Abstract
Solvent “lens” effects for the reaction kinetics of NO2 can be evaluated on the basis of published Henry’s law constants for nitrogen dioxide in various solvents. Water-to-organic solvent partition coefficients were derived from Henry’s law constants and used to assess the tendencies of NO2 toward fleeing the aqueous environments and concentrating in biological hydrophobic media. It is concluded, based only on the estimated aqueous medium-to-cell membrane partition coefficient for NO2, that such tendencies will be relatively small, and that they may account for an acceleration of chemical reactions in biological hydrophobic media with reaction kinetics that are first order on NO2 by a factor of approximately 3 ± 1. Thus, kinetic effects due to mass action will be relatively small but it is also important to recognize that because NO2 will tend to dissolve in cell membranes, reactions with cell membrane components will not be hindered by lack of physical solubility at these loci. In comparison to other gases, nitrogen dioxide is less hydrophobic than NO, O2 and N2.
Keywords: Nitrogen dioxide, partition coefficient, Henry’s law constant, solubility, kinetics, reactivity, compartmentation
Introduction
Exposures to nitrogen dioxide (NO2) can occur in industrial and occupational settings, and during war and acts of terror involving the use of explosives. Moreover, NO2 is present in polluted air [1–4] and also can be formed endogenously [5–11]. The reaction of peroxynitrite with carbon dioxide, reactions catalyzed by myeloperoxidase using nitrite as the electron donor, and acidification of nitrite followed by oxidation of the resulting nitric oxide (NO) are among the biological processes that can result in the endogenous formation of NO2 [5;7–9;12]. Nitrogen dioxide is involved in biological oxidations, including the nitration of protein tyrosine residues and possibly also the nitration of membrane lipids, reactions with low molecular weight antioxidants, and the oxidation of thiol residues. Cell signaling and covalent modifications mediated by NO2 will depend in part on its physicochemical properties relevant to passive transport, such as its ability to diffuse and penetrate into membranes, its relative solubility in membranes and aqueous solutions, and its ability to chemically react within the membranous or aqueous milieu. Despite the biological importance of NO2, it has been recently pointed out that the relative solubility of NO2 in membranes and aqueous medium is unknown [13–15], revealing the lack of even the most essential parameter necessary to interpret the reactivity of NO2 across different cellular and biological compartments. In contrast, the solubility of NO in several solvents has been studied in detail and this has led to the proposal for the existence of an acceleration or “lens” effect [13;14] whereby the hydrophobicity of NO helps focus some of the chemical reactivity of NO, such as its autoxidation, in cell membranes and lipid particles. This brief exercise presented here represents an attempt to estimate the relative solubility of NO2 in biological membranes and any possible ensuing acceleration or “lens” effects in NO2 reaction kinetics.
Methods
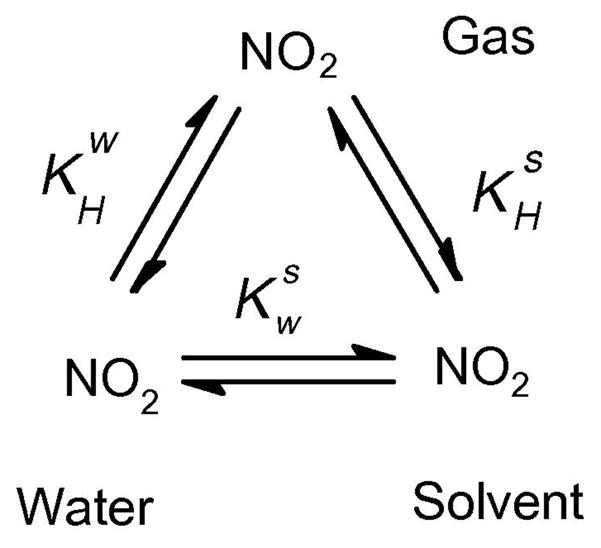
Solvent “lens” effects [13;14] for the reaction kinetics of NO2 are evaluated herein on the basis water-to-organic solvent partition coefficients ( ) as these coefficients may result in high, compartmentalized concentrations of NO2, that thereby increase the rates of reaction of NO2 in these compartments. Values of were derived from Henry’s law constants for NO2 ( ) calculated from published solubility studies of gaseous NO2 in various solvents [16;17] (see equations 5, 6 and 8 below, for operations applied in this process). When necessary, data were corrected for temperature to 37 °C using equation 7 and published enthalpies of solution (Δsol H) for NO2 in various solvents [17;18].
Results
On Solvent “Lens” Effects in Reaction Kinetics
Moller et al. studied the acceleration of the autoxidation of NO when NO is allowed to partition between water and organic media [13]. Since under a wide range of experimental conditions the rate law for the autoxidation of NO was found to be
| (1) |
and diffusion across compartments is not rate-limiting, these authors interpreted the acceleration they observed as arising from the larger concentration of NO in the organic media resulting from the hydrophobicities of NO and O2 [13]. It is important to recognize that solvent effects on the rate constant k could either amplify or dampen effects due to reactants’ hydrophobicities since the rate constant is subject to solvent effects. However, it is probable that hydropobicity is the dominant factor in the “lens” effect since the rate constant in equation (1) in the solvent carbon tetrachloride has been found to be close to that in aqueous solution [14;19].
On Estimating the Solvent “Lens” effect for NO2
Studies on the solubility of NO2 are experimentally challenging and it is not surprising that related literature is rather scarce. The solubility studies for NO2 are complicated by the reactivity of NO2 and N2O4 with many solvents. [Caution: The authors are aware of one explosion with fire that occurred in a research laboratory while using NO2 in tetrahydrofuran.] It is also important to consider that NO2 dimerizes to form N2O4 and the latter can react with traces of water present in solvents (Equations 2–4).
| (2) |
| (3) |
| (4) |
Despite the complications arising from the hydrolysis reaction (Equation 4), water is perhaps the most studied solvent, owing to the environmental importance of NO2, and a number of reactive uptake studies have been conducted in water from which Henry’s law constants for NO2 in water can be derived [18;20]. In contrast, few gaseous NO2-organic solvent partitions [16;17] had been measured and apparently, no organic solvent-to-water partition coefficients for NO2 had been reported to date.
The solubility of a gas in a solvent can be described in different ways, but usually, the Henry’s law constant is used to this effect. Thus, for NO2, we can write equation (5):
| (5) |
where [NO2]s is the molar concentration of NO2 in organic solvent s and pNO2 is the partial pressure of NO2 in the gas phase in units of atmospheres. When the solvent s is water (w), we write . Water-to-organic solvent partition coefficients ( ) can then be calculated by dividing the Henry’s law constant for organic solvent ( ) by the equivalent for water ( ) using equation (6):
| (6) |
Cheung et al.[18] compared literature values for for NO2 with values they obtained and recommend for 20 °C and for 3 °C. From these two values, using equation (7), one can calculate an approximate enthalpy of solution (Δsol H) for NO2 in water of −20 kJ mol−1 and extrapolate for 37 °C.
| (7) |
We identified two published solubility studies for gaseous NO2 in organic solvents[16;17] and the data published in these articles are summarized in Table 1. Mendiara et al. published Henry’s law constants in terms of molar fractions and in units of atm ( ) [16] and these were converted to (units of M atm−1) using equation 8, which is valid for dilute solutions, where MWs and ρs represent the molecular weight and the density of solvent s, respectively.
Table 1.
Henry’s law constants for nitrogen dioxide in various solvents and water-to-organic solvent partition coefficients at 37 °C. From left to right, second column: Henry’s law constants (M atm−1) at temperatures specified in the corresponding reference; third column: Henry’s law constants for 37 °C calculated using Eq. (7) as indicated in the text; fourth column, water-to-organic solvent partition coefficients calculated using Eq. (6) with values for and for 37 °C.
| (8) |
Values of were extrapolated to 37 °C using Δsol H = −12 kJ mol−1 for 1,1,2,2-tetrachloroethane [17], chloroform and carbon tetrachloride. Δsol H = −11 kJ mol−1 was used for n-decane [17]; Δsol H = −9 kJ mol−1 was used for nitrate esters[17] and Δsol H = −20 kJ mol−1, which was derived from data recommended by Cheung et al. [18], was used for water. Nitrate esters refer to 1,4- and 2-3-butylene glycol dinitrate, 1,2-propylene glycol dinitrate, and glyceryl trinitrate, all of which exhibited similar solubility properties [17]. Although not specified by Lur’e et al. [17], it is assumed here that these authors used pure chemicals (and not mixtures of isomers) in their study and that they refer to the linear isomer of decane (i.e. n-decane) and to the 1,1,2,2-tetrachloroethane isomer of tetrachloroethane that is produced at an industrial scale and is unreactive towards NO2.
Henry’s law constants for NO2, , shown in Table 1, are 1.2 to 6.7 times larger for organic solvents compared to water. The relative insensitivity of to changes in solvent suggests the NO2-solvent interactions are of a very general type, as has been previously interpreted for NO [21]. This slight preference that NO2 has for dissolving in organic solvents relative to water has implications, for example, on reactive uptake of gas phase NO2 by the lung epithelial lining fluid layer (ELF) that is covered by a thin film composed primarily of phospholipids and surfactant proteins and must be taken into account when modeling the local dose of NO2 in the respiratory tract. Thus, for example, it becomes important to compare Henry’s law constants for NO2 in water to those in organic solvents since a higher solubility of NO2 in the lipids near the air/lung ELF interface will tend to enhance NO2 reactive uptake [18] by providing a subphase where NO2 can dissolve that is vicinal to biological reactive targets (if all other experimental parameters remained unchanged).
Henry’s law constants and water-to-organic solvent partition coefficients for N2, O2, NO, and NO2 are shown on Table 2 [22–24]. In order to simplify comparisons, only the same or similar solvents for which these constants are known for NO2 are shown. It is interesting to note that NO2 is more soluble in all solvents, including water, than the other gases. However, since the most marked difference occurs when the solvent is water, this results in a lower hydrophobicity for NO2.
Table 2.
Henry’s law constants (M atm−1) and water-to-organic solvent partition coefficients for N2, O2, NO, and NO2 at 25 °C [16,17,21–24].
| Solvent | N2 |
O2 |
NO |
NO2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCl4 | 0.663 | 10.1 | 1.24 | 9.90 | 1.43 | 7.35 | 1.49 | 1.21 | ||||||||
| CHCl3 | 0.552 | 8.40 | 1.16 | 9.26 | 1.62a | 8.3a | 1.99 | 1.62 | ||||||||
| 1,1,2,2-Tetrachloroethane | — | — | — | — | — | — | 7.34 | 5.95 | ||||||||
| n-Decane | 0.623 | 9.52 | 1.13 | 9.01 | 1.27a | 6.49a | 5.96 | 4.85 | ||||||||
| n-Hexane | 1.06 | 16.2 | 1.53 | 12.2 | 1.80 | 9.26 | — | — | ||||||||
| Nitrate esters | — | — | — | — | — | — | 4.33 | 3.52 | ||||||||
| 1-Propyl nitrate ester | 0.589 | 9.00b | 0.832b | 6.62 | — | — | — | — | ||||||||
| Nitrobenzene | — | — | — | — | 0.786 | 4.03 | — | — | ||||||||
| Waterc | 0.0656 | 1 | 0.125 | 1 | 0.195 | 1 | 1.23 | 1 | ||||||||
Calculated using Abraham’s solvation equations.
At 20 °C.
.
In order to compare NO2 to NO (since biological “lens” effects have been reported for the latter [13;14]) we begin by selecting a value for NO at 37 °C. The most recent values for NO compiled by Sander [20] as well as a recent determination by Zacharia et al. [25] average 1.9×10−3 M atm−1 at 25 °C. Then, to correct this value to 37 °C, we employ the enthalpy of solution Δsol H for NOin water, taken as −13 kJ mol−1, which is the average of values compiled by Sander [20] and the heat of solution derived from solubility data at various temperatures given in Perry and Green [26]. Then, using equation (6), one arrives at for NO at 37 °C.
Henry’s law constants and water-to-organic solvent partition coefficients for NO and NO2 at 37 °Care shown in Table 3. Henry’s law constants for NO at 25 °C and enthalpies of solvation in several solvents, which were used here for temperature corrections to 37 °C, were reported by Shaw et al. [27]. In addition, the solvation equation of Abraham [21;28] was used as predictor for the partition coefficients for NO in chloroform and n-decane since experimental data for NO in these solvents are not available but are available for NO2. Since the enthalpies of solvation are unknown for chloroform and n-decane, the values for carbon tetrachloride and n-hexane, respectively, were used as surrogate values to calculate . We are now in a position to compare values for NO with NO2 at 37 °C (Table 3). In order to facilitate comparisons, when experimental data are not available for the same solvent for NO and NO2, experimental values for related solvents are shown in Table 3.
Table 3.
Henry’s law constants (M atm−1) for NO and water-to-organic solvent partition coefficients for NO and NO2 in various solvents at 37 °C.
| Solvent | NO |
NO2 | |||
|---|---|---|---|---|---|
| ; 37 °C | |||||
| CCl4 | 1.38 | 8.3 | 1.2 | ||
| CHCl3 | 1.58 | 10 | 1.9 | ||
| 1,1,2,2-Tetrachloroethane | — | — | 6.7 | ||
| Cyclohexane | 1.77 | 11 | — | ||
| n-Hexane | 1.74 | 11 | — | ||
| n-Decane | 1.23 | 7.7 | 5.5 | ||
| Nitrate esters | — | — | 4.2 | ||
| Nitrobenzene | 0.76 | 4.8 | — | ||
| Water | 0.16 | 1.0 | 1.0 | ||
Discussion
One can notice that all values are higher than one, indicating that both NO and NO2 are more soluble in organic solvents than they are in water, more so NO than is NO2. However, even for NO, the water-to-organic solvent relative solubilities are not very pronounced. Nevertheless, there will be some thermodynamic tendency for NO and NO2 to flee the aqueous environment where they will most likely be produced. Thus, NO is 7.7–11 times more soluble in hydrocarbons, 8.3 times more soluble in carbon tetrachloride, 10 times more soluble in chloroform, and 4.8 times more soluble in nitrobenzene than it is in water. Indeed, the “lens” effect or acceleration of the autoxidation of NO in liposomes by a factor of ~ 30 relative to aqueous solution has been explained using water-to-liposome partition coefficients of 3.6 and 3.2 for NO and O2, respectively [13;14;29]. Thus, when one considers the form of the rate expression in equation (1), a theoretical acceleration factor of (3.6)2(3.2) = 41, which is not very different from the observed value of ~30, can be calculated. It is important to recognize that it is because the rate expression is second order in NO and first order in O2 and that because both NO and O2 are relatively hydrophobic it is that the overall effect results in a large acceleration factor.
In comparison to NO, the relative solubilities of NO2 for organic solvents are less pronounced. NO2 is 5.5 times more soluble in the hydrocarbon n-decane, 1.2 times more soluble in carbon tetrachloride, 1.9 times more in chloroform, and 6.7 times more soluble in 1,1,2,2-tetrachloroethane than it is in water. Going towards more polar solvents, NO2 is 4.2 times more soluble in the nitrate esters, 1,4- and 2-3-butylene glycol dinitrate, 1,2-propylene glycol dinitrate, and glyceryl trinitrate than it is in water. Thus, for NO2 in these solvents, the water-to-organic solvent partition coefficients vary from 1.2 to 6.7, suggesting that NO2 is slightly hydrophobic but less so than is NO.
Averaging across the hydrocarbons, the for NO is 9.9 while the for NO2 in n-decane is 5.5; across the chlorocarbons, the average for NO is 9.2 while the average for NO2 is 3.3. The for NO in nitrobenzene (4.8) is 14% larger than the for NO2 in nitrate esters (4.2).
Water-to-solvent partition coefficients, in particular those for 1-octanol and chloroform, are often used in predicting solubilities and derived properties for various chemical species in and across cell membranes [30]. The paucity of solubility data for NO2 does not allow for the estimation of or using, for example, the solvation equations of Abraham as has been done for NO (5.5 and 8.3, respectively[21]; however, since, on the average, water-to-solvent partition coefficients for NO2 are about 40% of those for NO and are bracketed between 1.2 and 6.7, and the solvent the NO2-solvent interactions are of a very general type and do not appear to change much across solvents of various types, it is reasonable to expect the partition coefficient for water to the fatty acyl chains region of the cell membrane to be 3 ± 1.
Salting Out Effects
The biological aqueous medium ionic strength will result in a small salting out effect for the uncharged NO2 radical. Salting out effects for uncharged species like NO2 usually are small and obey a simple model for ionic strengths up to 5 M (equation 9) [31].
| (9) |
where γ is the activity coefficient for the uncharged species, b is a constant that is typically between 0 and 0.3 for small dissolved gases, and I is the ionic strength, usually less than 0.25 M for biological fluids [32]. Thus, 0 ≤ γ ≤ 1.19 for NO2 and since this effect will occur only in the aqueous phase, the net effect will be an increase in for NO2 of as much as 19 %.
Rate Laws for Reactions of NO2 with Biological Target Molecules
Rate laws for chemical reactions of NO2 (and its dimer N2O4 with which it trends to exist in equilibrium) with substrate molecules generally have the form qiven in equation (10)
| (10) |
where S represents the biological target molecule, and k1 and k2 are observed rate constants for the first and second order terms, respectively[33]. The second order term occurs not because the reaction has a termolecular component but instead because the reaction involves N2O4. This relationship occurs because [NO2]2 and [N2O4] are linearly related through the equilibrium constant for reaction 2 (equation 11).
| (11) |
On The Formation of N2O4
Because of equation (11), the [NO2] is one of the parameters that determine whether or not N2O4 will participate in these reactions. The equilibrium constant K11 for the dissociation of N2O4 is relatively insensitive to solvent effects and values obtained in various solvents range from 0.25×10−4 to 4.6×10−4 M at 37 °C [16;34–36]. The solution equilibria favor the formation of the dimer relative to the gas phase where K11 is 1.46×10−2 M [37] so it is important to evaluate whether or not N2O4 may play a role in the biological reactions of NO2. If one assumes [NO2] does not surpass 0.1 μM (a rather high concentration for biological systems, even for the less reactive NO [9]), then, one can calculate [NO2]/[N2O4]= K11/[NO2] will be between 250 and 4600. Thus, the equilibrium mixture will contain between 0.02 and 0.4% N2O4 on a molar basis. There is no doubt that there will be little N2O4 formed at equilibrium. However, it is unlikely that equilibrium will be reached, and consequently, that there will be even less N2O4 formed compared to equilibrium conditions. This is because the relatively high reactivity of NO2 for biological molecules will outcompete dimerization to form N2O4 since biological molecules can react with NO2 with rate constants [38] that although they can be smaller than the rate constant for the dimerization of NO2 (kN2O4 = 9×108 M−1s−1 in water [39]), the biological molecules can be present in concentrations that are much larger than NO2 can achieve in tissues and more than compensate for the difference in rate constants. The rates of disappearance of NO2 due to its dimerization (R12) and due to reaction with a biological substrate S (R13) can be expressed as depicted in equations 12 and 13, respectively.
| (12) |
| (13) |
As an example, one can evaluate R13/R12 = 0.5(kS/kN2O4)([S]/[NO2]) when [NO2] = 0.1×10−6 M, a high [NO2] that represents a favorable biological scenario for dimerization to N2O4. Now, let us consider that the substrate that will react with NO2 is intracellular glutathione (GSH) ([GSH]intracellular ≈ 2 mM; kGSH = 2×107 M−1s−1 [38]). Evaluation of R13/R12 for these values of rate constants and concentrations yields 200 revealing that it is very unlikely that any significant N2O4 will be formed even under conditions where high [NO2] is produced that favor formation of N2O4. Hence, it would be safe to assume that formation of N2O4 in biological systems will be very inefficient, that reaction products resulting from reactions of biological molecules with N2O4 will be negligible, and that N2O4 will not function as a substantial reservoir for NO2.
On The Formation of N2O3
NO2 can react with NO to form dinitrogen trioxide (N2O3) in a reversible reaction as depicted in reaction 14
| (14) |
This equilibrium has been studied by Shaw et. al. [27] and using their data one can calculate K14 = 0.34×10−4 M in carbon tetrachloride, and 2.2×10−4 in acetonitrile, at 37 °C. In water, K14 = 7.3×10−5 M at 25 °C [40] and assuming Δ14H = 62 kJmol−1 (as in acetonitrile [24]), one can calculate K14 = 1.9×10−4 M at 37 °C. As we observed with K11, K14 is also relatively insensitive to solvent effects. Moreover, K11 ≈ K14. The relative amount of NO2 that will react with NO to form N2O3 at equilibrium will depend on the concentrations of both of these reactants. For [NO] = 0.1 μM, one can calculate [NO2]/[N2O3]= K14/[NO] will be between 340 and 2200. Thus, only 0.05 to 0.3% of NO2 will form N2O3 at equilibrium when [NO] = 0.1 μM. The rate constant for the reaction of NO2 with NO (kN2O3 = 1.1×109 M−1s−1 in water [40]) to form N2O3 is also similar to the rate constant for the dimerization of NO2 (kN2O4 = 9×108 M−1s−1 [39]) to form N2O4. The rate laws for these processes are given in equations 15 and 16, respectively.
| (15) |
| (16) |
The overall case for N2O3 formation is thus similar to the case for N2O4, however, it is likely that [NO2] < [NO] and that R15 > R16, because of the higher reactivity of NO2 for biological molecules as compared to NO. Consequently, although conditions will not favor formation of either N2O3 or N2O4, N2O3 will generally be more likely to form than will be N2O4.
On the Reactions of NO2 with Target Molecules in Cell Membranes
As seen above, the reactions of NO2 with biological molecules follow rate laws that are first order in NO2 and first order in the biological molecule for environmentally and biologically relevant concentrations of NO2. Any gain in mass action due to gains in [NO2] in the hydrophobic region of the cell membrane will be modest because we estimate a water to the hydrophobic region of the cell membrane partition coefficient of 3 ± 1 (Figure 2). Reactions of NO2 in cell membranes will proceed with rates that are consistent with rate constants, concentrations for biological molecules in the cell membrane, and an estimated three-fold increase in [NO2].
Figure 2.
Conclusions
NO2 has a slight preference to dissolve in organic solvents compared to water and this may have some implications, for example, during reactive uptake of NO2 by the lung epithelial lining fluid. NO2 is 1.2 times more soluble in CCl4, 1.9 times in CHCl3, 4.2 times more soluble in the nitrate esters, 1,4- and 2-3-butylene glycol dinitrate, 1,2-propylene glycol dinitrate, and glyceryl trinitrate, 5.5 times in n-decane, and 6.7 times more soluble in 1,1,2,2-tetrachloroethane than it is in water, and the preference for solvent increases by approximately 19% when the ionic strength of the aqueous medium is taken into account. Henry’s law constants indicate NO2 is more soluble than NO in all solvents studied here with the most marked difference in water, resulting in a lower hydrophobicity for NO2, as compared to NO. The available data on the solubility of NO2 leads us to conclude that “lens” effects due to the slight hydrophobicity of NO2 will be small, probably around a factor of approximately 3 ± 1. Thus, only a small acceleration of NO2 reactions is expected to occur in hydrophobic cellular compartments (if all other experimental parameters remained unchanged). Nitrogen dioxide is less hydrophobic than NO, O2 and N2. Due to the low concentrations of NO2 that can be achieved in biological systems and in relation to the dissociation constant of N2O4, it is unlikely that N2O4, at any time, represents any significant reservoir of NO2, or significantly contributes to the reactivity of NO2. Finally, although biological conditions do not favor formation of either N2O3 or N2O4, it is estimated that N2O3 will generally be more likely to form than will be N2O4.
Figure 1.
Acknowledgments
This work was supported by National Institute of Health grant HL 54696. We are indebted to Professors Sara N. Mendiara and Luis J. Perissinotti from the Department of Chemistry of University of Mar del Plata, Argentina for critically reading our manuscript.
Reference List
- 1.Velsor LW, Ballinger CA, Patel J, Postlethwait EM. Influence of epithelial lining fluid lipids on NO2-induced membrane oxidation and nitration. Free Radical Biol Med. 2003;34:720–733. doi: 10.1016/s0891-5849(02)01370-9. [DOI] [PubMed] [Google Scholar]
- 2.Postlethwait EM, Langford SD, Jacobson LM, Bidani A. NO2 reactive absorption substrates in rat pulmonary surface lining fluids. Free Radical Biol Med. 1995;19:553–563. doi: 10.1016/0891-5849(95)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Postlethwait EM, Bidani A. Mechanisms of pulmonary NO2 absorption. Toxicology. 1994;89:217–237. doi: 10.1016/0300-483x(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 4.Postlethwait EM, Bidani A. Reactive uptake governs the pulmonary air space removal of inhaled nitrogen dioxide. J Appl Physiol. 1990;68:594–603. doi: 10.1152/jappl.1990.68.2.594. [DOI] [PubMed] [Google Scholar]
- 5.Squadrito GL, Pryor WA. Mapping the Reaction of Peroxynitrite with CO2: Energetics, Reactive Species, and Biological Implications. Chem Res Toxicol. 2002;15:885–895. doi: 10.1021/tx020004c. [DOI] [PubMed] [Google Scholar]
- 6.Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, Pryor WA. Reaction of Uric Acid with Peroxynitrite and Implications for the Mechanism of Neuroprotection by Uric Acid. Arch Biochem Biophys. 2000;376:333–337. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]
- 7.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radical Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 8.Burner U, Furtmuller PG, Kettle AJ, Koppenol WH, Obinger C. Mechanism of reaction of myeloperoxidase with nitrite. J Biol Chem. 2000;275:20597–20601. doi: 10.1074/jbc.M000181200. [DOI] [PubMed] [Google Scholar]
- 9.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsch M, Korth HG, Sustmann R, De Groot H. The pathobiochemistry of nitrogen dioxide. Biol Chem. 2002;383:389–399. doi: 10.1515/BC.2002.043. [DOI] [PubMed] [Google Scholar]
- 11.Wardman P. Nitrogen dioxide in biology: correlating chemical kinetics with biological effects. N-Centered. Radicals. In: Alfassi ZB, editor. The chemistry of N-centered radicals. Wiley; New York: pp. 155–179. [Google Scholar]
- 12.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA. Free radical biology and medicine: it’s a gas, man! Am J Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 13.Möller MN, Li Q, Lancaster JR, Denicola A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life. 2007;59:243–248. doi: 10.1080/15216540701311147. [DOI] [PubMed] [Google Scholar]
- 14.Möller MN, Li Q, Vittur DA, Robinson JM, Lancaster JR, Denicola A. Membrane “lens” effect: Focusing the formation of reactive nitrogen oxides from the NO/O2 reaction. Chemical Research in Toxicology. 2007;20:709–714. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 15.Lim CH, Dedon PC, Deen WM. Kinetic Analysis of Intracellular Concentrations of Reactive Nitrogen Species. Chem Res Toxicol. 2008;21:2134–2147. doi: 10.1021/tx800213b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendiara SN, Perissinotti LJ. Dissociation equilibrium of dinitrogen tetroxide in organic solvents: An electron paramagnetic resonance measurement. Applied Magnetic Resonance. 2003;25:323–346. [Google Scholar]
- 17.Lur’e BA, Arkhipov IV, Apal’kova VN. The N2O4 = 2NO2 Equilibrium in Solutions. Russian Journal of Physical Chemistry. 1986;60:1144–1147. [Google Scholar]
- 18.Cheung JL, Li YQ, Boniface J, Shi Q, Davidovits P, Worsnop DR, Jayne JT, Kolb CE. Heterogeneous interactions of NO2 with aqueous surfaces. Journal of Physical Chemistry A. 2000;104:2655–2662. [Google Scholar]
- 19.Nottingham WC, Sutter JR. Kinetics of the oxidation of nitric oxide by chlorine and oxygen in nonaqueous media. Int J Chem Kinet. 1986;18:1289–1302. [Google Scholar]
- 20.Sander R. Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry (Version 3) . 1999 http://www.henrys-law.org.
- 21.Abraham MH, Gola JMR, Cometto-Muniz JE, Cain WS. The solvation properties of nitric oxide. Perkin. 2000;2:2067–2070. [Google Scholar]
- 22.Battino R, editor. IUPAC Solubility Data Series, Vol. 7: Oxygen and Ozone. Pergamon Press; New York: pp. 1–559. [Google Scholar]
- 23.Battino R, Rettich TR, Tominaga T. The solubility of nitrogen and air in liquids. J Phys Chem Ref Data. 1984;13:563–600. [Google Scholar]
- 24.Vosper AJ, Shaw AW. Dinitrogen trioxide. IX. Stability of dinitrogen trioxide in solution. J Chem Soc. 1971;A:1592–1595. [Google Scholar]
- 25.Zacharia IG, Deen WM. Diffusivity and solubility of nitric oxide in water and saline. Annals of Biomedical Engineering. 2005;33:214–222. doi: 10.1007/s10439-005-8980-9. [DOI] [PubMed] [Google Scholar]
- 26.Perry RH, Green DW, editors. Perry’s Chemical Engineer’s Handbook. McGraw-Hill; New York: [Google Scholar]
- 27.Shaw AW, Vosper AJ. Solubility of nitric oxide in aqueous and nonaqueous solvents. J Chem Soc, Faraday Trans 1. 1977;73:1239–1244. [Google Scholar]
- 28.Abraham MH, Acree WE., Jr Correlation and prediction of partition coefficients between the gas phase and water, and the solvents dodecane and undecane. New J Chem. 2004;28:1538–1543. [Google Scholar]
- 29.Möller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005;280:8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 30.Sangster J. Octanol-water partition coefficients: fundamentals and physical chemistry. John Wiley and Sons; Chichester: 1997. [Google Scholar]
- 31.Butler JN. Ionic equilibrium: solubility and pH calculations. Wiley-Interscience; New York: 1998. [Google Scholar]
- 32.Alberty RA. Biochemical thermodynamics: Applications of Mathematica. Methods Biochem Anal. 2006;48:1–458. [PubMed] [Google Scholar]
- 33.Giamalva DH, Kenion GB, Church DF, Pryor WA. Rates and mechanisms of reactions of nitrogen dioxide with alkenes in solution. J Am Chem Soc. 1987;109:7059–7063. [Google Scholar]
- 34.Boughriet A, Wartel M. Thermodynamic and kinetic constants relative to some molecular equilibria of oxygenated nitrogen compounds and nitrogen oxychlorides in aprotic media. Application to the mechanistic study of aromatic nitration with dinitrogen tetroxide and nitrogen sesquioxide. J Electroanal Chem Interfacial Electrochem. 1988;251:127–141. [Google Scholar]
- 35.Miaskiewicz K, Kecki Z. EPR study of the dinitrogen tetroxide dissociation in organic solvents. J Solution Chem. 1985;14:665–673. [Google Scholar]
- 36.Redmond TF, Wayland BB. Dimerization of nitrogen dioxide in solution: a comparison of solution thermodynamics with the gas phase. J Phys Chem. 1968;72:1626–1629. [Google Scholar]
- 37.Hisatsune IC. Thermodynamic properties of some oxides of nitrogen. J Phys Chem. 1961;65:2249–2253. [Google Scholar]
- 38.Ford E, Hughes MN, Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radical Biol Med. 2002;32:1314–1323. doi: 10.1016/s0891-5849(02)00850-x. [DOI] [PubMed] [Google Scholar]
- 39.Grätzel M, Henglein A, Lilie J, Beck G. Pulse radiolytic study of some elementary processes of nitrite ion oxidation and reduction. Ber Bunsenges Phys Chem. 1969;73:646–653. [Google Scholar]
- 40.Grätzel M, Taniguchi S, Henglein A. Pulse radiolytic study of NO oxidation and of the equilibrium N2O3 = NO + NO2 in aqueous solution. Ber Bunsenges Phys Chem. 1970;74:488–492. [Google Scholar]


