 062558, Dobleman Head and Neck Cancer Institute Grant #61905, startup funds of Cell Signaling and Tumor Angiogenesis Laboratory at BTNRH to AS, AACR-AstraZeneca Scholar-in-Training Award to CSB and also supported (in part) by NIH grant EY16995 and Retinal Research Foundation to NS.
References
- 1.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Garner A. Ocular angiogenesis. Int Rev Exp Pathol. 1986;28:249–306. [PubMed] [Google Scholar]
- 3.Jampol LM, Ebroon DA, Goldbaum MH. Peripheral proliferative retinopathies: an update on angiogenesis, etiologies and management. Surv Ophthalmol. 1994;38:519–540. doi: 10.1016/0039-6257(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi H, Tamaki Y, Ishii N, et al. Identification of a novel vascular endothelial growth factor receptor 2 inhibitor and its effect for choroidal neovascularization in vivo. Curr Eye Res. 2008;33:1002–1010. doi: 10.1080/02713680802492440. [DOI] [PubMed] [Google Scholar]
- 6.Deissler HL, Lang GE. Effect of VEGF165 and the VEGF aptamer pegaptanib (Macugen) on the protein composition of tight junctions in microvascular endothelial cells of the retina. Klin Monatsbl Augenheilkd. 2008;225:863–867. doi: 10.1055/s-2008-1027767. [DOI] [PubMed] [Google Scholar]
- 7.Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 8.Ingber D, Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988;59:44–51. [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 10.Maragoudakis ME, Missirlis E, Karakiulakis GD, Sarmonica M, Bastakis M, Tsopanoglou N. Basement membrane biosynthesis as a target for developing inhibitors of angiogenesis with anti-tumor properties. Kidney Int. 1993;43:147–150. doi: 10.1038/ki.1993.24. [DOI] [PubMed] [Google Scholar]
- 11.Pedchenko V, Zent R, Hudson BG. Alpha(v)beta3 and alpha(v)beta5 integrins bind both the proximal RGD site and non-RGD motifs within noncollagenous (NC1) domain of the alpha3 chain of type IV collagen: implication for the mechanism of endothelia cell adhesion. J Biol Chem. 2004;279:2772–2780. doi: 10.1074/jbc.M311901200. [DOI] [PubMed] [Google Scholar]
- 12.Colorado PC, Torre A, Kamphaus G, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 13.Sudhakar A, Nyberg P, Keshamouni VG, et al. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J Clin Invest. 2005;115:2801–2810. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–178. [PubMed] [Google Scholar]
- 15.Borza CM, Pozzi A, Borza DB, et al. Integrin alpha3beta1, a novel receptor for alpha3(IV) noncollagenous domain and a trans-dominant Inhibitor for integrin alphavbeta3. J Biol Chem. 2006;281:20932–20939. doi: 10.1074/jbc.M601147200. [DOI] [PubMed] [Google Scholar]
- 16.Boosani CS, Sudhakar A. Cloning, purification, and characterization of a non-collagenous anti-angiogenic protein domain from human alpha1 type IV collagen expressed in Sf9 cells. Protein Expr Purif. 2006;49:211–218. doi: 10.1016/j.pep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res. 2001;130:123–132. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 18.Boosani CS, Mannam AP, Cosgrove D, et al. Regulation of COX-2 mediated signaling by α3 type IV noncollagenous domain in tumor angiogenesis. Blood. 2007;110:1168–1177. doi: 10.1182/blood-2007-01-066282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munugalavadla V, Borneo J, Ingram DA, Kapur R. p85alpha subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood. 2005;106:103–109. doi: 10.1182/blood-2004-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells. J Immunol Methods. 2002;265:97–110. doi: 10.1016/s0022-1759(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 21.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 22.Bagley RG, Walter-Yohrling J, Cao X, et al. Endothelial precursor cells as a model of tumor endothelium: characterization and comparison with mature endothelial cells. Cancer Res. 2003;63:5866–5873. [PubMed] [Google Scholar]
- 23.McCawley LJ, Matrisian LM. Tumor progression: defining the soil round the tumor seed. Curr Biol. 2001;11:R25–R27. doi: 10.1016/s0960-9822(00)00038-5. [DOI] [PubMed] [Google Scholar]
- 24.Huang Q, Sheibani N. High Glucose Promotes Retinal Endothelial Cell Migration through Activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008 doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkman J, Haudenschild C. Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K. 1980;100:346–353. [PubMed] [Google Scholar]
- 26.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 27.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 28.Roberts MS, Woods AJ, Shaw PE, Norman JC. ERK1 associates with alpha vbeta 3 integrin and regulates cell spreading on vitronectin. J Biol Chem. 2002;19:19. doi: 10.1074/jbc.M208607200. [DOI] [PubMed] [Google Scholar]
- 29.Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 30.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase- promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker PM, Verin AD, Booth MA, Liu F, Birukova A, Garcia JG. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1500–L1511. doi: 10.1152/ajplung.2001.281.6.L1500. [DOI] [PubMed] [Google Scholar]
- 32.Kuo CJ, LaMontagne KR, Jr, Garcia-Cardena G, et al. Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J Cell Biol. 2001;152:1233–1246. doi: 10.1083/jcb.152.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grethe S, Ares MP, Andersson T, Porn-Ares MI. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L) Exp Cell Res. 2004;298:632–642. doi: 10.1016/j.yexcr.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Sudhakar A, Boosani CS. Signaling Mechanisms of Endogenous Angiogenesis Inhibitors Derived from Type IV Collagen. Gene Regulation and System Biology. 2007;1:217–226. doi: 10.4137/grsb.s345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyberg P, Xie L, Sugimoto H, et al. Characterization of the anti-angiogenic properties of arresten, an alpha1beta1 integrin-dependent collagen-derived tumor suppressor. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith LE, Kopchick JJ, Chen W, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 37.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 38.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 39.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–291. [PMC free article] [PubMed] [Google Scholar]
- 41.Tobe T, Okamoto N, Vinores MA, et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998;39:180–188. [PubMed] [Google Scholar]
- 42.Tobe T, Ortega S, Luna JD, et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantel I, Zografos L, Ambresin A. Early clinical experience with ranibizumab for occult and minimally classic neovascular membranes in age-related macular degeneration. Ophthalmologica. 2008;222:321–323. doi: 10.1159/000144075. [DOI] [PubMed] [Google Scholar]
- 44.Konstantinidis L, Mantel I, Pournaras JA, Zografos L, Ambresin A. Intravitreal ranibizumab (Lucentis(R)) for the treatment of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008 doi: 10.1007/s00417-008-0995-0. [DOI] [PubMed] [Google Scholar]
- 45.Siemann DW, Shi W. Dual targeting of tumor vasculature: combining Avastin and vascular disrupting agents (CA4P or OXi4503) Anticancer Res. 2008;28:2027–2031. [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen QD, Shah SM, Hafiz G, et al. A phase I trial of an IV-administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113:1522, e1521–e1522, e1514. doi: 10.1016/j.ophtha.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 47.Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 48.Lu F, Adelman RA. Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization? Graefes Arch Clin Exp Ophthalmol. 2009;247:171–177. doi: 10.1007/s00417-008-0936-y. [DOI] [PubMed] [Google Scholar]
- 49.Lima ESR, Kachi S, Akiyama H, et al. Recombinant non-collagenous domain of alpha2(IV) collagen causes involution of choroidal neovascularization by inducing apoptosis. J Cell Physiol. 2006;208:161–166. doi: 10.1002/jcp.20645. [DOI] [PubMed] [Google Scholar]