Abstract
Thyroid eye disease (TED) is an inflammatory condition of the orbit closely associated with Graves' disease. During the course of TED, fibrosis can develop around the extraocular muscles, and excess extracellular matrix and fat accumulates in the periorbital space. This dramatic remodeling results in protrusion of the eye, also known as exophthalmos. Current treatments are sometimes effective in alleviating the symptoms of the disease, but there remains a demand for treatments that prevent or reverse the pathological alterations of orbital tissues. Such treatments may become available as a result of research aimed at understanding the mechanism by which Graves' disease leads to specific remodeling of orbital tissues. Recent findings have uncovered the importance of intercellular communication between autoreactive T cells and orbital fibroblasts. When orbital fibroblasts are activated, possibly by Graves' disease–related autoantibodies, they release T cell chemoattractants, initiating an interaction in which these cells activate each other. These interactions ultimately result in fibroblasts expressing extracellular matrix molecules, proliferating and differentiating into myofibroblasts or lipofibroblasts. Although the mechanisms underlying these processes are not completely understood, several currently available therapeutic strategies might interrupt the signaling between B and T cells and fibroblasts, thereby treating the clinical manifestations of TED.
Introduction
Graves' disease is an autoimmune condition in which the thyroid-stimulating hormone receptor (TSH-R) displayed on thyrocytes is targeted by autoantibodies, inducing the production of excess thyroid hormone (1). Besides hyperthyroidism, up to 60% of patients with Graves' disease develop a manifestation localizing to the orbit called Graves' ophthalmopathy or thyroid eye disease (TED) (2,3). TED occurs more frequently in patients older than 50 years of age and in males, smokers, and those with microvascular disease (2,4). TED affects many eye functions, including wetting of the ocular surface, eye motility, optic nerve function, and eyelid anatomy (2,5). The most dramatic pathological findings in end-stage TED include glycosaminoglycan (GAG) deposition (accompanied by dramatic swelling resulting from the water-binding capacity of these macromolecules) and fibrosis affecting the extraocular muscles and fat accumulation in the orbit (5–8). The increased volume of orbital connective tissue leads to protrusion of the eye (exophthalmos) (2,9). To date, there are no effective means of preventing the disease or reliably altering its course. Current therapeutic options include corticosteroids, external beam radiation, and surgery. These interventions are aimed at the consequences of the disease rather than targeting its cause, which remains poorly understood (5,6,10,11). They do nothing to prevent or reverse pathological remodeling of orbital tissues (10). Understanding the pathogenetic mechanisms underlying TED should yield the identification of predictive biomarkers of progressive disease and effective and specific treatments.
The close association of TED with Graves' disease of the thyroid provides clues to its etiology. The hyperthyroid condition itself does not impact directly on the accumulation of connective tissue within the orbit (1,2). The risk for developing TED is similar for euthyroid and hyperthyroid Graves' disease patients (2,12). Rather, the orbit constitutes a second target of autoimmunity. Therefore, understanding the role of the immune system in orbital inflammation is critical to developing treatment strategies for TED patients.
Cell Types Implicated in TED
From the little we know about TED, there are at least three cell types involved in the development and progression of the disease: B cells, T cells, and orbital fibroblasts. As in the hyperthyroidism of Graves' disease, B cells are important in the early stages of TED, producing antibodies against at least one self-antigen (1,13). However, development of TED is postulated to require a second initiating event that results in recruitment of activated T cells to the orbit (1). These T cells take on the role of amplifying the B cell response and are thought to be major contributors to disease progression (14). Cells (namely, orbital fibroblasts) that recruit the T cells into the orbit are critical players in the establishment of inflammation (15). Fibroblasts provoke T cell recruitment and then engage them in a cycle of brief reciprocal activation ending in tissue remodeling characteristic of TED (14). This review will explore the interactions among these three cell types that contribute to the disease phenotype and which may provide targets for therapeutic strategies.
B and T Cells
The most important cells for adaptive immunity, and thus for autoimmunity, are B and T cells. B cells, including two major subsets (B1 and B2), migrate and proliferate extensively and develop into antibody-secreting plasma cells when they encounter foreign antigen in the proper costimulatory context. Dual signals are required for B cell activation, differentiation, and the production of antibodies. One signal is provided by antigen binding to the B cell receptor. The second signal can be provided through interaction between CD40 on the surface of the B cell and CD40 ligand (CD40L, CD154) on the surface of a T cell (16–18). The interaction with CD40L stimulates T cell secretion of cytokines such as IL-4 that are critical to B cell activation and immunoglobulin class switching (19). Initially, differentiated B cells produce antibodies in the form of IgM, and the CD40–CD40L interaction is required for class switching to IgG or IgE. T cells, like B cells, are migratory and highly proliferative in response to antigen. However, T cells are more diverse than B cells, differentiating into many different types of effector cells, including CD8+ cytotoxic T cells and CD4+ helper T cells of the Th1, Th2, Th17, and Treg subsets (20). The context in which T cells recognize antigen is more constrained. Detection of antigen occurs through surface T cell receptors (TCRs) that recognize specific peptides presented in association with major histocompatibility complex (MHC) proteins on the surface of antigen-presenting cells (APCs). These include macrophages, dendritic cells, and B cells. T cells also require dual signals for activation. The first signal is provided by antigen/MHC binding to the TCR (1,21). The second signal is often a B7 molecule on the surface of an APC, possibly a B cell, which binds to CD28 on the T cell surface (1,17,21,22). CD40L can interact with CD40 on the surface of the APC, resulting in increased B7 expression. This in turn can lead to increased interaction with CD28 and further T cell activation (17,21,22). T cell activation results in proliferation and differentiation to an effector cell type. If one signal is present in the absence of another, the T cell will fail to become activated and may become refractory to future activation (1,23). This phenomenon, known as “peripheral anergy,” is one of several tolerance mechanisms preventing the development of autoimmunity (23,24).
Tolerance mechanisms sometimes fail and autoimmunity develops. Cells of the Th17 subset of T cells have been implicated in the onset of many autoimmune and inflammatory diseases, including arthritis, multiple sclerosis, psoriasis, and inflammatory bowel disease (20). It may be hypothesized that autoreactive Th17 cells infiltrate the orbit, where they activate B cells to secrete autoantibodies. Th17 cells are defined by their expression of IL-23R (20), and variants in the IL-23R gene have been shown to be strongly associated with the occurrence of TED (25). Further work is required to firmly identify the role of the Th17 subset in TED initiation. However, by an unknown mechanism, production of autoantibodies by autoreactive B cells does occur in TED patients (1,13). The TSH-R has been implicated as an autoantigen targeted in TED, largely based on the close association of this condition with the hyperthyroidism of Graves' disease (26). TSH-R is expressed on the surface of orbital fibroblasts, and TSH-R immunoreactivity has been demonstrated specifically in TED orbits. Insulin-like growth factor-1 receptor (IGF-1R) represents another self-antigen with a pathogenic role in TED (8,27). This receptor is displayed by orbital fibroblasts from patients with TED at a higher level than those from normal orbits (28). Further, antibody directed against the IGF-1R (a) provokes the synthesis of hyaluronan in orbital fibroblasts from TED, but not in those from normal orbits, and (b) induces orbital fibroblasts from patients with TED to produce the T cell chemoattractants IL-16 and RANTES (8,28). Patients with Graves' disease exhibiting anti-TSH-R antibody production may also develop antibodies against the IGF-1R and other orbital antigens. This may occur as a result of extraocular muscle inflammation and damage, as is the case with antibodies to extraocular muscle proteins, which are present in TED patients, but do not contribute to disease progression (29,30). Alternatively, production of autoantibodies against additional self-antigens may result from a process known as determinant spreading (31). Immunoglobulin synthesized by autoreactive B cells can bind an epitope of a macromolecular complex. Then, acting as an APC, the B cell processes peptides from this complex for presentation to specific T cells. Because it processed a macromolecular complex, the B cell may present specific peptides that do not replicate the antigen to which it originally bound, or the antigen against which its antibodies will be directed. However, T cells specific for the antigen will be able to activate the B cell. In this way, T cells can activate multiple B cell clones and produce several autoantibodies. This phenomenon may underlie the large variety of autoantibodies detected in patients with autoimmune disease. Since TED is an autoimmune condition, therapeutic strategies designed to block the loss of immune tolerance and the inappropriate activation of B and T cells may constitute treatments for the condition in its earliest stages. For example, there are agents currently available that disrupt the B7–CD28 and CD40–CD40L interactions, as well as antibody therapies that deplete B and T cells (13,32–34). Rituximab (RTX), a monoclonal antibody directed against the B cell surface antigen CD20, is one such B cell–depleting agent (13). Case reports and one open pilot study demonstrate a marked amelioration of eye symptoms in TED patients treated with RTX (35–37), supporting a role for B cells in the pathogenesis of TED.
Orbital fibroblasts
TED occurs with the hyperthyroidism of Graves' disease, but the correlation is not perfect (2). Many patients never develop clinically important orbital disease. Some patients with TED fail to develop hyperthyroidism. These variations in clinical presentation suggest that development of TED requires multiple “hits.” The initial hit could be the generation of an autoantibody within the orbit (26). However, this may be inadequate for triggering disease in some patients. Another autoantibody may represent a second hit. Additional factors might include the genetic predisposition of the individual, environmental factors such as tobacco smoke or infection (1,2,4,8). Once sufficient hits occur, orbital fibroblasts can be activated to initiate inflammation characteristic of TED. Greater understanding of these provocative factors should enhance our development of effective treatment strategies.
Fibroblasts were once viewed as mere structural elements of the microenvironment. They were thought only to produce extracellular matrix components, but otherwise to play no role in tissue homeostasis. Now, it has become apparent that fibroblasts are highly interactive “sentinel cells,” capable of detecting danger signals and communicating these to cells of the “professional” immune system. Fibroblasts respond directly to these signals by proliferating and differentiating into effector cells (1,38,39). They respond to immune stimulation and actively participate in inflammation pathways through their synthesis of chemokines, cytokines, and lipid mediators. Orbital fibroblasts are not only responsible for generating GAGs and other macromolecules, but they also differentiate to either myofibroblasts (i.e., scar-forming cells) or to lipofibroblasts (i.e., adipocytes) (40–43). These attributes implicate fibroblasts as effectors in the progression of TED. The earliest stage of TED involves infiltration of the orbit by T cells (5,6,10,11,44,45). Evidence supports the concept that infiltrating T lymphocytes activate fibroblasts. In doing so, they promote the synthesis of extracellular matrix, differentiation to adipocytes, and myofibroblast proliferation (14). T cell–fibroblast interactions are mediated through costimulatory molecules, adhesion molecules, and cytokines, including IFNγ, IL-1β, and TNFα (14,46). GAG and prostaglandin synthesis in orbital fibroblasts is upregulated by CD40 engagement (39,47,48). However, fibroblasts might also play a role in initiating the early stage T cell infiltration of the orbit. Stimulated fibroblasts secrete multiple cytokines, including IL-6, which stimulates B cell differentiation. IL-16 and RANTES are chemoattractants that initiate T cell migration (15,48–50). Fibroblasts can also function as APCs, providing a second signal for lymphocyte activation. Those fibroblasts from patients with TED constitutively express MHC II and CD40 (39,51), both of which are upregulated by IFNγ (46,48). They can also drive proliferation of autologous T cells (52). T cell responsiveness to fibroblasts may mediate T cell infiltration in early disease and amplify inflammation.
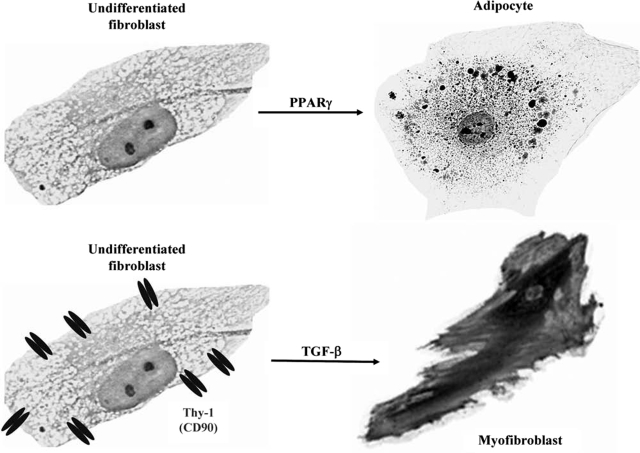
Fibroblasts differ from one tissue to another (39,41,47,48,53). Those present in a particular tissue, such as the orbit, might derive from one of several sources. They could transdifferentiate from epithelial cells, muscle cells, or even endothelium (54). Alternatively, they could be recruited from the bone marrow as circulating “fibrocytes” (55,56). The origin of activated fibroblasts involved in the pathogenesis of TED remains uncertain. Orbital fibroblasts exist as multiple subsets (53,57). Each can differ in morphology, proliferation, biosynthetic capacity, and cell surface marker expression. Two major subsets of orbital fibroblast exist (41,49,53) based on their expression of the surface protein Thy-1 (CD90). The balance between Thy-1–negative and Thy-1–positive fibroblasts in the orbit is essential for normal regulation of inflammation since these subsets have distinct biosynthetic capabilities, differing in cytokine, collagen, and prostaglandin E2 (PGE2) production (49). However, this balance may also underlie the development and progression of TED. As shown in Figure 1, depending on their pericellular environment and phenotype, fibroblasts can differentiate into myofibroblasts or lipofibroblasts (40,41). Myofibroblasts participate in normal wound healing and in the pathogenesis of fibrosis, such as that occurring in orbital connective tissue in TED (42). The presence of lipofibroblasts is usually indicative of pathology. In TED, they result in excess orbital fat deposition (58). The potential for terminal differentiation in fibroblasts depends on the expression of Thy-1. TGF-β triggers the differentiation of Thy-1–positive fibroblasts into myofibroblasts, identified by their relatively high level of alpha‐smooth muscle actin (α-SMA) expression (40). Thy-1–negative fibroblasts preferentially differentiate into adipocytes, as evidenced by staining with oil red O (40,41). Lipid accumulation is driven by the ligation of peroxisome proliferator activated receptor γ (PPARγ) with an agonist.
FIG. 1.
Fibroblasts can be stimulated to differentiate into myofibroblasts or lipofibroblasts. This potential may depend on expression of Thy-1 (CD90). TGF-β triggers the differentiation of Thy-1–positive fibroblasts into myofibroblasts, while PPARγ activation induces adipocyte differentiation of mainly Thy-1–negative cells.
T Cell–Fibroblast Interactions
PPARγ is a nuclear receptor that functions as a transcription factor. It binds lipid ligands, dimerizes with retinoid-X receptor alpha (RXRα), translocates to the nucleus, and alters the transcription of genes containing peroxisome proliferator responsive elements (59). These genes include those that dampen inflammation by decreasing TNFα, IL-6, and IL-8 production (60). Other PPARγ genes are also important to adipogenesis. PPARγ ligands trigger the differentiation of fibroblasts to lipofibroblasts (61–65). These agonists promote glucose uptake; thus, they have a therapeutic value in Type 2 diabetes. Insulin-sensitizing drugs, such as ciglitazone, pioglitazone, and rosiglitazone, bind to and activate PPARγ (61–64,66). Naturally occurring PPARγ ligands include lysophosphatidic acid, some fatty acids, prostaglandin D2 (PGD2), and 15-deoxy-prostaglandin J2 (15d-PGJ2) (60,62,66,67). PGD2 and 15d-PGJ2 are derived from arachidonic acid (68). Their synthesis is dependent on the activity of cyclooxygenase-2 (Cox-2) and PGD synthase enzymes (68,69). Activated T cells express both Cox-2 and PGD synthase; they also produce 15d-PGJ2 (58,70,71).
The response of orbital fibroblasts to PPARγ agonists provides a potentially important link between these cells and T cell activation. 15d-PGJ2 treatment of Thy-1–negative orbital fibroblasts from patients with TED initiates adipocytic differentiation (40). T cells from these patients constitutively express Cox-2 and secrete high levels of 15d-PGJ2 (58). It is thus possible to envision a mechanism through which T cell infiltration of the orbit could result in adipocytic differentiation of fibroblasts. In fact, coculture of these fibroblasts with activated T cells results in accumulation of cytoplasmic lipid droplets in the fibroblasts. Understanding these complex interactions may lead to the development of new therapeutic strategies for TED. Blockade of PPARγ-dependent adipogenesis may alleviate fat expansion in this disease (72).
Conclusions
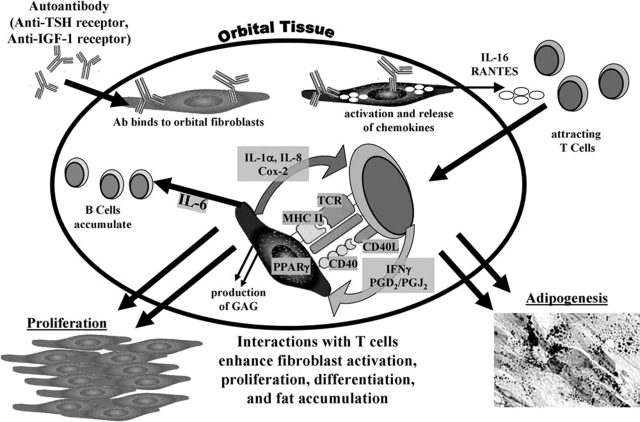
A summary of our current model for the pathogenesis of TED is depicted in Figure 2. The key elements involve binding and activation of orbital fibroblasts by autoantibodies, produced by autoreactive B cell–derived plasma cells (13). Trafficking of T cells to the orbit ultimately leads to complex interactions of fibroblasts with these T cells and the deposition of extracellular matrix molecules. Proliferation and differentiation of fibroblast subsets into either fat-laden adipocytes (14) or scar-forming myofibroblasts culminates in the increased orbital connective tissue volume and remodeling that underlie the clinical manifestations of TED (9). Although the disease mechanisms through which TED develops are not fully elucidated, several potential therapeutic strategies might interrupt fibroblast, T cell, and B cell activation in the orbit. General suppression of immune function, such as occurs with corticosteroids, has produced inconsistent results and is associated with undesirable side effects (5,6,10). To avoid these side effects, it is important to target more selective aspects of the disease. The development of autoantibodies against IGF-1R may be prevented through the use of agents that deplete B cell and T cell numbers (13,34) or that disrupt the B7–CD28 and CD40–CD40L interactions that are essential to T and B cell activation (32,33,48). Once orbital fibroblasts have been activated and the amplification of the disease process has begun, antagonists to cytokine and chemokine receptors may be used to interrupt communications between fibroblasts and lymphocytes that drive morphological changes (73). Finally, PPARγ signaling may be blocked to prevent fibroblast adipogenesis and fat accumulation, either by inhibiting Cox-2 activity in the T cells (58,71,74), thus eliminating release of natural PPARγ agonists, or through the use of direct PPARγ antagonists (72). Targeting more specific aspects of the disease based on expanded understanding of its pathophysiology should provide many opportunities to prevent or alleviate progression of TED.
FIG. 2.
One current model focuses on TED being triggered by activation of orbital fibroblasts by autoantibodies. These autoantibodies could be specific for antigens such as TSH-R and/or IGF-1R. Activated orbital fibroblasts release chemokines, including IL-16 and RANTES, which traffic T cells into the orbit. These lymphocytes then interact with fibroblasts, potentially activating each other, further promoting cytokine production (IFNγ, PGD2, and 15d-PGJ2) and secretion of T cell-activating factors by the fibroblasts (IL-1α, IL-8, and products of Cox-2 activity). Fibroblasts are also stimulated to secrete IL-6, which stimulates B cell differentiation. The interactions of fibroblasts with T cells result in the deposition of extracellular matrix molecules, fibroblast proliferation, and differentiation.
Acknowledgments
This research was supported by EY014564, EY017123, EY011708, DE011390, EY08976, DK063121, T32 HL66988, and the Research to Prevent Blindness Foundation. The continued support of the Bell Charitable Foundation is gratefully acknowledged.
References
- 1.Prabhakar BS. Bahn RS. Smith TJ. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev. 2003;24:802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 2.Burch HB. Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–793. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 3.Heufelder AE. Weetman AP. Ludgate M. Bahn RS. Pathogenesis of Graves' ophthalmopathy. In: Prummel MF, editor; Wiersinga WM, editor; Mourits MP, editor; Heufelder AE, editor. Recent Developments in Graves' Ophthalmopathy. Kluwer Academic Publishers; Boston: 2000. pp. 15–37. [Google Scholar]
- 4.Bartalena L. Marcocci C. Pinchera A. Graves' ophthalmopathy: a preventable disease? Eur J Endocrinol. 2002;146:457–461. doi: 10.1530/eje.0.1460457. [DOI] [PubMed] [Google Scholar]
- 5.Bahn RS. Heufelder AE. Pathogenesis of Graves' ophthalmopathy. N Engl J Med. 1993;329:1468–1475. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 6.Feldon SE. Weiner JM. Clinical significance of extraocular muscle volumes in Graves' ophthalmopathy: a quantitative computed tomography study. Arch Ophthalmol. 1982;100:1266–1269. doi: 10.1001/archopht.1982.01030040244006. [DOI] [PubMed] [Google Scholar]
- 7.Nunery WR. Nunery CW. Martin RT. Truong TV. Osborn DR. The risk of diplopia following orbital floor and medial wall decompression in subtypes of ophthalmic Graves' disease. Ophthal Plast Reconstr Surg. 1997;13:153–160. doi: 10.1097/00002341-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Smith TJ. Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 9.Hatton MP. Rubin PA. The pathophysiology of thyroid-associated ophthalmopathy. Ophthalmol Clin North Am. 2002;15:113–119. doi: 10.1016/s0896-1549(01)00004-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu D. Feldon SE. Thyroid ophthalmopathy. Ophthalmol Clin North Am. 1992;5:597–622. [Google Scholar]
- 11.Wiersinga WM. Prummel MF. Pathogenesis of Graves' ophthalmopathy—current understanding. J Clin Endocrinol Metab. 2001;86:501–503. doi: 10.1210/jcem.86.2.7338. [DOI] [PubMed] [Google Scholar]
- 12.Kim JM. LaBree L. Levin L. Feldon SE. The relation of Graves' ophthalmopathy to circulating thyroid hormone status. Br J Ophthalmol. 2004;88:72–74. doi: 10.1136/bjo.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Fassi D. Nielsen CH. Hasselbalch HC. Hegedus L. The rationale for B lymphocyte depletion in Graves' disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. Eur J Endocrinol. 2006;154:623–632. doi: 10.1530/eje.1.02140. [DOI] [PubMed] [Google Scholar]
- 14.Feldon SE. Park DJ. O'Loughlin CW. Nguyen VT. Landskroner-Eiger S. Chang D. Thatcher TH. Phipps RP. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves' ophthalmopathy. Invest Ophthalmol Vis Sci. 2005;46:3913–3921. doi: 10.1167/iovs.05-0605. [DOI] [PubMed] [Google Scholar]
- 15.Pritchard J. Horst N. Cruikshank W. Smith TJ. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–950. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 16.Noelle RJ. Roy M. Shepherd DM. Stamenkovic I. Ledbetter JA. Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin D. Zhang L. Wang R. Radvanyi L. Haudenschild C. Fang Q. Kehry MR. Shi Y. Ligation of CD28 in vivo induces CD40 ligand expression and promotes B cell survival. J Immunol. 1999;163:4328–4334. [PubMed] [Google Scholar]
- 18.Ray DM. Akbiyik F. Bernstein SH. Phipps RP. CD40 engagement prevents peroxisome proliferator-activated receptor gamma agonist-induced apoptosis of B lymphocytes and B lymphoma cells by an NF-kappaB-dependent mechanism. J Immunol. 2005;174:4060–4069. doi: 10.4049/jimmunol.174.7.4060. [DOI] [PubMed] [Google Scholar]
- 19.Kehry MR. Hodgkin PD. Helper T cells: delivery of cell contact and lymphokine-dependent signals to B cells. Semin Immunol. 1993;5:393–400. doi: 10.1006/smim.1993.1045. [DOI] [PubMed] [Google Scholar]
- 20.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Van Gool SW. Vandenberghe P. de Boer M. Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 22.Howland KC. Ausubel LJ. London CA. Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 23.Powell JD. The induction and maintenance of T cell anergy. Clin Immunol. 2006;120:239–246. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Melchers F. Anergic B cells caught in the act. Immunity. 2006;25:864–867. doi: 10.1016/j.immuni.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Huber AK. Jacobson EM. Jazdzewski K. Concepcion ES. Tomer Y. IL-23R is a major susceptibility gene for Graves' ophthalmopathy: the IL-23/Th17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab. 2008;93:1077–1081. doi: 10.1210/jc.2007-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahn RS. Dutton CM. Natt N. Joba W. Spitzweg C. Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 27.Smith TJ. The putative role of fibroblasts in the pathogenesis of Graves' disease: evidence for the involvement of the insulin-like growth factor-1 receptor in fibroblast activation. Autoimmunity. 2003;36:409–415. doi: 10.1080/08916930310001603000. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard J. Han R. Horst N. Cruikshank WW. Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170:6348–6354. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 29.Khoo TK. Bahn RS. Pathogenesis of Graves' ophthalmopathy: the role of autoantibodies. Thyroid. 2007;17:1013–1018. doi: 10.1089/thy.2007.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YJ. Clarke EM. Shepherd P. Prevalence and significance of antibodies reactive with eye muscle membrane antigens in sera from patients with Graves' ophthalmopathy and other thyroid and nonthyroid diseases. Thyroid. 1998;8:167–174. doi: 10.1089/thy.1998.8.167. [DOI] [PubMed] [Google Scholar]
- 31.Dai YD. Carayanniotis G. Sercarz E. Antigen processing by autoreactive B cells promotes determinant spreading. Cell Mol Immunol. 2005;2:169–175. [PubMed] [Google Scholar]
- 32.Nogid A. Pham DQ. Role of abatacept in the management of rheumatoid arthritis. Clin Ther. 2006;28:1764–1778. doi: 10.1016/j.clinthera.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Huang W. Sinha J. Newman J. Reddy B. Budhai L. Furie R. Vaishnaw A. Davidson A. The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum. 2002;46:1554–1562. doi: 10.1002/art.10273. [DOI] [PubMed] [Google Scholar]
- 34.Chatenoud L. Anti-CD3 antibodies: towards clinical antigen-specific immunomodulation. Curr Opin Pharmacol. 2004;4:403–407. doi: 10.1016/j.coph.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 35.El Fassi D. Nielsen CH. Hasselbalch HC. Hegedus L. Treatment-resistant severe, active Graves' ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16:709–710. doi: 10.1089/thy.2006.16.709. [DOI] [PubMed] [Google Scholar]
- 36.Salvi M. Vannucchi G. Campi I. Rossi S. Bonara P. Sbrozzi F. Guastella C. Avignone S. Pirola G. Ratiglia R. Beck-Peccoz P. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154:511–517. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 37.Salvi M. Vannucchi G. Campi I. Curro N. Dazzi D. Simonetta S. Bonara P. Rossi S. Sina C. Guastella C. Ratiglia R. Beck-Peccoz P. Treatment of Graves' disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 38.Smith TJ. Insights into the role of fibroblasts in human autoimmune diseases. Clin Exp Immunol. 2005;141:388–397. doi: 10.1111/j.1365-2249.2005.02824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RS. Smith TJ. Blieden TM. Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 40.Koumas L. Smith TJ. Feldon S. Blumberg N. Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith TJ. Koumas L. Gagnon A. Bell A. Sempowski GD. Phipps RP. Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 42.Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res. 1981;54:183–194. [PubMed] [Google Scholar]
- 43.Kumar S. Coenen MJ. Scherer PE. Bahn RS. Evidence for enhanced adipogenesis in the orbits of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 2004;89:930–935. doi: 10.1210/jc.2003-031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weetman AP. Cohen S. Gatter KC. Fells P. Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989;75:222–227. [PMC free article] [PubMed] [Google Scholar]
- 45.Drexhage HA. Weetman AP. Heufelder AE. Feldon SE. Future research in Graves' ophthalmopathy. In: Prummel MF, editor; Wiersinga WM, editor; Mourits MP, editor; Heufelder AE, editor. Recent Developments in Graves' Ophthalmopathy. Kluwer Academic Publishers; Boston: 2000. p. 187. [Google Scholar]
- 46.Heufelder AE. Bahn RS. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993;23:10–17. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 47.Cao HJ. Wang HS. Zhang Y. Lin HY. Phipps RP. Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273:29615–29625. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 48.Sempowski GD. Rozenblit J. Smith TJ. Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998;274:C707–C714. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 49.Koumas L. Smith TJ. Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1- subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32:477–485. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 50.Sciaky D. Brazer W. Center DM. Cruikshank WW. Smith TJ. Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol. 2000;164:3806–3814. doi: 10.4049/jimmunol.164.7.3806. [DOI] [PubMed] [Google Scholar]
- 51.Heufelder AE. Smith TJ. Gorman CA. Bahn RS. Increased induction of HLA-DR by interferon-gamma in cultured fibroblasts derived from patients with Graves' ophthalmopathy and pretibial dermopathy. J Clin Endocrinol Metab. 1991;73:307–313. doi: 10.1210/jcem-73-2-307. [DOI] [PubMed] [Google Scholar]
- 52.Otto EA. Ochs K. Hansen C. Wall JR. Kahaly GJ. Orbital tissue-derived T lymphocytes from patients with Graves' ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996;81:3045–3050. doi: 10.1210/jcem.81.8.8768872. [DOI] [PubMed] [Google Scholar]
- 53.Smith TJ. Sempowski GD. Wang HS. Del Vecchio PJ. Lippe SD. Phipps RP. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80:2620–2625. doi: 10.1210/jcem.80.9.7673404. [DOI] [PubMed] [Google Scholar]
- 54.Postlethwaite AE. Shigemitsu H. Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733–738. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- 55.Lama VN. Phan SH. The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quan TE. Cowper S. Wu SP. Bockenstedt LK. Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Fries KM. Blieden T. Looney RJ. Sempowski GD. Silvera MR. Willis RA. Phipps RP. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72:283–292. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 58.Feldon SE. O'Loughlin CW. Ray DM. Landskroner-Eiger S. Seweryniak KE. Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol. 2006;169:1183–1193. doi: 10.2353/ajpath.2006.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willson TM. Brown PJ. Sternbach DD. Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 60.Daynes RA. Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 61.Rangwala SM. Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Kliewer SA. Lenhard JM. Willson TM. Patel I. Morris DC. Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 63.Barak Y. Nelson MC. Ong ES. Jones YZ. Ruiz-Lozano P. Chien KR. Koder A. Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 64.Viles-Gonzalez JF. Choi BG. Fuster V. Badimon JJ. Peroxisome proliferator-activated receptor ligands in atherosclerosis. Expert Opin Investig Drugs. 2004;13:1393–1403. doi: 10.1517/13543784.13.11.1393. [DOI] [PubMed] [Google Scholar]
- 65.Harris SG. Padilla J. Koumas L. Ray D. Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 66.Forman BM. Tontonoz P. Chen J. Brun RP. Spiegelman BM. Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 67.Yu K. Bayona W. Kallen CB. Harding HP. Ravera CP. McMahon G. Brown M. Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 68.Shibata T. Kondo M. Osawa T. Shibata N. Kobayashi M. Uchida K. 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J Biol Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 69.Smith WL. Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001;107:1491–1495. doi: 10.1172/JCI13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pablos JL. Santiago B. Carreira PE. Galindo M. Gomez-Reino JJ. Cyclooxygenase-1 and -2 are expressed by human T cells. Clin Exp Immunol. 1999;115:86–90. doi: 10.1046/j.1365-2249.1999.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L. Zhang L. Yi Y. Kang HK. Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat Med. 2004;10:411–415. doi: 10.1038/nm1005. [DOI] [PubMed] [Google Scholar]
- 72.Rieusset J. Touri F. Michalik L. Escher P. Desvergne B. Niesor E. Wahli W. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocrinol. 2002;16:2628–2644. doi: 10.1210/me.2002-0036. [DOI] [PubMed] [Google Scholar]
- 73.Ribeiro S. Horuk R. The clinical potential of chemokine receptor antagonists. Pharmacol Ther. 2005;107:44–58. doi: 10.1016/j.pharmthera.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Kawamori T. Rao CV. Seibert K. Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]